Heteroleptic [Cu(P^P)(N^N)][PF6] Compounds with Isomeric Dibromo-1,10-Phenanthroline Ligands
Abstract
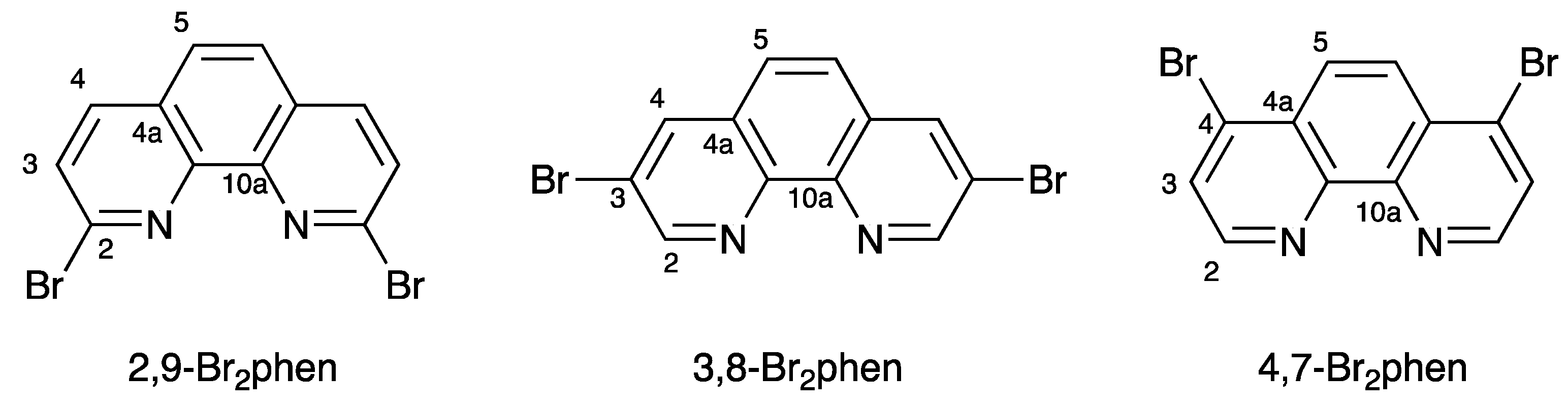
1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of Copper(I) Complexes
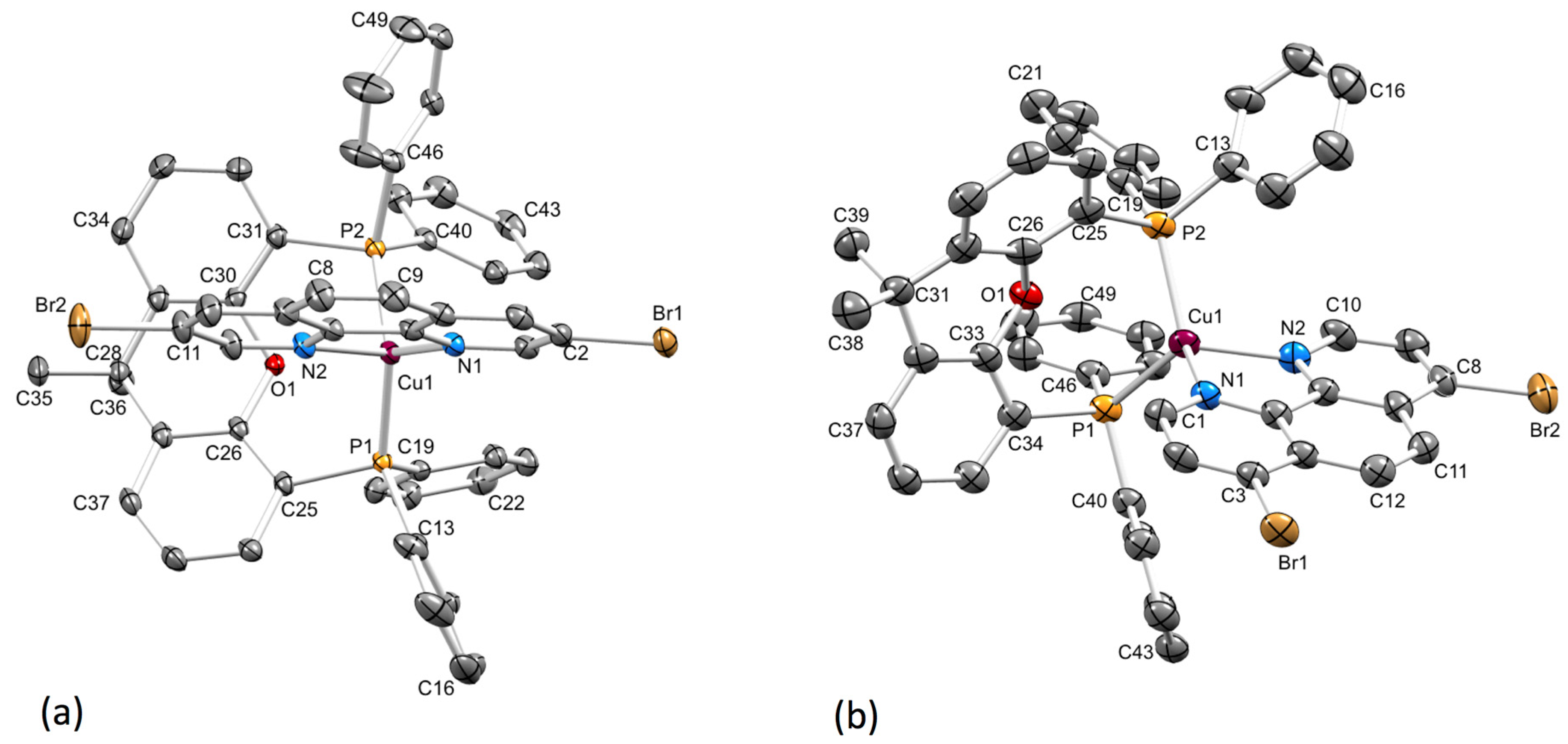
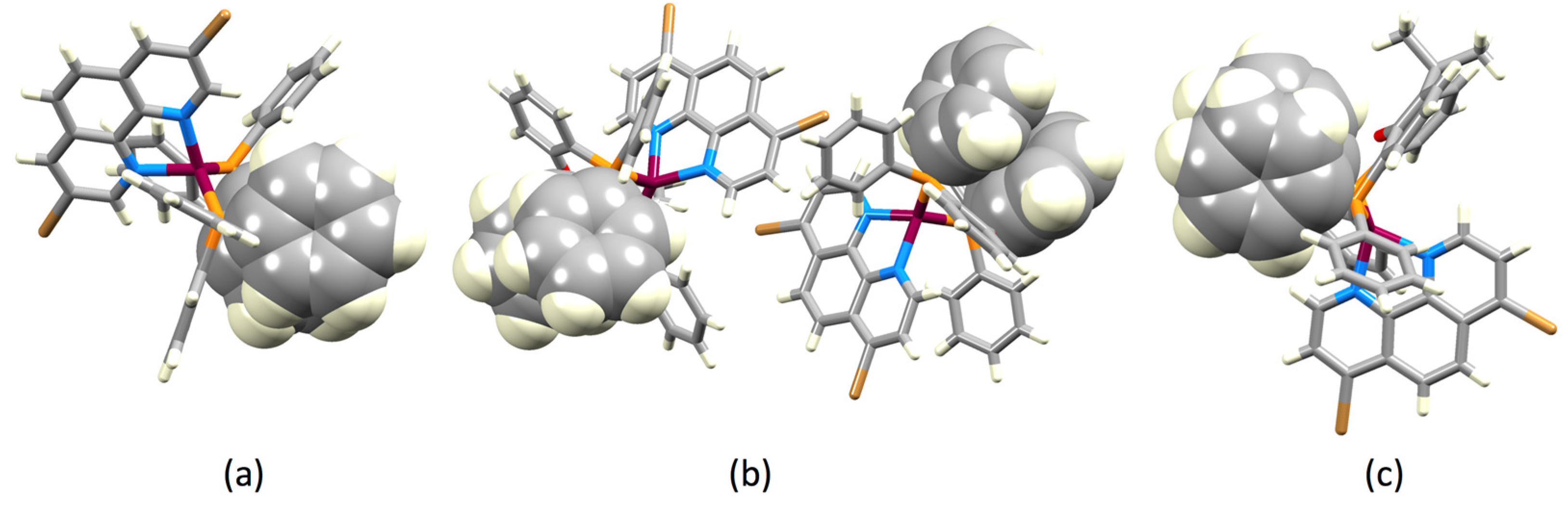
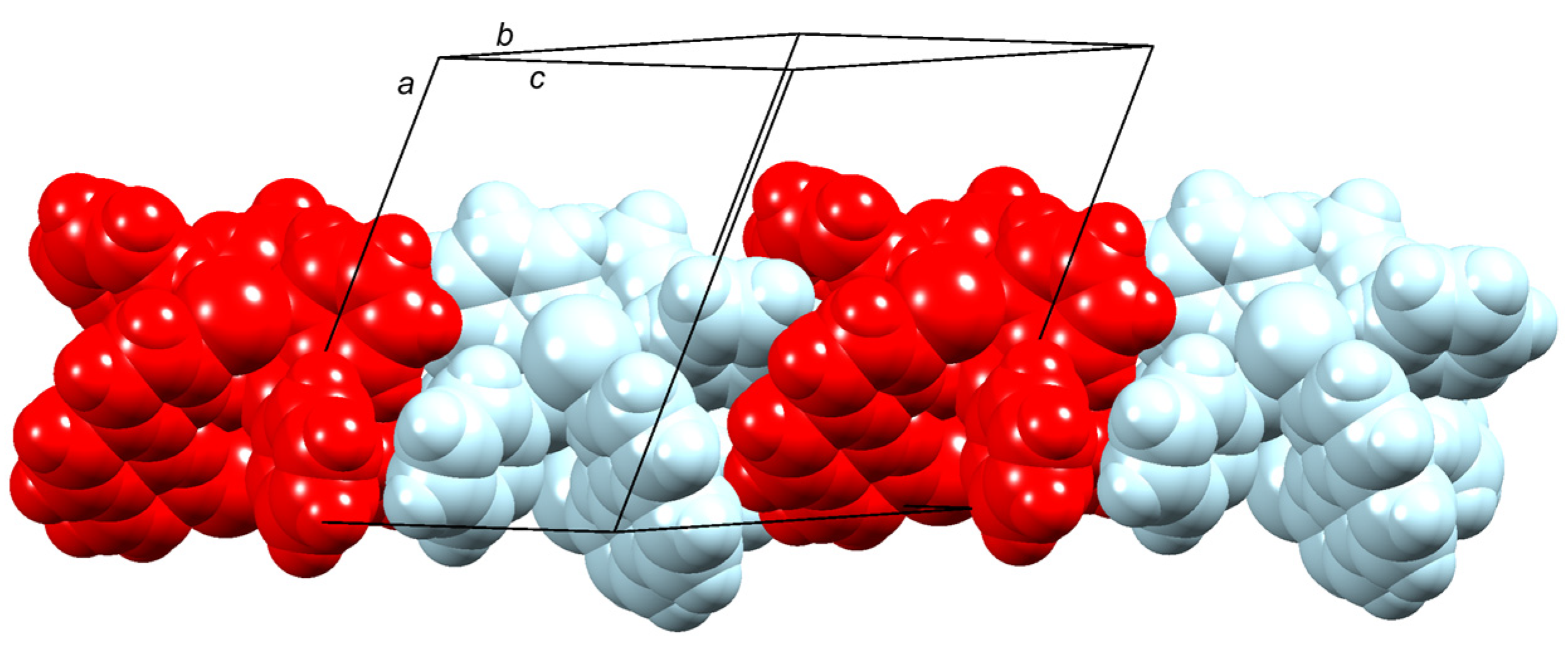
2.2. Crystal Structures
2.3. Electrochemistry
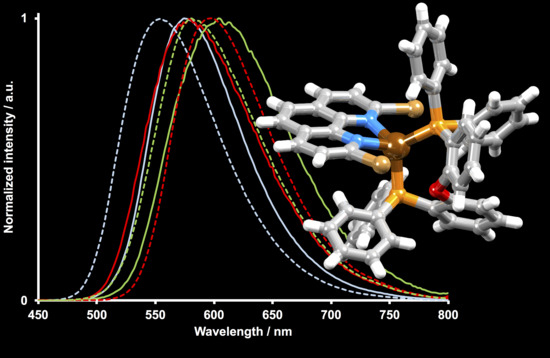
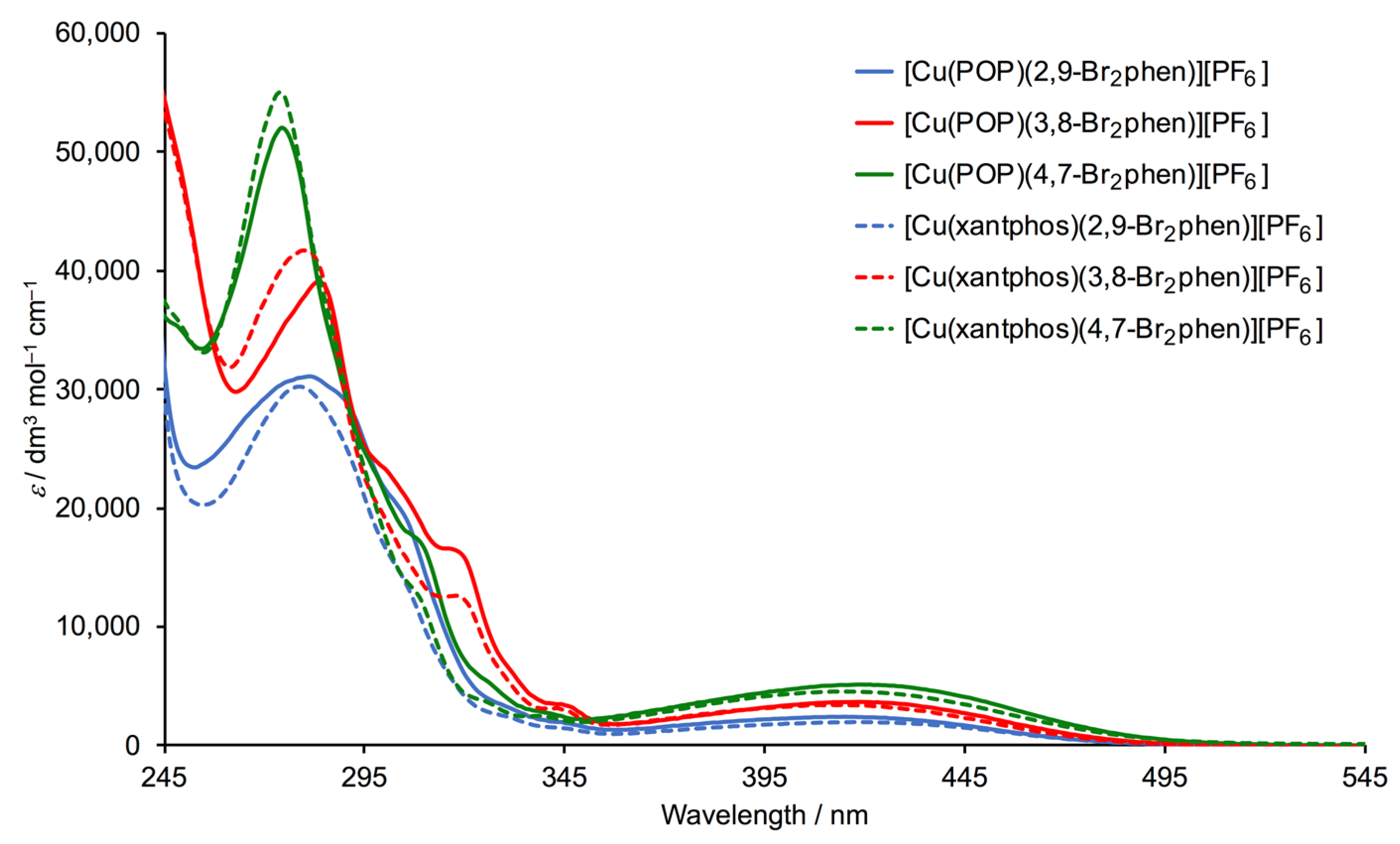
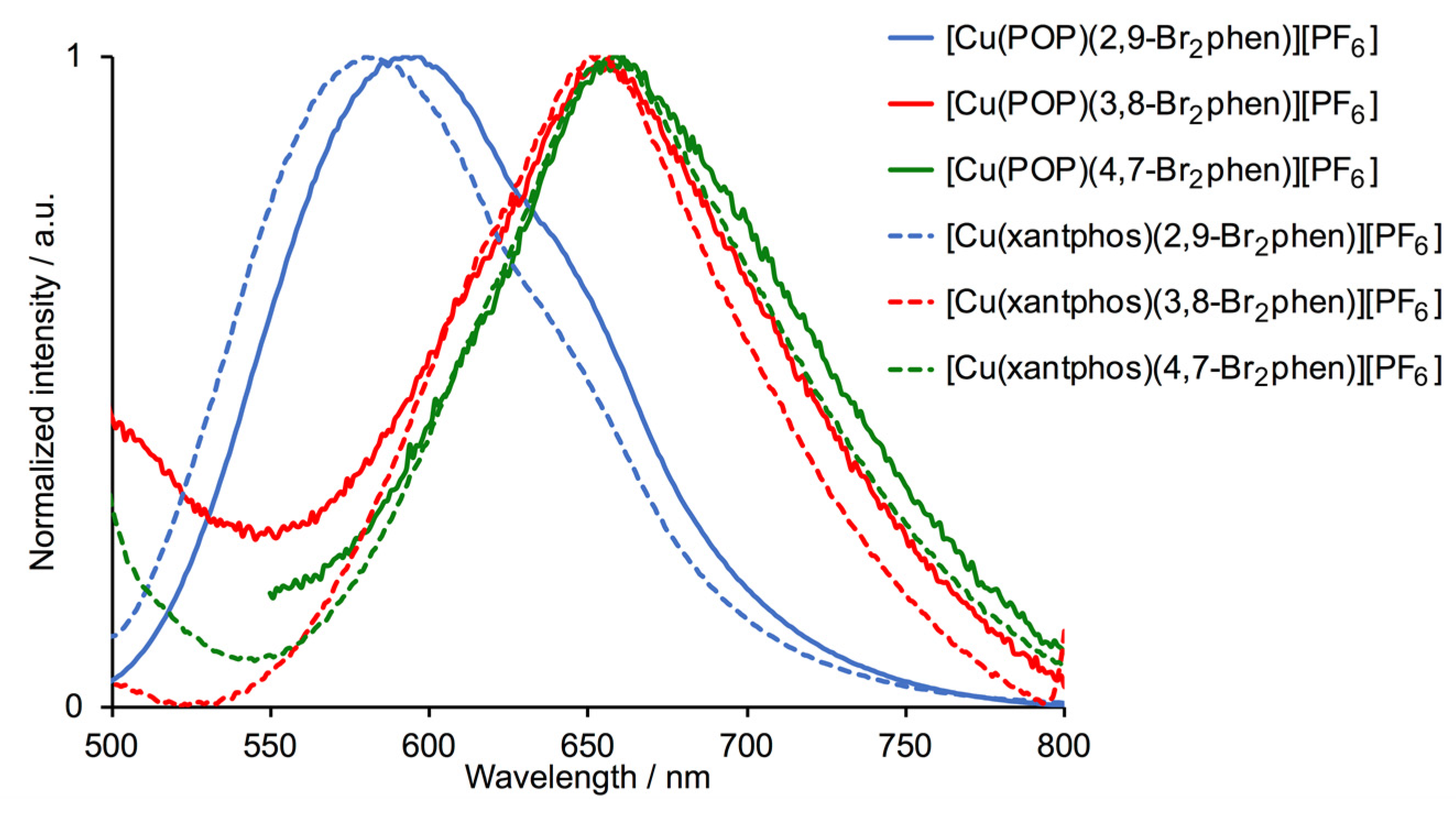
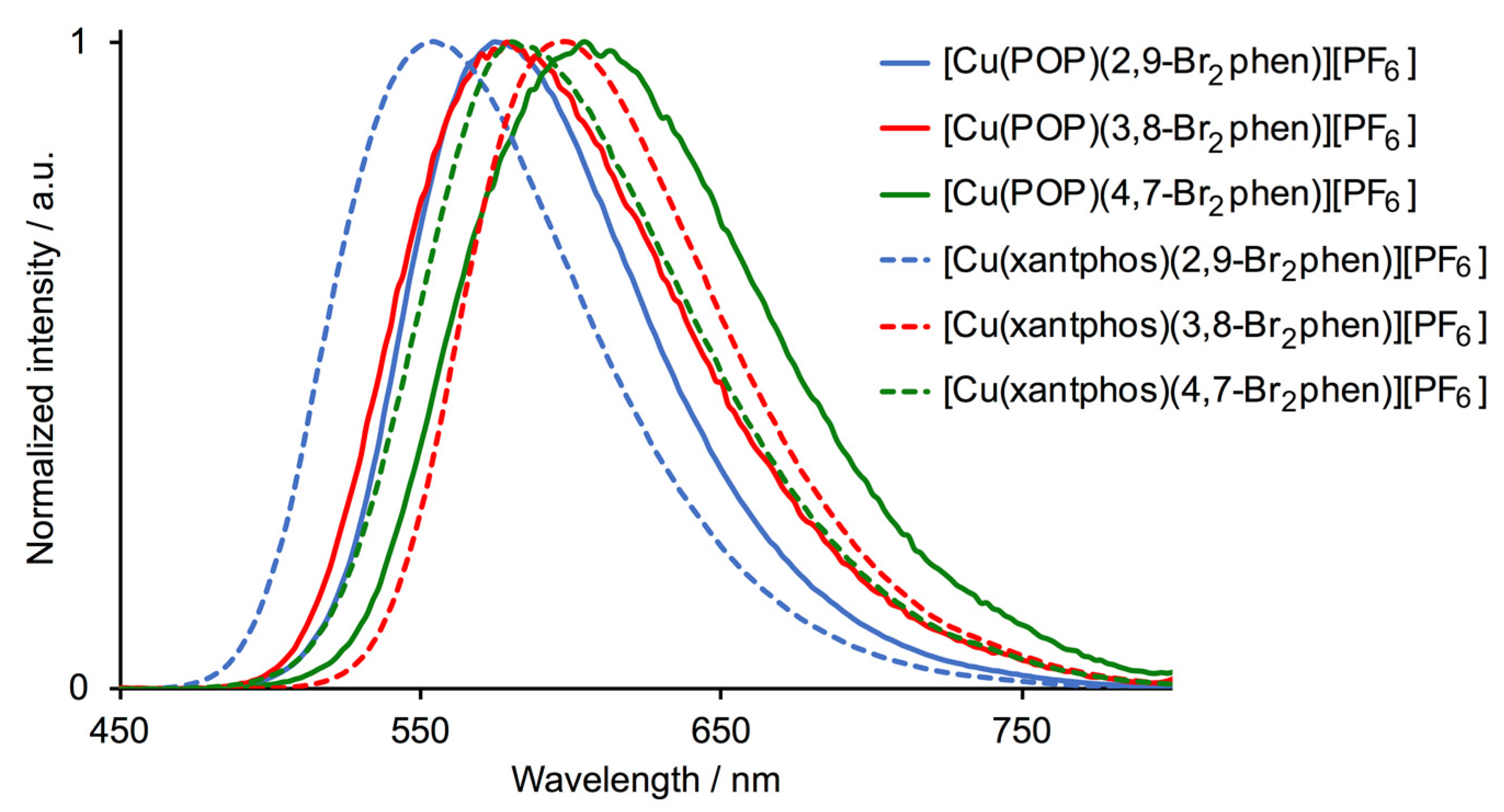
2.4. Photophysical Properties
3. Materials and Methods
3.1. General
3.2. General Procedures for Copper(I) Compound Synthesis
3.3. [Cu(POP)(2,9-Br2phen)][PF6]
3.4. [Cu(POP)(3,8-Br2phen)][PF6]
3.5. [Cu(POP)(4,7-Br2phen)][PF6]
3.6. [Cu(xantphos)(2,9-Br2phen)][PF6]
3.7. [Cu(xantphos)(3,8-Br2phen)][PF6]
3.8. [Cu(xantphos)(4,7-Br2phen)][PF6]
3.9. Crystallography
3.10. [Cu(POP)(2,9-Br2phen)][PF6]·0.5Et2O
3.11. [Cu(POP)(3,8-Br2phen)][PF6]·0.8CH2Cl2·0.9H2O
3.12. [Cu(POP)(4,7-Br2phen)][PF6]·CH2Cl2
3.13. [Cu(xantphos)(2,9-Br2phen)][PF6]·1.1CH2Cl2
3.14. [Cu(xantphos)(3,8-Br2phen)][PF6]·1.1CH2Cl2·0.7Et2O
3.15. [Cu(xantphos)(4,7-Br2phen)][PF6]·CH2Cl2·0.9Et2O
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Buckner, M.T.; McMillin, D.R. Photoluminescence from copper(I) complexes with low-lying metal-to-ligand charge transfer excited states. J. Chem. Soc. Chem. Commun. 1978, 17, 759–761. [Google Scholar] [CrossRef]
- Rader, R.A.; McMillin, D.R.; Buckner, M.T.; Matthews, T.G.; Casadonte, D.J.; Lengel, R.K.; Whittaker, S.B.; Darmon, L.M.; Lytle, F.E. Photostudies of 2,2’-bipyridine bis(triphenylphosphine)copper(1+), 1,10-phenanthroline bis(triphenylphosphine)copper(1+), and 2,9-dimethyl-1,10-phenanthroline bis(triphenylphosphine)copper(1+) in solution and in rigid, low-temperature glasses. Simultaneous multiple emissions from intraligand and charge-transfer states. J. Am. Chem. Soc. 1981, 103, 5906–5912. [Google Scholar] [CrossRef]
- Yersin, H.; Czerwieniec, R.; Shafikov, M.Z.; Suleymanova, A.F. TADF Material Design: Photophysical Background and Case Studies Focusing on CuI and AgI Complexes. ChemPhysChem 2017, 18, 3508–3535. [Google Scholar] [CrossRef] [PubMed]
- Leitl, M.J.; Krylova, V.A.; Djurovich, P.I.; Thompson, M.E.; Yersin, H. Phosphorescence versus Thermally Activated Delayed Fluorescence. Controlling Singlet–Triplet Splitting in Brightly Emitting and Sublimable Cu(I) Compounds. J. Am. Chem. Soc. 2014, 136, 16032–16038. [Google Scholar] [CrossRef] [PubMed]
- Czerwieniec, R.; Leitl, M.J.; Homeier, H.H.H.; Yersin, H. Cu(I) complexes—Thermally activated delayed fluorescence. Photophysical approach and material design. Coord. Chem. Rev. 2016, 325, 2–28. [Google Scholar] [CrossRef]
- Costa, R.D.; Ortí, E.; Bolink, H.J.; Monti, F.; Accorsi, G.; Armaroli, N. Luminescent ionic transition-metal complexes for light-emitting electrochemical cells. Angew. Chem. Int. Ed. 2012, 51, 8178–8211. [Google Scholar] [CrossRef]
- Elie, M.; Gaillard, S.; Renaud, J.-L. Luminescent Cationic Copper(I) Complexes: Synthesis, Photophysical Properties and Application in Light-Emitting Electrochemical Cells. In Light-Emitting Electrochemical Cells: Concepts, Advances and Challenges; Costa, R.D., Ed.; Springer: Cham, Switzerland, 2017; Chapter 11; pp. 287–327. [Google Scholar] [CrossRef]
- Fresta, E.; Costa, R.D. Beyond traditional light-emitting electrochemical cells—A review of new device designs and emitters. J. Mater. Chem. C 2017, 5, 5643–5675. [Google Scholar] [CrossRef]
- Housecroft, C.E.; Constable, C.E. Over the LEC rainbow: Colour and stability tuning of cyclometallated iridium(III) complexes in light-emitting electrochemical cells. Coord. Chem. Rev. 2017, 350, 155–177. [Google Scholar] [CrossRef]
- Alkan-Zambada, M.; Keller, S.; Martínez-Sarti, L.; Prescimone, A.; Junquera-Hernández, J.M.; Constable, E.C.; Bolink, H.J.; Sessolo, M.; Ortí, E.; Housecroft, C.E. [Cu(P^P)(N^N)][PF6] compounds with bis(phosphane) and 6-alkoxy, 6-alkylthio, 6-phenyloxy and 6-phenylthio-substituted 2,2′-bipyridine ligands for light-emitting electrochemical cells. J. Mater. Chem. C 2018, 6, 8460–8471. [Google Scholar] [CrossRef]
- Leoni, E.; Mohanraj, J.; Holler, M.; Mohankumar, M.; Nierengarten, I.; Monti, F.; Sournia-Saquet, A.; Delavaux-Nicot, B.; Nierengarten, J.-F.; Armaroli, N. Heteroleptic copper(I) complexes prepared from phenanthroline and bis-phosphine ligands: Rationalization of the photophysical and electrochemical properties. Inorg. Chem. 2018, 57, 15537–15549. [Google Scholar] [CrossRef]
- Zhang, Y.; Schulz, M.; Wächtler, M.; Karnahl, M.; Dietzek, B. Heteroleptic diimine-diphosphine Cu(I) complexes as an alternative towards noble-metal based photosensitizers: Design strategies, photophysical properties and perspective applications. Coord. Chem. Rev. 2018, 356, 127–146. [Google Scholar] [CrossRef]
- Keller, S.; Constable, E.C.; Housecroft, C.E.; Neuburger, M.; Prescimone, A.; Longo, G.; Pertegás, A.; Sessolo, M.; Bolink, H.J. [Cu(bpy)(P^P)]+ containing light-emitting electrochemical cells: Improving performance through simple substitution. Dalton Trans. 2014, 43, 16593–16596. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.; Pertegás, A.; Longo, G.; Martinez, L.; Cerdá, J.; Junquera-Hernández, J.M.; Prescimone, A.; Constable, E.C.; Housecroft, C.E.; Ortí, E.; et al. Shine bright or live long: Substituent effects in [Cu(N^N)(P^P)]+-based light-emitting electrochemical cells where N^N is a 6-substituted 2,2′-bipyridine. J. Mater. Chem. C 2016, 4, 3857–3871. [Google Scholar] [CrossRef]
- Fresta, E.; Volpi, G.; Milanesio, M.; Garino, C.; Barolo, C.; Costa, R.D. Novel Ligand and Device Designs for Stable Light-Emitting Electrochemical Cells Based on Heteroleptic Copper(I) Complexes. Inorg. Chem. 2018, 57, 10469–10479. [Google Scholar] [CrossRef]
- Keller, S.; Prescimone, A.; Bolink, H.J.; Sessolo, M.; Longo, G.; Martínez-Sarti, L.; Junquera-Hernández, J.M.; Constable, E.C.; Ortí, E.; Housecroft, C.E. Luminescent copper(I) complexes with bisphosphane and halogen-substituted 2,2’-bipyridine ligands. Dalton Trans. 2018, 47, 14263–14276. [Google Scholar] [CrossRef]
- Yuasa, J.; Dan, M.; Kawai, T. Phosphorescent properties of metal-free diphosphine ligands and effects of copper binding. Dalton Trans. 2013, 42, 16096–16101. [Google Scholar] [CrossRef]
- Costa, R.D.; Tordera, D.; Ortí, E.; Bolink, H.J.; Schönle, J.; Graber, S.; Housecroft, C.E.; Constable, E.C.; Zampese, J.A. Copper(I) complexes for sustainable light-emitting electrochemical cells. J. Mater. Chem. 2011, 21, 16108–16118. [Google Scholar] [CrossRef]
- Brunner, F.; Graber, S.; Baumgartner, Y.; Häussinger, D.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. The effects of introducing sterically demanding aryl substituents in [Cu(N^N)(P^P)]+ complexes. Dalton Trans. 2017, 46, 6379–6391. [Google Scholar] [CrossRef]
- Kaeser, A.; Mohankumar, M.; Mohanraj, J.; Monti, F.; Holler, M.; Cid, J.J.; Moudam, O.; Nierengarten, I.; Karmazin-Brelot, L.; Duhayon, C.; et al. Heteroleptic Copper(I) Complexes Prepared from Phenanthroline and Bis-Phosphine Ligands. Inorg. Chem. 2013, 52, 12140–12151. [Google Scholar] [CrossRef]
- Graber, S.; Doyle, K.; Neuburger, M.; Housecroft, C.E.; Constable, E.C.; Costa, R.D.; Ortí, E.; Repetto, D.; Bolink, H.J. A supramolecularly-caged ionic iridium(III) complex yielding bright and very stable solid-state light-emitting electrochemical cells. J. Am. Chem. Soc. 2008, 130, 14944–14945. [Google Scholar] [CrossRef]
- Bünzli, A.M.; Constable, E.C.; Housecroft, C.E.; Prescimone, A.; Zampese, J.A.; Longo, G.; Gil-Escrig, L.; Pertegás, A.; Bolink, H.J. Exceptionally long-lived light-emitting electrochemical cells: Multiple intra-cation π-stacking interactions in [Ir(C^N)2(N^N)][PF6] emitters. Chem. Sci. 2015, 6, 2843–2852. [Google Scholar] [CrossRef] [PubMed]
- Casadonte, D.J.; McMillin, D.R. Hindered Internal Conversion in Rigid Media. Thermally Nonequilibrated 3IL and 3CT Emissions from [Cu(5-X-phen)(PPh3)2]+ and [Cu(4,7-X2-phen)(PPh3)2]+ Systems in a Glass at 77 K. J. Am. Chem. Soc. 1987, 109, 331–337. [Google Scholar] [CrossRef]
- Li, X.-L.; Ai, Y.-B.; Yang, B.; Chen, J.; Tan, M.; Xin, X.-L.; Shi, Y.-H. Syntheses, structures and photophysical properties of a series of luminescent copper(I) mixed-ligand complexes. Polyhedron 2012, 35, 47–54. [Google Scholar] [CrossRef]
- Xin, X.-L.; Chen, M.; Ai, Y.-B.; Yang, F.; Li, X.-L.; Li, F. Aggregation-Induced Emissive Copper(I) Complexes for Living Cell Imaging. Inorg. Chem. 2014, 53, 2922–2931. [Google Scholar] [CrossRef]
- Feng, X.-Y.; Xin, X.-L.; Guo, Y.-M.; Chen, L.-L.; Liang, Y.-Y.; Xu, M.; Li, X.-L. Synthesis, structure and solid luminescence of copper(I)–bromodiimine-diphosphine complexes. Polyhedron 2015, 101, 23–28. [Google Scholar] [CrossRef]
- Brown-Xu, S.; Fumanal, M.; Gourlaouen, C.; Gimeno, L.; Quatela, A.; Thobie-Gautier, C.; Blart, E.; Planchat, A.; Riobé, F.; Monnereau, C.; et al. Intriguing Effects of Halogen Substitution on the Photophysical Properties of 2,9-(Bis)halo-Substituted Phenanthrolinecopper(I) Complexes. Inorg. Chem. 2019, 58, 7730–7745. [Google Scholar] [CrossRef]
- Yang, L.; Powell, D.R.; Houser, R.P. Structural variation in copper(I) complexes with pyridylmethylamide ligands: Structural analysis with a new four-coordinate geometry index, τ4. Dalton Trans. 2007, 955–964. [Google Scholar] [CrossRef]
- Yang, L.; Feng, J.-K.; Ren, A.-M.; Zhang, M.; Ma, Y.-G.; Liu, X.-D. Structures, Electronic States and Electroluminescent Properties of a Series of CuI Complexes. Eur. J. Inorg. Chem. 2005, 1867–1879. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. 2016, B72, 171–179. [Google Scholar] [CrossRef]
- Cuttell, D.G.; Kuang, S.-M.; Fanwick, P.E.; McMillin, D.R.; Walton, R.A. Simple Cu(I) Complexes with Unprecedented Excited-State Lifetimes. J. Am. Chem. Soc. 2002, 124, 6–7. [Google Scholar] [CrossRef]
- Smith, C.S.; Branham, C.W.; Marquardt, B.J.; Mann, K.R. Oxygen Sensing by Luminescence Quenching in Crystals of Cu(xantphos)(phen)+ Complexes. J. Am. Chem. Soc. 2010, 132, 14079–14085. [Google Scholar] [CrossRef] [PubMed]
- Kubas, G.J.; Monzyk, B.; Crumbliss, A.L. Tetrakis(acetonitrile)copper(I) hexafluorophosphate. Inorg. Synth. 1979, 19, 90–92. [Google Scholar] [CrossRef]
- Guo, H.C.; Zheng, R.H.; Jiang, H.J. Improved Synthesis of 2,9-Dichloro-1,10-phenanthroline. Org. Prep. Proced. Internat. 2012, 44, 392–396. [Google Scholar] [CrossRef]
- Thomas, S.W.; Venkatesan, K.; Müller, P.; Swager, T.M. Dark-field Oxidative Addition-based Chemosensing: New Bis-cyclometalated Pt(II) COmplexes and Phosphorescent Detection of Cyanogen Halides. J. Am. Chem. Soc. 2006, 128, 16641–16648. [Google Scholar] [CrossRef] [PubMed]
- Software for the Integration of CCD Detector System Bruker Analytical X-ray Systems; Bruker AXS: Madison, WI, USA, 2013.
- Sheldrick, G.M. ShelXT-Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. Olex2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Cryst. 2015, C27,, 3–8. [Google Scholar] [CrossRef]
- Palatinus, L.; Chapuis, G. Superflip—A Computer Program for the Solution of Crystal Structures by Charge Flipping in Arbitrary Dimensions. J. Appl. Cryst. 2007, 40, 786–790. [Google Scholar] [CrossRef]
- Palatinus, L.; Prathapa, S.J.; van Smaalen, S. EDMA: A Computer Program for Topological Analysis of Discrete Electron Densities. J. Appl. Cryst. 2012, 45, 575–580. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: Visualization and Analysis of Crystal Structures. J. Appl. Cryst. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New Features for the Visualization and Investigation of Crystal Structures. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Spek, A.L. Platon Squeeze: A Tool for the Calculation of the Disordered Solvent Contribution to the Calculated Structure Factors. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 9–18. [Google Scholar] [CrossRef] [PubMed]


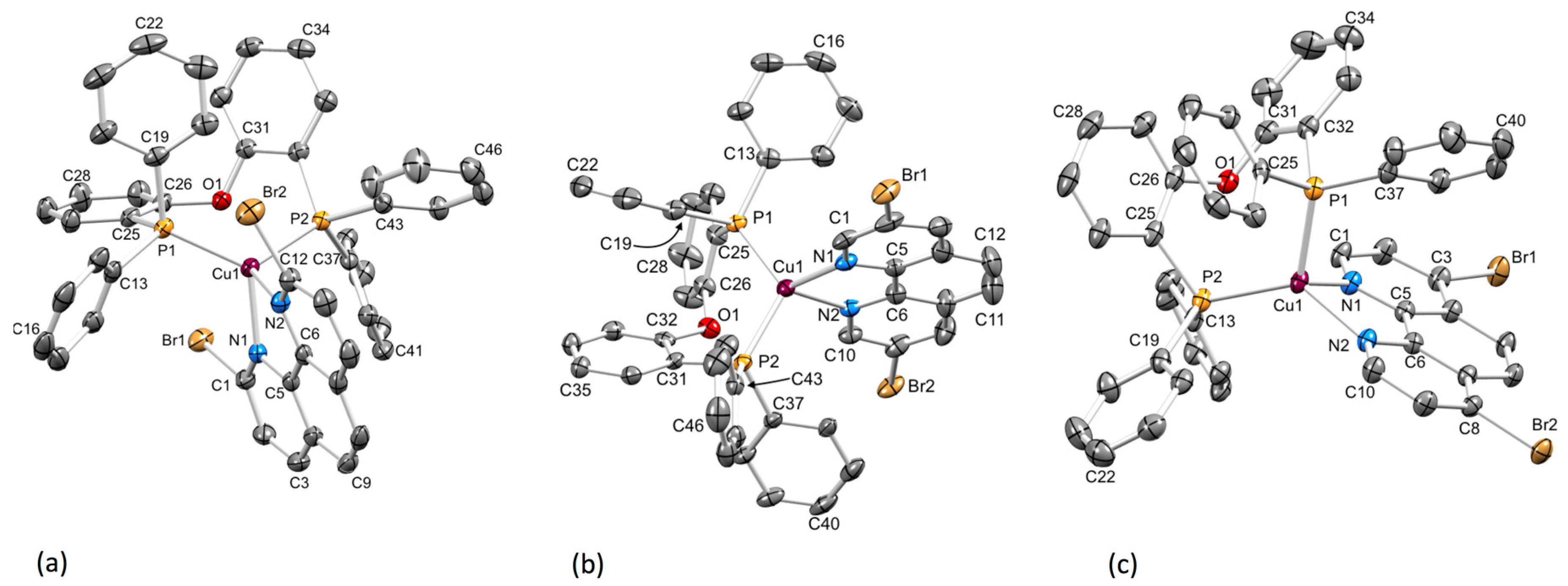

| [Cu(P^P)(N^N)]+ Cation 1 | Cu–N/Å | Cu–P/Å | P–Cu–P/Deg | N–Cu–N/Deg | τ4 2 |
|---|---|---|---|---|---|
| [Cu(POP)(2,9-Br2phen)]+ Cation 1 | 2.100(2), 2.135(2) | 2.2742(7), 2.2416(7) | 118.00(3) | 79.61(8) | 0.87 |
| [Cu(POP)(2,9-Br2phen)]+ Cation 2 | 2.149(2), 2.129(2) | 2.2849(7), 2.2704(7) | 116.47(3) | 78.46(9) | 0.87 |
| [Cu(POP)(3,8-Br2phen)]+ | 2.055(6), 2.083(5) | 2.2468(17), 2.2332(18) | 115.65(7) | 80.9(2) | 0.88 |
| [Cu(POP)(4,7-Br2phen)]+ Cation 1 | 2.0797(19), 2.0607(19) | 2.2622(7), 2.2377(7) | 112.37(2) | 80.22(7) | 0.84 |
| [Cu(POP)(4,7-Br2phen)]+ Cation 2 | 2.088(2), 2.0683(19) | 2.2694(7), 2.2370(7) | 112.52(3) | 80.34(8) | 0.85 |
| [Cu(xantphos)(2,9-Br2phen)]+ | 2.115(4), 2.115(4) 3 | 2.295(2), 2.2523(18) | 117.97(8) | 79.2(2) | 0.83 |
| [Cu(xantphos)(3,8-Br2phen)]+ | 2.079(2), 2.122(2) | 2.2495(7), 2.2752(7) | 116.57(3) | 80.45(8) | 0.87 |
| [Cu(xantphos)(4,7-Br2phen)]+ | 2.075(4), 2.069(5) | 2.2619(15), 2.2591(15) | 115.36(6) | 80.29(16) | 0.87 |
| Cation in [Cu(P^P)(N^N)][PF6] | E1/2ox/V | Epc − Epa/mV | Ipc/Ipa | Epca | E1/2red/V b |
|---|---|---|---|---|---|
| [Cu(POP)(2,9-Br2phen)]+ | +0.98 | −2.23, −2.10, −1.92, −1.83 | |||
| [Cu(POP)(3,8-Br2phen)]+ | +0.86 | 120 | 0.13 | −2.20, −1.94, −1.83 | |
| [Cu(POP)(4,7-Br2phen)]+ | +0.79 | 130 | 0.12 | −2.17, −1.93, −1.84 | |
| [Cu(xantphos)(2,9-Br2phen)]+ | +0.97 | 105 | 0.19 | −2.08, −1.85, −1.74 | |
| [Cu(xantphos)(3,8-Br2phen)]+ | +0.87 | −2.19, −1.90, −1.81 | |||
| [Cu(xantphos)(4,7-Br2phen)]+ | +0.95 | −2.17, −1.87, −1.77 |
| Cation in [Cu(P^P)(N^N)][PF6] | λmax/nm (εmax/dm3 mol–1 cm–1) | |
|---|---|---|
| Ligand-Based Absorptions | MLCT | |
| [Cu(POP)(2,9-Br2phen)]+ | 281 (31,100), 304 sh (19,800), 346 (2900) | 415 (2400) |
| [Cu(POP)(3,8-Br2phen)]+ | 283 (39,100), 302 sh (22,500), 319 sh (16,200), 346 (2900) | 418 (3700) |
| [Cu(POP)(4,7-Br2phen)]+ | 274 (52,000), 309 sh (17,000), 327 sh (5000) | 418 (5100) |
| [Cu(xantphos)(2,9-Br2phen)]+ | 280 (30,200), 304 sh (19,800), 346 (2900) | 415 (2400) |
| [Cu(xantphos)(3,8-Br2phen)]+ | 282 (41,700), 320 sh (12,500), 346 (2900) | 416 (3400) |
| [Cu(xantphos)(4,7-Br2phen)]+ | 275 (54,500), 309 sh (12,200), 327 sh (5000) | 420 (4500) |
| Cation in [Cu(P^P)(N^N)][PF6] | Solution | Powder | |||
|---|---|---|---|---|---|
| λexc/nm | λemmax/nm | λemmax/nm a | PLQY/% | τ1/2/μs | |
| [Cu(POP)(2,9-Br2phen)]+ | 415 | 596 | 574 | 24 | 6.3 |
| [Cu(POP)(3,8-Br2phen)]+ | 420 | 655 | 578 | 3 | 0.9 |
| [Cu(POP)(4,7-Br2phen)]+ | 425 | 660 | 604 | 7 | 1.7 |
| [Cu(xantphos)(2,9-Br2phen)]+ | 425 | 582 | 554 | 45 | 9.9 |
| [Cu(xantphos)(3,8-Br2phen)]+ | 410 | 653 | 598 | 21 | 8.4 |
| [Cu(xantphos)(4,7-Br2phen)]+ | 420 | 657 | 580 | 12 | 4.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nohara, I.; Keller, A.; Tarassenko, N.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. Heteroleptic [Cu(P^P)(N^N)][PF6] Compounds with Isomeric Dibromo-1,10-Phenanthroline Ligands. Inorganics 2020, 8, 4. https://doi.org/10.3390/inorganics8010004
Nohara I, Keller A, Tarassenko N, Prescimone A, Constable EC, Housecroft CE. Heteroleptic [Cu(P^P)(N^N)][PF6] Compounds with Isomeric Dibromo-1,10-Phenanthroline Ligands. Inorganics. 2020; 8(1):4. https://doi.org/10.3390/inorganics8010004
Chicago/Turabian StyleNohara, Isaak, Aramis Keller, Nikolai Tarassenko, Alessandro Prescimone, Edwin C. Constable, and Catherine E. Housecroft. 2020. "Heteroleptic [Cu(P^P)(N^N)][PF6] Compounds with Isomeric Dibromo-1,10-Phenanthroline Ligands" Inorganics 8, no. 1: 4. https://doi.org/10.3390/inorganics8010004
APA StyleNohara, I., Keller, A., Tarassenko, N., Prescimone, A., Constable, E. C., & Housecroft, C. E. (2020). Heteroleptic [Cu(P^P)(N^N)][PF6] Compounds with Isomeric Dibromo-1,10-Phenanthroline Ligands. Inorganics, 8(1), 4. https://doi.org/10.3390/inorganics8010004