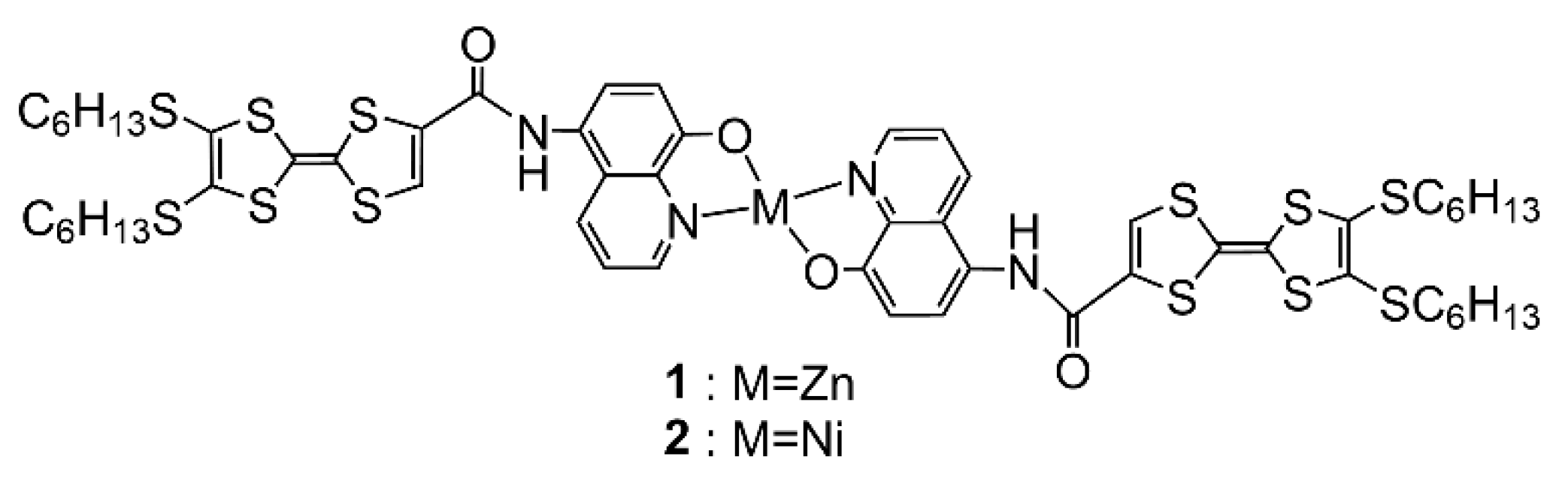
Synthesis and Physical Properties of Tetrathiafulvalene-8-Quinolinato Zinc(II) and Nickel(II) Complexes
Abstract
1. Introduction
2. Results and Discussion
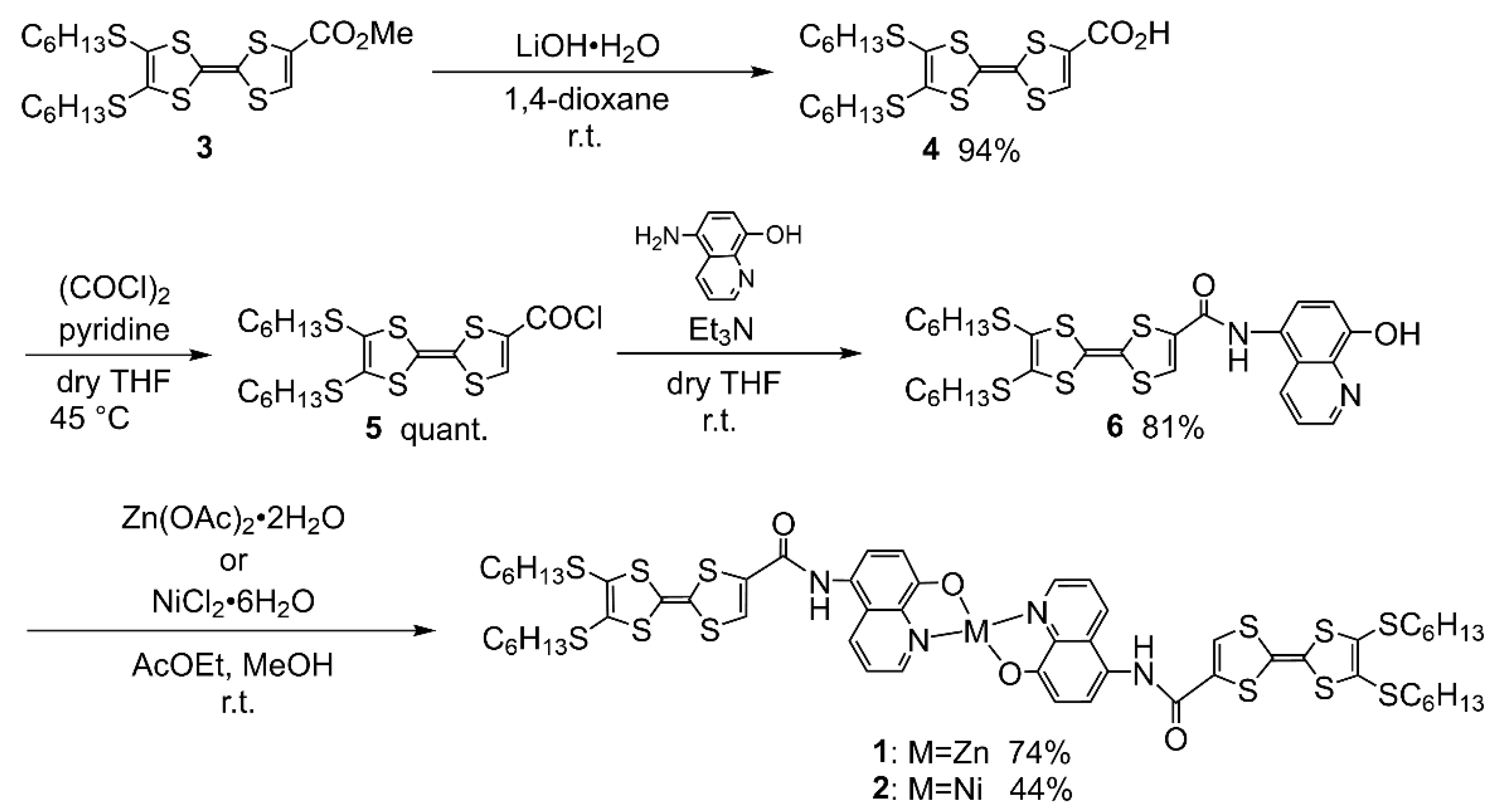
2.1. Synthesis
2.2. Electrochemical Properties of 1 and 2
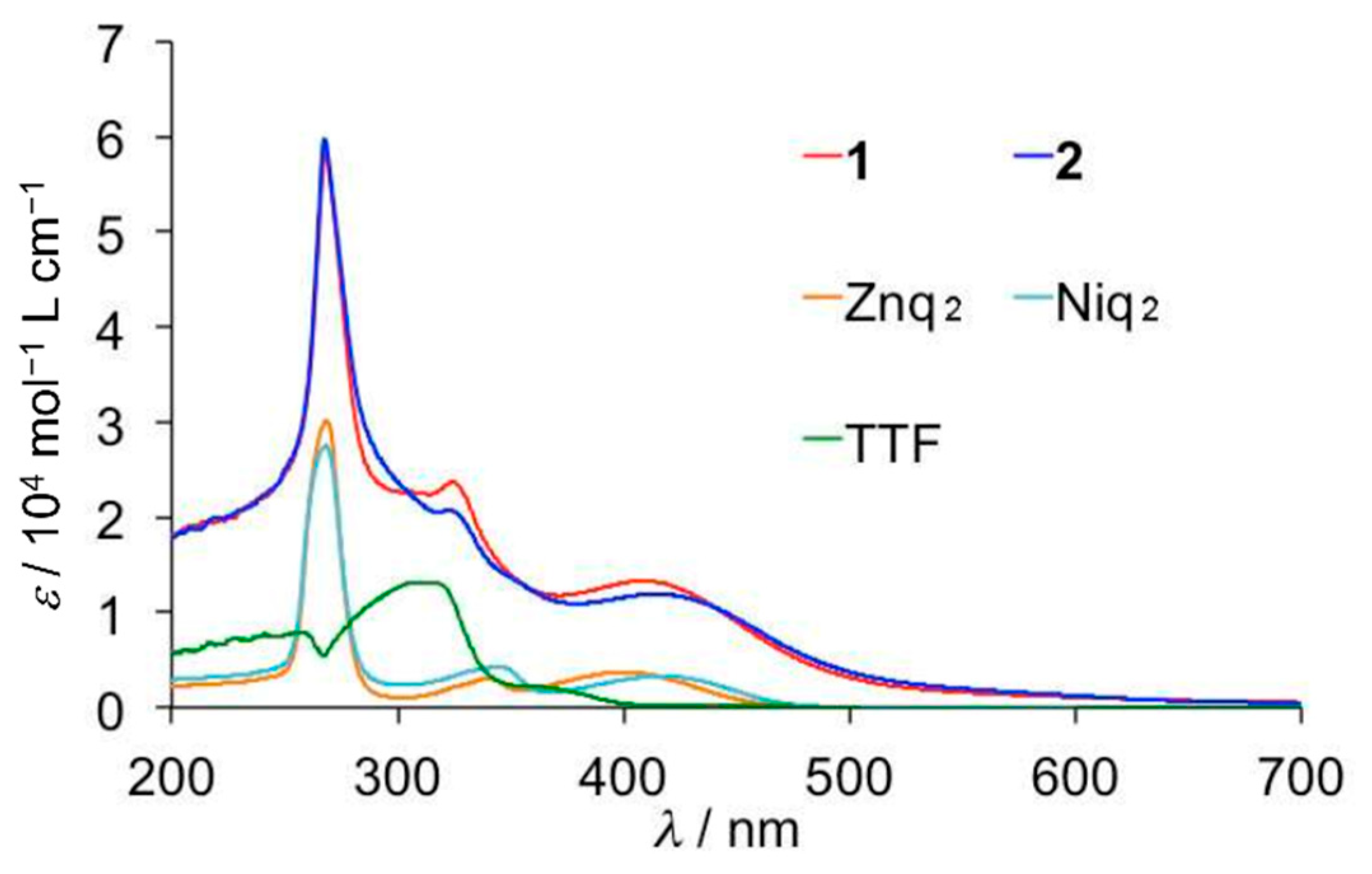
2.3. UV-Vis Absorption Spectra and Emission Spectra
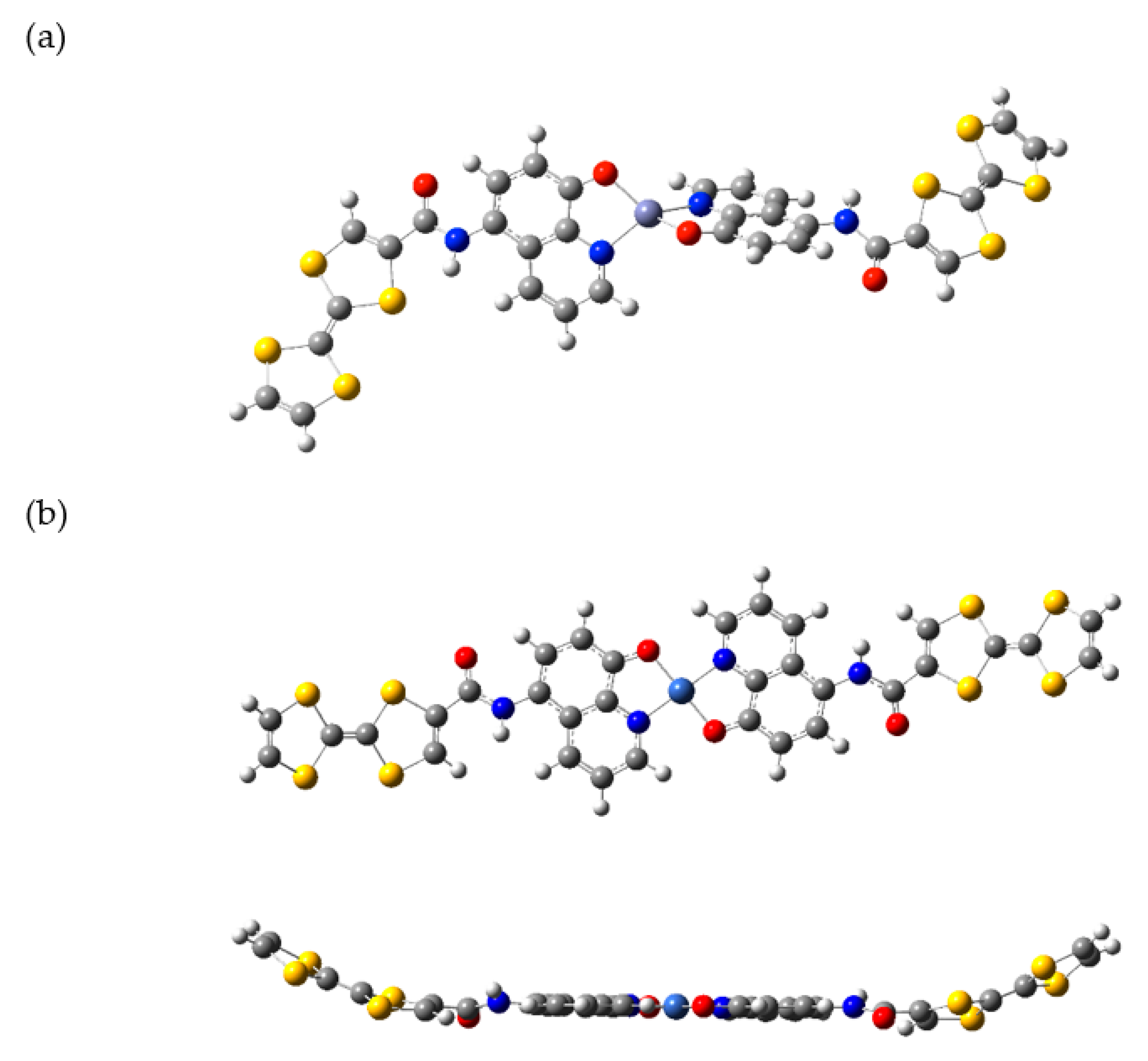
2.4. Molecular Orbital Calculation
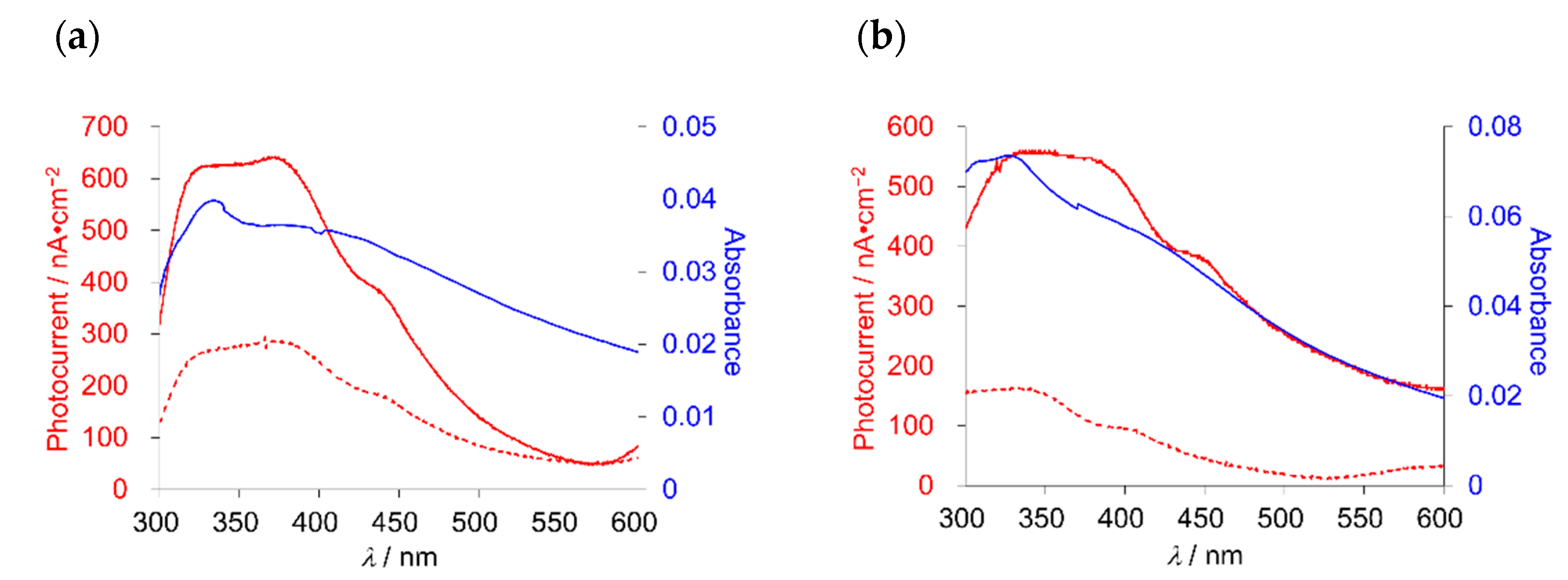
2.5. Photoelectric Conversion Functionalities of Thin Films
3. Conclusions
4. Materials and Methods
4.1. General
4.2. Synthesis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brunetti, F.G.; Lopez, J.L.; Atienza, C.; Martin, N. π-Extended TTF: A versatile molecule for organic electronics. J. Mater. Chem. 2012, 22, 4188–4205. [Google Scholar] [CrossRef]
- Gorgues, A.; Hudhomme, P.; Salle, M. Highly functionalized tetrathiafulvalenes: Riding along the synthetic trail from electrophilic alkynes. Chem. Rev. 2004, 104, 5151–5184. [Google Scholar] [CrossRef] [PubMed]
- Segura, J.L.; Martin, N. New concepts in tetrathiafulvalene chemistry. Angew. Chem. Int. Ed. Engl. 2001, 40, 1372–1409. [Google Scholar] [CrossRef]
- Wenger, S.; Bouit, P.-A.; Chen, Q.; Teuscher, J.; Censo, D.D.; Humphry-Baker, R.; Moser, J.-E.; Delgado, J.L.; Martín, N.; Zakeeruddin, S.M.; et al. Efficient Electron Transfer and Sensitizer Regeneration in Stable π-Extended Tetrathiafulvalene-Sensitized Solar Cells. J. Am. Chem. Soc. 2010, 132, 5164–5169. [Google Scholar] [CrossRef] [PubMed]
- Yamada, J.; Sugimoto, T. TTF Chemistry: Fundamentals and Applications of Tetrathiafulvalene; Kodansha-Springer: Tokyo, Japan, 2004. [Google Scholar]
- Biswas, C.; Katturi, N.K.; Duvva, N.; Giribabu, L.; Soma, V.R.; Raavi, S.S.K. Multistep Electron Injection Dynamics and Optical Nonlinearity Investigations of π-Extended Thioalkyl-Substituted Tetrathiafulvalene Sensitizers. J. Phys. Chem. C 2020, 124, 24039–24051. [Google Scholar] [CrossRef]
- Mogensen, J.; Michaels, H.; Roy, R.; Broløs, L.; Kilde, M.D.; Freitag, M.; Nielsen, M.B. Indenofluorene-Extended Tetrathiafulvalene Scaffolds for Dye-Sensitized Solar Cells. Eur. J. Org. Chem. 2020, 2020, 6127–6134. [Google Scholar] [CrossRef]
- Fujiwara, H.; Sugishima, Y.; Tsujimoto, K. Synthesis, structure, and photoelectrochemical properties of new tetrathiafulvalene-diphenyl-1,3,4-oxadiazole dyads. Tetrahedron Lett. 2008, 49, 7200–7203. [Google Scholar] [CrossRef]
- Fujiwara, H.; Sugishima, Y.; Tsujimoto, K. Synthesis, structure and physical properties of a new TTF derivative containing a PPD part. J. Phys. Conf. Ser. 2008, 132, 012025. [Google Scholar] [CrossRef]
- Fujiwara, H.; Yokota, S.; Hayashi, S.; Takemoto, S.; Matsuzaka, H. Development of photofunctional materials using TTF derivatives containing a 1,3-benzothiazole ring. Phys. B 2010, 405, S15–S18. [Google Scholar] [CrossRef]
- Yokota, S.; Tsujimoto, K.; Hayashi, S.; Pointillart, F.; Ouahab, L.; Fujiwara, H. CuII and CuI Coordination Complexes Involving Two Tetrathiafulvalene-1,3-benzothiazole Hybrid Ligands and Their Radical Cation Salts. Inorg. Chem. 2013, 52, 6543–6550. [Google Scholar] [CrossRef]
- Kishi, Y.; Cornet, L.; Pointillart, F.; Riobé, F.; Lefeuvre, B.; Cador, O.; Le Guennic, B.; Maury, O.; Fujiwara, H.; Ouahab, L. Luminescence and Single-Molecule-Magnet Behaviour in Lanthanide Coordination Complexes Involving Benzothiazole-Based Tetrathiafulvalene Ligands. Eur. J. Inorg. Chem. 2018, 2018, 458–468. [Google Scholar] [CrossRef]
- Fujiwara, H.; Tsujimoto, K.; Sugishima, Y.; Takemoto, S.; Matsuzaka, H. New fluorene-substituted TTF derivatives as photofunctional materials. Phys. B 2010, 405, S12–S14. [Google Scholar] [CrossRef]
- Tsujimoto, K.; Ogasawara, R.; Kishi, Y.; Fujiwara, H. TTF-fluorene dyads and their M(CN)2−(M = Ag, Au) salts designed for photoresponsive conducting materials. New J. Chem. 2014, 38, 406–418. [Google Scholar] [CrossRef]
- Tsujimoto, K.; Ogasawara, R.; Fujiwara, H. Photocurrent generation based on new tetrathiafulvalene-BODIPY dyads. Tetrahedron Lett. 2013, 54, 1251–1255. [Google Scholar] [CrossRef]
- Tsujimoto, K.; Ogasawara, R.; Nakagawa, T.; Fujiwara, H. Photofunctional Conductors Based on TTF-BODIPY Dyads Bearing p-Phenylene and p-Phenylenevinylene Spacers. Eur. J. Inorg. Chem. 2014, 2014, 3960–3972. [Google Scholar] [CrossRef]
- Tang, C.W.; Vanslyke, S.A. Organic Electroluminescent Diodes. Appl. Phys. Lett. 1987, 51, 913–915. [Google Scholar] [CrossRef]
- Chen, C.H.; Shi, J. Metal chelates as emitting materials for organic electroluminescence. Coord. Chem. Rev. 1998, 171, 161–174. [Google Scholar] [CrossRef]
- Colle, M.; Dinnebier, R.E.; Brutting, W. The structure of the blue luminescent d-phase of tris(8-hydroxyquinoline)aluminium(III) (Alq3). Chem. Commun. 2002, 2908–2909. [Google Scholar] [CrossRef]
- Hung, L.S.; Chen, C.H. Recent progress of molecular organic electroluminescent materials and devices. Mater. Sci. Eng. R-Rep. 2002, 39, 143–222. [Google Scholar] [CrossRef]
- Ballardini, R.; Varani, G.; Indelli, M.T.; Scandola, F. Phosphorescent 8-quinolinol metal chelates. Excited-state properties and redox behavior. Inorg. Chem. 1986, 25, 3858–3865. [Google Scholar] [CrossRef]
- Tsuboi, T.; Nakai, Y.; Torii, Y. Photoluminescence of bis(8-hydroxyquinoline) zinc (Znq2) and magnesium (Mgq2). Cent. Eur. J. Phys. 2012, 10, 524–528. [Google Scholar] [CrossRef]
- Vivo, P.; Jukola, J.; Ojala, M.; Chukharev, V.; Lemmetyinen, H. Influence of Alq3/Au cathode on stability and efficiency of a layered organic solar cell in air. Sol. Energy Mater. Sol. Cells 2008, 92, 1416–1420. [Google Scholar] [CrossRef]
- Kao, P.-C.; Chu, S.-Y.; Huang, H.-H.; Tseng, Z.-L.; Chen, Y.-C. Improved efficiency of organic photovoltaic cells using tris (8-hydroxy-quinoline) aluminum as a doping material. Thin Solid Films 2009, 517, 5301–5304. [Google Scholar] [CrossRef]
- Wang, C.S.; Bryce, M.R.; Batsanov, A.S.; Stanley, C.F.; Beeby, A.; Howard, J.A.K. Synthesis, spectroscopy and electrochemistry of phthalocyanine derivatives functionalised with four and eight peripheral tetrathiafulvalene units. J. Chem. Soc. Perkin Trans. 1997, 2, 1671–1678. [Google Scholar] [CrossRef]
- Parg, R.P.; Kilburn, J.D.; Petty, M.C.; Pearson, C.; Ryan, T.G. A semiconducting Langmuir-Blodgett film of a non-amphiphilic bis-tetrathiafulvalene derivative. J. Mater. Chem. 1995, 5, 1609–1615. [Google Scholar] [CrossRef]
- Heuzé, K.; Fourmigué, M.; Batail, P.; Canadell, E.; Auban-Senzier, P. Directing the Structures and Collective Electronic Properties of Organic Conductors: The Interplay of π-Overlap Interactions and Hydrogen Bonds. Chem. Eur. J. 1999, 5, 2971–2976. [Google Scholar] [CrossRef]
- Devic, T.; Avarvari, N.; Batail, P. A Series of Redox Active, Tetrathiafulvalene-Based Amidopyridines and Bipyridines Ligands: Syntheses, Crystal Structures, a Radical Cation Salt and Group 10 Transition-Metal Complexes. Chem. Eur. J. 2004, 10, 3697–3707. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Friedrich, A.; Uhlemann, E.; Weller, F. Chelate von Chinolin-8-ol-Derivaten. XII. Die Kristall- und Molekülstruktur von Bis(7-isopropylchinolin-8-olato)nickel(II). Z. Anorg. Allg. Chem. 1992, 615, 39–42. [Google Scholar] [CrossRef]
- Xiong, R.-G.; Zou, J.-L.; You, X.-Z.; Wu, Q.-J.; Huang, X.-Y. The synthesis, characterization and crystal structure of trans-bis-(4-methylpyridine)bis(8-quinolinolato-N1,O8)nickel(II)monohydrate. Transit. Met. Chem. 1995, 20, 498–500. [Google Scholar] [CrossRef]
- Merritt, L.L.; Cady, R.T.; Mundy, B.W. The crystal structure of zinc 8-hydroxyquinolinate dihydrate. Acta Crystallogr. 1954, 7, 473–476. [Google Scholar] [CrossRef]
- Chen, Z.-F.; Zhang, M.; Shi, S.-M.; Huang, L.; Liang, H.; Zhou, Z.-Y. Diaquabis(8-quinolinolato-[kappa]2N,O)zinc(II). Acta Crystallogr. Sect. E 2003, 59, m814–m815. [Google Scholar] [CrossRef]
- Grice, K.A.; Griffin, G.B.; Cao, P.S.; Saucedo, C.; Niyazi, A.H.; Aldakheel, F.A.; Sterbinsky, G.E.; LeSuer, R.J. Elucidating the Solution-Phase Structure and Behavior of 8-Hydroxyquinoline Zinc in DMSO. J. Phys. Chem. A 2018, 122, 2906–2914. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Wei, T.X.; Huang, C.H.; Cao, H. The photoelectrochemical study of a series of ionically combined bischromophore transition metal complexes in LB films. J. Mater. Chem. 2000, 10, 625–630. [Google Scholar] [CrossRef]
- Nariai, H.; Masuda, Y.; Sekido, E. Structural Characterization of Several Metal(II) 2-Methyl-8-quinolinolato Complexes in Several Organic Solvents. Bull. Chem. Soc. Jpn. 1984, 57, 3077–3082. [Google Scholar] [CrossRef]
| Compound | Ered | Eox1 | Eox2 | E2 − E1 |
|---|---|---|---|---|
| 1 (M = Zn) | −1.19 b | +0.56 | +0.92 | 0.36 |
| 2 (M = Ni) | −1.16 b | +0.59 | +0.90 | 0.31 |
| BTM-TTF | +0.48 | +0.82 | 0.34 | |
| Znq2 | −1.17 b | |||
| Niq2 | −1.02 b |
| Compound | λmax (nm) | ε (104 mol−1 L cm−1) | λem (nm) |
|---|---|---|---|
| 1 (M = Zn) | 268, 323, 402 | 5.1 | 404 |
| 2 (M = Ni) | 268, 322, 405 | 4.5 | 404 |
| TTF | 312 | 1.3 | |
| Znq2 | 268, 340, 400 | 3.9 | 404 |
| Niq2 | 268, 342, 416 | 3.6 | 404 |
| Compound | Bias Voltage vs. Ag/AgCl (V) | Maximum Photocurrent (Imax/nA cm−2) | Light Power (W/mW cm−2) | λ (nm) | Absorbance of Thin Film (Abs) | ηmax (%) |
|---|---|---|---|---|---|---|
| 1 | 0 | 265 | 11.4 | 334 | 0.040 | 0.099 |
| 1 | −0.2 | 622 | 11.4 | 334 | 0.040 | 0.23 |
| 2 | 0 | 163 | 9.2 | 325 | 0.073 | 0.044 |
| 2 | −0.2 | 522 | 9.2 | 325 | 0.073 | 0.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsujimoto, K.; Yamamoto, S.; Fujiwara, H. Synthesis and Physical Properties of Tetrathiafulvalene-8-Quinolinato Zinc(II) and Nickel(II) Complexes. Inorganics 2021, 9, 11. https://doi.org/10.3390/inorganics9020011
Tsujimoto K, Yamamoto S, Fujiwara H. Synthesis and Physical Properties of Tetrathiafulvalene-8-Quinolinato Zinc(II) and Nickel(II) Complexes. Inorganics. 2021; 9(2):11. https://doi.org/10.3390/inorganics9020011
Chicago/Turabian StyleTsujimoto, Keijiro, Shinya Yamamoto, and Hideki Fujiwara. 2021. "Synthesis and Physical Properties of Tetrathiafulvalene-8-Quinolinato Zinc(II) and Nickel(II) Complexes" Inorganics 9, no. 2: 11. https://doi.org/10.3390/inorganics9020011
APA StyleTsujimoto, K., Yamamoto, S., & Fujiwara, H. (2021). Synthesis and Physical Properties of Tetrathiafulvalene-8-Quinolinato Zinc(II) and Nickel(II) Complexes. Inorganics, 9(2), 11. https://doi.org/10.3390/inorganics9020011






