Sol-Gel Combustion Synthesis, Crystal Structure and Luminescence of Cr3+ and Mn4+ Ions in Nanocrystalline SrAl4O7
Abstract
:1. Introduction
2. Results and Discussion
2.1. Evolution of Phase Composition, Crystal Structure, and Microstructural Parameters
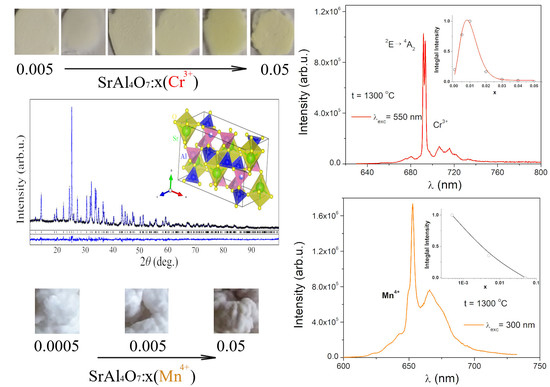
2.2. Luminescent Properties
3. Materials and Methods
3.1. Samples Preparation
3.2. Physical–Chemical Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lai, J.; Qiu, J.; Wang, Q.; Zhou, D.; Long, Z.; Yang, Y.; Hu, S.; Li, X.; Pi, J.; Wang, J. Disentangling site occupancy, cation regulation, and oxidation state regulation of the broadband near infrared emission in a chromium-doped SrGa4O7 phosphor. Inorg. Chem. Front. 2020, 7, 2313–2321. [Google Scholar] [CrossRef]
- Jisha, V.T. Sol gel synthesis and characterization of the SrAl4O7:Mn nano phosphors. Acta Cienc. Indica 2016, XLII, 105–109. [Google Scholar]
- Jisha, V.T.; Meera, M.R. Mn and Dy doped strontium aluminate’s optical properties—A comparative study. Mater. Today Proc. 2021, 37, 2470–2473. [Google Scholar] [CrossRef]
- Katsumata, T.; Sasajima, K.; Nabae, T.; Komuro, S.; Morikawa, T. Characteristics of strontium aluminate crystals used for long-duration phosphors. J. Am. Ceram. Soc. 1998, 81, 413–416. [Google Scholar] [CrossRef]
- Leanenia, M.S.; Lutsenko, E.V.; Rzheutski, M.V.; Yablonskii, G.P.; Naghiyev, T.G.; Ganbarova, H.B.; Tagiev, O.B. High photoluminescence stability of CaGa4O7:Eu3+ red phosphor in wide excitation intensity interval. Opt. Mat. 2016, 54, 45–49. [Google Scholar] [CrossRef]
- Li, P.; Peng, M.; Yin, X.; Ma, Z.; Dong, G.; Zhang, Q.; Qiu, J. Temperature dependent red luminescence from a distorted Mn4+ site in CaAl4O7:Mn4+. Opt. Expr. 2013, 21, 18943–18948. [Google Scholar] [CrossRef]
- Liu, S.X.; Xiong, F.B.; Lin, H.F.; Meng, X.G.; Lian, S.Y.; Zhu, W.Z. A deep red-light-emitting phosphor Mn4+:CaAl4O7 for warm white LEDs. Opt. Stuttg. 2018, 170, 178–184. [Google Scholar] [CrossRef]
- Park, J.; Kim, G.; Kim, Y.J. Luminescent properties of CaAl4O7 powders doped with Mn4+ ions. Ceram. Int. 2013, 39, S623–S626. [Google Scholar] [CrossRef]
- Puchalska, M.; Bolek, P.; Kot, K.; Zych, E. Luminescence of Bi3+ and Bi2+ ions in novel Bi-doped SrAl4O7 phosphor. Opt. Mater. 2020, 107, 109999. [Google Scholar] [CrossRef]
- Singh, V.; Kaur, S.; Jayasimhadri, M. Luminescence properties of orange emitting CaAl4O7:Sm3+ phosphor for solid state lighting applications. Solid State Sci. 2019, 101, 106049. [Google Scholar] [CrossRef]
- Singh, D.; Tanwar, V.; Simantilleke, A.P.; Bhagwan, S.; Mari, B.; Kadyan, P.S.; Singh, K.C.; Singh, I. Synthesis and enhanced luminescent characterization of SrAl4O7:Eu2+,RE3+ (RE = Nd, Dy) nanophosphors for light emitting applications. J. Mater. Sci. Mater. Electron. 2016, 27, 5303–5308. [Google Scholar] [CrossRef]
- Singh, D.; Tanwar, V.; Samantilleke, A.P.; Mari, B.; Bhagwan, S.; Singh, K.C.; Kadyan, P.S.; Singh, I. Synthesis of Sr(1−x−y)Al4O7:Eux2+,Lny3+ (Ln = Dy, Y, Pr) nanophosphors using rapid gel combustion process and their down conversion characteristics. Electron. Mater. Lett. 2017, 13, 222–229. [Google Scholar] [CrossRef]
- Yerpude, A.N.; Dhoble, S.J. Combustion synthesis of SrAl4O7:Eu2+, Dy3+, Gd3+ long lasting phosphor. IOP Conf. Ser. Mater. Sci. Eng. 2015, 73, 012126. [Google Scholar] [CrossRef]
- Sharma, S.K.; Pitale, S.S.; Malik, M.M.; GunduRao, T.K.; Chawla, S.; Qureshi, M.S.; Dubey, R.N. Spectral and defect analysis of Cu-doped combustion synthesized new SrAl4O7 phosphor. J. Lumin. 2010, 130, 240–248. [Google Scholar] [CrossRef]
- Chen, T.; Yang, X.; Xia, W.; Li, W.; Xiao, S. Deep-red emission of Mn4+ and Cr3+ in (Li1−xAx)2MgTiO4 (A = Na and K) phosphor: Potential application as W-LED and compact spectrometer. Ceram. Int. 2017, 43, 6949–6954. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.; Sun, L.; Devakumar, B.; Wang, S.; Sun, Q.; Guo, H.; Li, B.; Huang, X. Far-red-emitting double-perovskite CaLaMgSbO6:Mn4+ phosphors with high photoluminescence efficiency and thermal stability for indoor plant cultivation LEDs. RSC Adv. 2018, 8, 31666–31672. [Google Scholar] [CrossRef] [Green Version]
- Capron, M.; Douy, A. Strontium dialuminate SrAl4O7: Synthesis and stability. J. Am. Ceram. Soc. 2002, 85, 3036–3040. [Google Scholar] [CrossRef]
- Lindop, A.J.; Goodwin, D.W. The refined structure of SrO.2Al2O3. Acta Crystallogr. Sect. B 1972, 28, 2625–2626. [Google Scholar] [CrossRef]
- Villars, P.; Cenzual, K. Pearson’s Crystal Data—Crystal Structure Database for Inorganic Compounds; ASM International: Novelty, OH, USA, 2016. [Google Scholar]
- Adachi, S. Review—Mn4+ vs Cr3+: A comparative study as activator ions in red and deep red-emitting phosphors. ECS J. Solid State Sci. Technol. 2020, 9, 026003. [Google Scholar] [CrossRef]
- Luchechko, A.; Vasyltsiv, V.; Zhydachevskyy, Y.; Kushlyk, M.; Ubizskii, S.; Suchocki, A. Luminescence spectroscopy of Cr3+ ions in bulk single crystalline β-Ga2O3. J. Phy. D Appl. Phys. 2020, 53, 354001. [Google Scholar] [CrossRef]
- Mykhaylyk, V.; Kraus, H.; Zhydachevskyy, Y.; Tsiumra, V.; Luchechko, A.; Wagner, A.; Suchocki, A. Multimodal non-contact luminescence thermometry with Cr-doped oxides. Sensors 2020, 20, 5259. [Google Scholar] [CrossRef] [PubMed]
- Akselrud, L.; Grin, Y. WinCSD: Software package for crystallographic calculations (Version 4). J. Appl. Crystallogr. 2014, 47, 803–805. [Google Scholar] [CrossRef]











| Lattice Parameters, Residuals | Atom, Sites | x/a | y/b | z/c | Biso/eq, Å2 |
|---|---|---|---|---|---|
| SrAl4O7:xCr3+ | |||||
| a = 13.0462(9) Å b = 9.0221(7) Å c = 5.5439(4) Å β = 106.209(2)° V = 626.6(2) Å3 | Sr, 4e | 0 | 0.8113(4) | 1/4 | 1.00(6) |
| Al1, 8f | 0.1663(6) | 0.0857(8) | 0.2940(15) | 0.7(2) | |
| Al2, 8f | 0.1259(6) | 0.0857(8) | 0.2655(15) | 0.6(2) | |
| O1, 4e | 0 | 0.516(2) | 1/4 | 0.7(5) | |
| O2, 8f | 0.1252(9 | 0.0376(12) | 0.540(3) | 1.6(4) | |
| O3, 8f | 0.1281(8) | 0.2554(13) | 0.179(2) | 0.7(3) | |
| O4, 8f | 0.1936(11) | 0.4433(11) | 0.595(2) | 1.5(4) | |
| RI = 0.056; RP = 0.190 | |||||
| SrAl4O7:xMn4+ | |||||
| a = 13.0469(8) Å b = 9.0227(6) Å c = 5.5443(3) Å β = 106.197(2)° V = 626.8(1) Å3 | Sr, 4e | 0 | 0.8115(4) | 1/4 | 0.99(6) |
| Al1, 8f | 0.1672(6) | 0.0853(7) | 0.2974(13) | 0.9(2) | |
| Al2, 8f | 0.1246(6) | 0.4390(8) | 0.2660(13) | 1.0(2) | |
| O1, 4e | 0 | 0.5218(15) | 1/4 | 1.7(5) | |
| O2, 8f | 0.1243(8) | 0.0374(11) | 0.544(2) | 1.2(3) | |
| O3, 8f | 0.1260(7) | 0.2567(12) | 0.170(2) | 0.8(3) | |
| O4, 8f | 0.1904(9) | 0.4434(10) | 0.597(2) | 1.3(4) | |
| RI = 0.057; RP = 0.172 | |||||
| Atoms | Distances [Å] | Atoms | Distances [Å] | Atoms | Distances [Å] |
|---|---|---|---|---|---|
| SrAl4O7:xCr3+ | |||||
| Sr-O3 × 2 | 2.570(11) | Al1-O2 | 1.659(14) | Al2-O3 | 1.731(14) |
| Sr-O2 × 2 | 2.634(12) | Al1-O3 | 1.679(13) | Al2-O1 | 1.763(10) |
| Sr-O1 | 2.660(15) | Al1-O2 | 1.755(14) | Al2-O4 | 1.794(14) |
| Sr-O2 × 2 | 2.821(12) | Al1-O4 | 1.78(2) | Al2-O4 | 1.805(15) |
| Sr-Al1 × 2 | 3.256(8) | Al1-Al2 | 3.109(11) | Al2-O1 | 2.899(8) |
| Sr-Al1 × 2 | 3.319(8) | Al1-Al2 | 3.114(12) | Al2-Al2 × 2 | 2.979(11) |
| Sr-Al2 × 2 | 3.656(8) | Al1-Sr | 3.256(8) | Al2-Al1 | 3.109(11) |
| Sr-O4 × 2 | 3.688(13) | Al1-Sr | 3.319(8) | Al2-Al1 | 3.114(12) |
| Sr-Al2 × 2 | 3.725(8) | Al1-O4 | 3.441(15) | Al2-O2 | 3.130(15) |
| SrAl4O7:xMn4+ | |||||
| Sr-O3 × 2 | 2.524(9) | Al1-O2 | 1.671(13) | Al2-O3 | 1.731(12) |
| Sr-O2 × 2 | 2.614(10) | Al1-O3 | 1.722(12) | Al2-O1 | 1.769(9) |
| Sr-O1 | 2.614(14) | Al1-O2 | 1.754(12) | Al2-O4 | 1.784(13) |
| Sr-O2 × 2 | 2.822(10) | Al1-O4 | 1.805(14) | Al2-O4 | 1.798(13) |
| Sr-Al1 × 2 | 3.257(7) | Al1-Al2 | 3.101(10) | Al2-O1 | 2.891(7) |
| Sr-Al1 × 2 | 3.338(7) | Al1-Al2 | 3.127(10) | Al2-Al2 × 2 | 2.983(10) |
| Sr-Al2 × 2 | 3.653(7) | Al1-Sr | 3.257(7) | Al2-Al1 | 3.101(10) |
| Sr-O4 × 2 | 3.653(11) | Al1-Sr | 3.338(7) | Al2-Al1 | 3.127(10) |
| Sr-Al2 × 2 | 3.724(8) | Al1-O4 | 3.486(13) | Al2-O2 | 3.157(13) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stadnik, V.; Hreb, V.; Luchechko, A.; Zhydachevskyy, Y.; Suchocki, A.; Vasylechko, L. Sol-Gel Combustion Synthesis, Crystal Structure and Luminescence of Cr3+ and Mn4+ Ions in Nanocrystalline SrAl4O7. Inorganics 2021, 9, 89. https://doi.org/10.3390/inorganics9120089
Stadnik V, Hreb V, Luchechko A, Zhydachevskyy Y, Suchocki A, Vasylechko L. Sol-Gel Combustion Synthesis, Crystal Structure and Luminescence of Cr3+ and Mn4+ Ions in Nanocrystalline SrAl4O7. Inorganics. 2021; 9(12):89. https://doi.org/10.3390/inorganics9120089
Chicago/Turabian StyleStadnik, Vitalii, Vasyl Hreb, Andriy Luchechko, Yaroslav Zhydachevskyy, Andrzej Suchocki, and Leonid Vasylechko. 2021. "Sol-Gel Combustion Synthesis, Crystal Structure and Luminescence of Cr3+ and Mn4+ Ions in Nanocrystalline SrAl4O7" Inorganics 9, no. 12: 89. https://doi.org/10.3390/inorganics9120089
APA StyleStadnik, V., Hreb, V., Luchechko, A., Zhydachevskyy, Y., Suchocki, A., & Vasylechko, L. (2021). Sol-Gel Combustion Synthesis, Crystal Structure and Luminescence of Cr3+ and Mn4+ Ions in Nanocrystalline SrAl4O7. Inorganics, 9(12), 89. https://doi.org/10.3390/inorganics9120089