Photoluminescent Coordination Polymers Based on Group 12 Metals and 1H-Indazole-6-Carboxylic Acid
Abstract
:1. Introduction
2. Results and Discussion
2.1. Description of the Structures
2.1.1. Structural Description of [Zn(L)(H2O)]n (1)
2.1.2. Structural Description of [Cd2(HL)4]n (2)
2.2. Fourier Transformed Infrared (FTIR) Spectroscopy
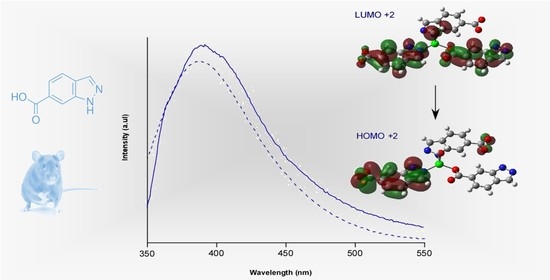
2.3. Luminescence Properties
3. Materials and Methods
3.1. Materials and Physical Measurements
3.2. Synthesis of [Zn(L)(H2O)]n (1)
3.3. Synthesis of [Cd2(HL)4]n (2)
3.4. Crystallographic Refinement and Structure Solution
3.5. Photophysical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- McKinlay, A.C.; Morris, R.E.; Horcajada, P.; Férey, G.; Gref, R.; Couvreur, P.; Serre, C. BioMOFs: Metal-organic frameworks for biological and medical applications. Angew. Chem. Int. Ed. 2010, 49, 6260–6266. [Google Scholar] [CrossRef]
- Tranchemontagne, D.J.; Tranchemontagne, J.L.; O’keeffe, M.; Yaghi, O.M. Secondary building units, nets and bonding in the chemistry of metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1257–1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Cui, Y.; Qian, G. Goal-directed design of metal–organic frameworks for liquid-phase adsorption and separation. Coord. Chem. Rev. 2019, 378, 310–332. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G. On the design of highly luminescent lanthanide complexes. Coord. Chem. Rev. 2015, 293–294, 19–47. [Google Scholar] [CrossRef]
- Chen, C.T.; Suslick, K.S. One-dimensional coordination polymers: Applications to material science. Coord. Chem. Rev. 1993, 128, 293–322. [Google Scholar] [CrossRef] [Green Version]
- Heravi, M.M.; Zadsirjan, V. Prescribed drugs containing nitrogen heterocycles: An overview. RSC Adv. 2020, 10, 44247–44311. [Google Scholar] [CrossRef]
- Horton, D.A.; Bourne, G.T.; Smythe, M.L. The combinatorial synthesis of bicyclic privileged structures or privileged substructures. Chem. Rev. 2003, 103, 893–930. [Google Scholar] [CrossRef]
- Zhang, S.G.; Liang, C.G.; Zhang, W.H. Recent advances in indazole-containing derivatives: Synthesis and biological perspectives. Molecules 2018, 23, 2783. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.; Zhang, Q.; Wang, Z.; Huang, G.; Li, S. Recent Advances in the Development of Indazole-based Anticancer Agents. ChemMedChem 2018, 13, 1490–1507. [Google Scholar] [CrossRef]
- Büchel, G.E.; Kossatz, S.; Sadique, A.; Rapta, P.; Zalibera, M.; Bucinsky, L.; Komorovsky, S.; Telser, J.; Eppinger, J.; Reiner, T.; et al. Cis -Tetrachlorido-bis(indazole)osmium(IV) and its osmium(III) analogues: Paving the way towards the cis -isomer of the ruthenium anticancer drugs KP1019 and/or NKP1339. Dalt. Trans. 2017, 46, 11925–11941. [Google Scholar] [CrossRef] [Green Version]
- Hawes, C.S.; Kruger, P.E. Discrete and polymeric Cu(ii) complexes featuring substituted indazole ligands: Their synthesis and structural chemistry. Dalt. Trans. 2014, 43, 16450–16458. [Google Scholar] [CrossRef]
- Long, B.F.; Huang, Q.; Wang, S.L.; Mi, Y.; Wang, M.F.; Xiong, T.; Zhang, S.C.; Yin, X.H.; Hu, F.L. Five new cobalt(II) complexes based on indazole derivatives: Synthesis, DNA binding and molecular docking study. J. Coord. Chem. 2019, 72, 645–663. [Google Scholar] [CrossRef]
- Furman, J.D.; Burwood, R.P.; Tang, M.; Mikhailovsky, A.A.; Cheetham, A.K. Understanding ligand-centred photoluminescence through flexibility and bonding of anthraquinone inorganic-organic frameworks. J. Mater. Chem. 2011, 21, 6595–6601. [Google Scholar] [CrossRef]
- Yuan, W.Z.; Shen, X.Y.; Zhao, H.; Lam, J.W.Y.; Tang, L.; Lu, P.; Wang, C.; Liu, Y.; Wang, Z.; Zheng, Q.; et al. Crystallization-induced phosphorescence of pure organic luminogens at room temperature. J. Phys. Chem. C 2010, 114, 6090–6099. [Google Scholar] [CrossRef]
- Cepeda, J.; Rodríguez-Diéguez, A. Tuning the luminescence performance of metal-organic frameworks based on d10metal ions: From an inherent versatile behaviour to their response to external stimuli. CrystEngComm 2016, 18, 8556–8573. [Google Scholar] [CrossRef]
- Solov’ev, K.N.; Borisevich, E.A. Intramolecular heavy-atom effect in the photophysics of organic molecules. Phys. Uspekhi 2005, 48, 231–253. [Google Scholar] [CrossRef]
- Seco, J.M.; Pérez-Yáñez, S.; Briones, D.; García, J.Á.; Cepeda, J.; Rodríguez-Diéguez, A. Combining Polycarboxylate and Bipyridyl-like Ligands in the Design of Luminescent Zinc and Cadmium Based Metal-Organic Frameworks. Cryst. Growth Des. 2017, 17, 3893–3906. [Google Scholar] [CrossRef]
- Cepeda, J.; Pérez-Yáñez, S.; Rodríguez-Diéguez, A. Luminescent Zn/Cd-based MOFS, CPS and their applications. In Advances in Materials Science Research; Wythers, M.C., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2017; Volume 28, ISBN 9781536109054. [Google Scholar]
- San Sebastian, E.; Rodríguez-Diéguez, A.; Seco, J.M.; Cepeda, J. Coordination Polymers with Intriguing Photoluminescence Behavior: The Promising Avenue for Greatest Long-Lasting Phosphors. Eur. J. Inorg. Chem. 2018, 2155–2174. [Google Scholar] [CrossRef]
- Pajuelo-Corral, O.; Rodríguez-Diéguez, A.; García, J.A.; San Sebastián, E.; Seco, J.M.; Cepeda, J. Chiral coordination polymers based on d10metals and 2-aminonicotinate with blue fluorescent/green phosphorescent anisotropic emissions. Dalt. Trans. 2018, 47, 8746–8754. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, K.Z.; Yan, D. Ultralong Persistent Room Temperature Phosphorescence of Metal Coordination Polymers Exhibiting Reversible pH-Responsive Emission. ACS Appl. Mater. Interfaces 2016, 8, 15489–15496. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, R.; Wang, S. Blue phosphorescent Zn(II) and orange phosphorescent Pt(II) complexes of 4,4′-diphenyl-6,6′-dimethyl-2,2′-bipyrimidine. Dalt. Trans. 2004, 35, 2073–2079. [Google Scholar] [CrossRef]
- Barbieri, A.; Accorsi, G.; Armaroli, N. Luminescent complexes beyond the platinum group: The d10 avenue. Chem. Commun. 2008, 2185–2193. [Google Scholar] [CrossRef]
- Hu, Z.; Deibert, B.J.; Li, J. Luminescent metal-organic frameworks for chemical sensing and explosive detection. Chem. Soc. Rev. 2014, 43, 5815–5840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leo, P.; Briones, D.; García, J.A.; Cepeda, J.; Orcajo, G.; Calleja, G.; Rodríguez-Diéguez, A.; Martínez, F. Strontium-Based MOFs Showing Dual Emission: Luminescence Thermometers and Toluene Sensors. Inorg. Chem. 2020, 59, 18432–18443. [Google Scholar] [CrossRef]
- Liu, J.; Zhuang, Y.; Wang, L.; Zhou, T.; Hirosaki, N.; Xie, R.-J. Achieving Multicolor Long-Lived Luminescence in Dye-Encapsulated Metal–Organic Frameworks and Its Application to Anticounterfeiting Stamps. ACS Appl. Mater. Interfaces 2018, 10, 1802–1809. [Google Scholar] [CrossRef]
- Sun, Y.-X.; Sun, W.-Y. Zinc(ii)– and cadmium(ii)–organic frameworks with 1-imidazole-containing and 1-imidazole-carboxylate ligands. CrystEngComm 2015, 17, 4045–4063. [Google Scholar] [CrossRef]
- Alvarez, S.; Avnir, D.; Llunell, M.; Pinsky, M. Continuous symmetry maps and shape classification. The case of six-coordinated metal compounds. New J. Chem. 2002, 26, 996–1009. [Google Scholar] [CrossRef]
- Volkringer, C.; Loiseau, T.; Guillou, N.; Fèrey, G.; Haouas, M.; Taulelle, F.; Audebrand, N.; Margiolaki, I.; Popov, D.; Burghammer, M.; et al. Structural transitions and flexibility during dehydration—Rehydration process in the MOF-type aluminum pyromellitate A12(OH)2[C1008H2](MIL-118). Cryst. Growth Des. 2009, 9, 2927–2936. [Google Scholar] [CrossRef]
- Boča, M.; Baran, P.; Boča, R.; Fuess, H.; Kickelbick, G.; Linert, W.; Renz, F.; Svoboda, I. Selective imidazolidine ring opening during complex formation of iron(III), copper(II), and zinc(II) with a multidentate ligand obtained from 2-pyridinecarboxaldehyde N-oxide and triethylenetetramine. Inorg. Chem. 2000, 39, 3205–3212. [Google Scholar] [CrossRef]
- Xiao, H.; Li, X.B.; Qin, G.F.; Xia, Y.; Zhou, G. Directed assembly of cobalt(II) 1-H-indazole-3-carboxylic acid coordination networks by bipyridine and its derivatives: Structural versatility, electrochemical properties, and antifungal activity. J. Iran. Chem. Soc. 2016, 13, 793–802. [Google Scholar] [CrossRef] [Green Version]
- Ohno, K.; Okimura, M.; Akai, N.; Katsumoto, Y. The effect of cooperative hydrogen bonding on the OH stretching-band shift for water clusters studied by matrix-isolation infrared spectroscopy and density functional theory. Phys. Chem. Chem. Phys. 2005, 7, 3005–3014. [Google Scholar] [CrossRef] [PubMed]
- Salinas-Castillo, A.; Calahorro, A.J.; Briones, D.; Fairen-Jiménez, D.; Gándara, F.; Mendicute-Fierro, C.; Seco, J.M.; Pérez-Mendoza, M.; Fernández, B.; Rodríguez-Diéguez, A. 2D-cadmium MOF and gismondine-like zinc coordination network based on the N-(2-tetrazolethyl)-4′-glycine linker. New J. Chem. 2015, 39, 3982–3986. [Google Scholar] [CrossRef] [Green Version]
- Pamei, M.; Puzari, A. Luminescent transition metal–organic frameworks: An emerging sensor for detecting biologically essential metal ions. Nano-Struct. Nano-Objects 2019, 19, 100364. [Google Scholar] [CrossRef]
- García-Valdivia, A.A.; Zabala-Lekuona, A.; Ramírez-Rodríguez, G.B.; Delgado-López, J.M.; Fernández, B.; Cepeda, J.; Rodríguez-Diéguez, A. 2D-Coordination polymers based on 1H-indazole-4-carboxylic acid and transition metal ions: Magnetic{,} luminescence and biological properties. CrystEngComm 2020, 22, 5086–5095. [Google Scholar] [CrossRef]
- García-Valdivia, A.A.; Pérez-Mendoza, M.; Choquesillo-Lazarte, D.; Cepeda, J.; Fernández, B.; Souto, M.; González-Tejero, M.; García, J.A.; Espallargas, G.M.; Rodríguez-Diéguez, A. Interpenetrated Luminescent Metal–Organic Frameworks based on 1H-Indazole-5-carboxylic Acid. Cryst. Growth Des. 2020, 20, 4550–4560. [Google Scholar] [CrossRef]
- Dobretsov, G.E.; Syrejschikova, T.I.; Smolina, N.V. On mechanisms of fluorescence quenching by water. Biophysics 2014, 59, 183–188. [Google Scholar] [CrossRef]
- Bruker Apex2. B.A.I. Bruker Apex2; Bruker AXS Inc.: Madison, WI, USA, 2004. [Google Scholar]
- Sheldrick, G.M. SADABS 1996, Program for Empirical Adsorption Correction. Available online: https://cmacd.myweb.cs.uwindsor.ca/Teaching/553-class/sadabs.pdf (accessed on 16 January 2021).
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]







| Compound | 1 | 2 |
|---|---|---|
| Formula | C8H6N2O3Zn | C32H20Cd2N8O8 |
| Mr (g mol−1) | 243.52 | 869.36 |
| Crystal system | monoclinic | triclinic |
| Space group | P21/n | P–1 |
| Temperature (K) | 100 (2) | 100 (2) |
| a (Å) | 9.774 (3) | 8.7080 (4) |
| b (Å) | 5.7633 (15) | 9.0640 (3) |
| c (Å) | 14.592 (4) | 19.4510 (7) |
| α (°) | 90 | 101.089 (1) |
| β (°) | 95.626 (7) | 90.961 (2) |
| λ (°) | 90 | 98.063 (1) |
| V (Å3) | 818.0 (4) | 1490.18 (1) |
| Z | 4 | 2 |
| ρ (g cm−3) | 1.977 | 1.937 |
| µ (mm−1) | 2.979 | 1.497 |
| Unique reflections | 1035 (817) | 7671 (5938) |
| Rint | 0.1342 | 0.0722 |
| GoF a | 1.082 | 1.107 |
| R1 b/wR2 c [I>2σ(I)] | 0.0777/0.0535 | 0.0604/0.0352 |
| R1 b/wR2 c [all data] | 0.1075/0.0994 | 0.0823/0.0672 |
| Largest difference in peak and hole (e Å−3) | 0.635 and −0.0656 | 1.062 and −1.046 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Valdivia, A.A.; Echenique-Errandonea, E.; Ramírez-Rodríguez, G.B.; Delgado-López, J.M.; Fernández, B.; Rojas, S.; Cepeda, J.; Rodríguez-Diéguez, A. Photoluminescent Coordination Polymers Based on Group 12 Metals and 1H-Indazole-6-Carboxylic Acid. Inorganics 2021, 9, 20. https://doi.org/10.3390/inorganics9030020
García-Valdivia AA, Echenique-Errandonea E, Ramírez-Rodríguez GB, Delgado-López JM, Fernández B, Rojas S, Cepeda J, Rodríguez-Diéguez A. Photoluminescent Coordination Polymers Based on Group 12 Metals and 1H-Indazole-6-Carboxylic Acid. Inorganics. 2021; 9(3):20. https://doi.org/10.3390/inorganics9030020
Chicago/Turabian StyleGarcía-Valdivia, Antonio A., Estitxu Echenique-Errandonea, Gloria B. Ramírez-Rodríguez, José M. Delgado-López, Belén Fernández, Sara Rojas, Javier Cepeda, and Antonio Rodríguez-Diéguez. 2021. "Photoluminescent Coordination Polymers Based on Group 12 Metals and 1H-Indazole-6-Carboxylic Acid" Inorganics 9, no. 3: 20. https://doi.org/10.3390/inorganics9030020
APA StyleGarcía-Valdivia, A. A., Echenique-Errandonea, E., Ramírez-Rodríguez, G. B., Delgado-López, J. M., Fernández, B., Rojas, S., Cepeda, J., & Rodríguez-Diéguez, A. (2021). Photoluminescent Coordination Polymers Based on Group 12 Metals and 1H-Indazole-6-Carboxylic Acid. Inorganics, 9(3), 20. https://doi.org/10.3390/inorganics9030020