Synthesis and Characterisation of Molecular Polarised-Covalent Thorium-Rhenium and -Ruthenium Bonds
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis and Isolation of the Th-Re and Th-Ru Complexes ThRe and ThRu
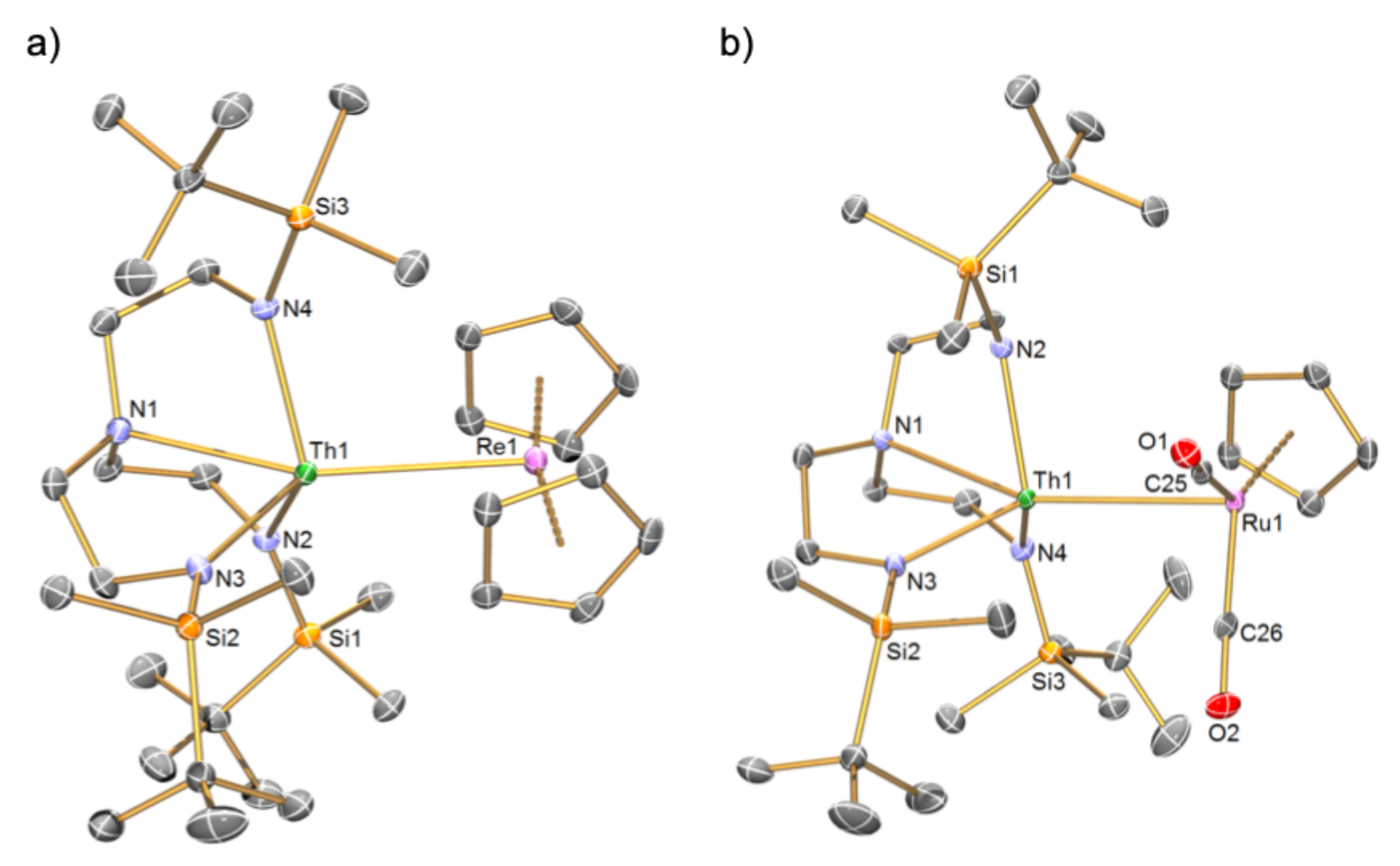
2.2. Solid-State Structures of the Th-Re and Th-Ru Complexes ThRe and ThRu
2.3. Spectroscopic and Analytical Characterisation of the Th-Re and Th-Ru Complexes ThRe and ThRu
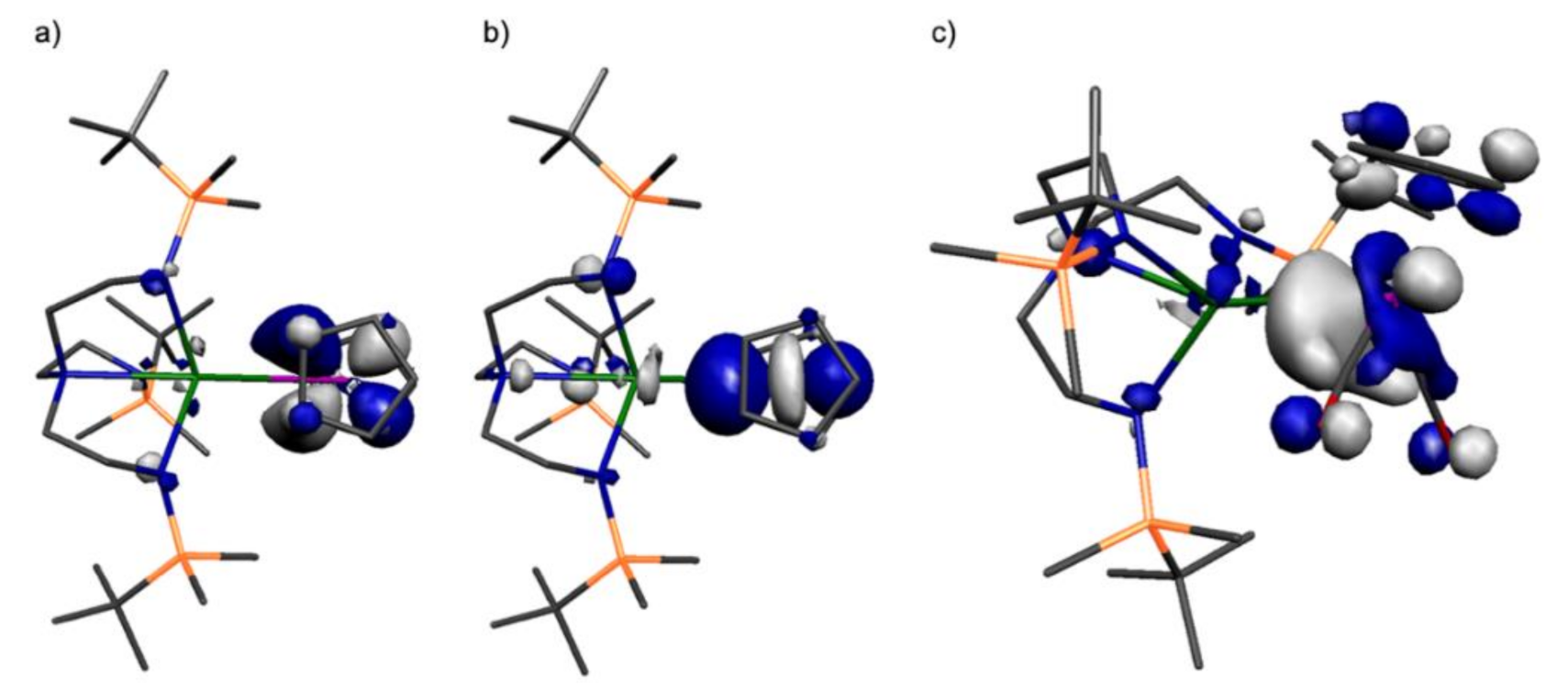
2.4. Quantum Chemical Computational Analysis of the Th-Re and Th-Ru Complexes ThRe and ThRu
3. Materials and Methods
3.1. General Materials and Methods
3.2. Quantum Chemical Calculations
3.3. Preparation of [Th(TrenDMBS)ReCp2] (ThRe)
3.4. Preparation of [Th(TrenDMBS)RuCp-(CO)2] (ThRu)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cotton, F.A.; Murillo, C.A.; Walton, R.A. (Eds.) Multiple Bonds between Metal Atoms, 3rd ed.; Springer: New York, NY, USA, 2005. [Google Scholar]
- Parkin, G. (Ed.) Metal-Metal Bonding; Springer: New York, NY, USA, 2010. [Google Scholar]
- Liddle, S.T. (Ed.) Molecular Metal-Metal Bonds: Compounds, Synthesis, Properties; Wiley VCH: Weinheim, Germany, 2015. [Google Scholar]
- Wheatley, N.; Kalck, P. Structure and Reactivity of Early-Late Heterobimetallic Complexes. Chem. Rev. 1999, 99, 3379–3420. [Google Scholar] [CrossRef] [PubMed]
- Chipman, J.A.; Berry, J.F. Paramagnetic Metal-Metal Bonded Heterometallic Complexes. Chem. Rev. 2020, 120, 2409–2447. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Y.; Morales-Martínez, R.; Zhong, J.; de Graaf, C.; Rodríguez-Fortea, A.; Poblet, J.M.; Echegoyen, L.; Feng, L.; Chen, N. U2@Ih(7)-C80: Crystallographic Characterization of a Long-Sought Dimetallic Actinide Endohedral Fullerene. J. Am. Chem. Soc. 2018, 140, 3907–3915. [Google Scholar] [CrossRef] [PubMed]
- Liddle, S.T.; Mills, D.P. Metal-Metal Bonds in f-Element Chemistry. Dalton Trans. 2009, 5592–5605. [Google Scholar] [CrossRef]
- Oelkers, B.; Butovskii, M.V.; Kempe, R. f-Element-Metal Bonding and the Use of the Bond Polarity to Build Molecular Intermetalloids. Chem. Eur. J. 2012, 18, 13566–13579. [Google Scholar] [CrossRef]
- Patel, D.; Liddle, S.T. f-Element-Metal Bond Chemistry. Rev. Inorg. Chem. 2012, 32, 1–22. [Google Scholar] [CrossRef]
- Butovskii, M.V.; Kempe, R. Rare earth-metal bonding in molecular compounds: Recent advances, challenges, and perspectives. New J. Chem. 2015, 39, 7544–7558. [Google Scholar] [CrossRef]
- Réant, B.L.L.; Liddle, S.T.; Mills, D.P. f-Element silicon and heavy tetrel chemistry. Chem. Sci. 2020, 11, 10871–10886. [Google Scholar] [CrossRef]
- Ayres, A.J.; Zegke, M.; Ostrowski, J.P.A.; Tuna, F.; McInnes, E.J.L.; Wooles, A.J.; Liddle, S.T. Actinide-transition metal bonding in heterobimetallic uranium− and thorium−molybdenum paddlewheel complexes. Chem. Commun. 2018, 54, 13515–13518. [Google Scholar] [CrossRef]
- Sternal, R.S.; Marks, T.J. Actinide-to-transition metal bonds. Synthesis, characterization, and properties of metal-metal bonded systems having the tris(cyclopentadienyl)actinide fragment. Organometallics 1987, 6, 2621–2623. [Google Scholar] [CrossRef]
- Nolan, S.P.; Porchia, M.; Marks, T.J. Organo-f-element thermochemistry. Actinide-Group 14 element and actinide-transition-element bond disruption enthalpies and stoichiometric/catalytic chemical implications thereof in heterobimetallic tris(cyclopentadienyl)uranium(IV) compounds. Organometallics 1991, 10, 1450–1457. [Google Scholar] [CrossRef]
- Bucaille, A.; Le Borgne, T.; Ephritikhine, M.; Daran, J.C. Synthesis and X-ray Crystal Structure of a Urana[1]ferrocenophane, the First Tris(1,1′-ferrocenylene) Metal Compound. Organometallics 2000, 19, 4912–4914. [Google Scholar] [CrossRef]
- Monreal, M.J.; Carver, C.T.; Diaconescu, P.L. Redox processes in a uranium bis(1,1′-diamidoferrocene) complex. Inorg. Chem. 2007, 46, 7226–7228. [Google Scholar] [CrossRef]
- Monreal, M.J.; Diaconescu, P.L. A weak interaction between iron and uranium in uranium alkyl complexes supported by ferrocene diamide ligands. Organometallics 2008, 27, 1702–1706. [Google Scholar] [CrossRef]
- Fortier, S.; Aguilar-Calderon, J.R.; Vlaisavljevich, B.; Metta-Magana, A.J.; Goos, A.G.; Botez, C.E. An N-Tethered Uranium(III) Arene Complex and the Synthesis of an Unsupported U-Fe Bond. Organometallics 2017, 36, 4591–4599. [Google Scholar] [CrossRef]
- Sternal, R.S.; Brock, C.P.; Marks, T.J. Metal-metal bonds involving actinides. Synthesis and characterization of a complex having an unsupported actinide to transition metal bond. J. Am. Chem. Soc. 1985, 107, 8270–8272. [Google Scholar] [CrossRef]
- Gardner, B.M.; Patel, D.; Cornish, A.D.; McMaster, J.; Lewis, W.; Blake, A.J.; Liddle, S.T. The Nature of Unsupported Uranium-Ruthenium Bonds: A Combined Experimental and Theoretical Study. Chem. Eur. J. 2011, 17, 11266–11273. [Google Scholar] [CrossRef]
- Gardner, B.M.; McMaster, J.; Lewis, W.; Liddle, S.T. Synthesis and structure of {N(CH2CH2NSiMe3)3}URe(η5-C5H5)2: A heterobimetallic complex with an unsupported uranium-rhenium bond. Chem. Commun. 2009, 2851–2853. [Google Scholar] [CrossRef]
- Gardner, B.M.; McMaster, J.; Moro, F.; Lewis, W.; Blake, A.J.; Liddle, S.T. An Unsupported Uranium-Rhenium Complex Prepared by Alkane Elimination. Chem. Eur. J. 2011, 17, 6909–6912. [Google Scholar] [CrossRef]
- Patel, D.; King, D.M.; Gardner, B.M.; McMaster, J.; Lewis, W.; Blake, A.J.; Liddle, S.T. Structural and theoretical insights into the perturbation of uranium-rhenium bonds by dative Lewis base ancillary ligands. Chem. Commun. 2011, 47, 295–297. [Google Scholar] [CrossRef]
- Patel, D.; Moro, F.; McMaster, J.; Lewis, W.; Blake, A.J.; Liddle, S.T. A Formal High Oxidation State Inverse-Sandwich Diuranium Complex: A New Route to f-Block-Metal Bonds. Angew. Chem. Int. Ed. 2011, 50, 10388–10392. [Google Scholar] [CrossRef]
- Napoline, J.W.; Kraft, S.J.; Matson, E.M.; Fanwick, P.E.; Bart, S.C.; Thomas, C.M. Tris(phosphinoamide)-Supported Uranium-Cobalt Heterobimetallic Complexes Featuring Co → U Dative Interactions. Inorg. Chem. 2013, 52, 12170–12177. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.L.; Lukens, W.W.; Lu, C.C.; Arnold, J. Photochemical Route to Actinide-Transition Metal Bonds: Synthesis, Characterization and Reactivity of a Series of Thorium and Uranium Heterobimetallic Complexes. J. Am. Chem. Soc. 2014, 136, 3647–3654. [Google Scholar] [CrossRef]
- Lu, E.; Wooles, A.J.; Gregson, M.; Cobb, P.J.; Liddle, S.T. A Very Short Uranium(IV)-Rhodium(I) Bond with Net Double-Dative Bonding Character. Angew. Chem. Int. Ed. 2018, 57, 6587–6591. [Google Scholar] [CrossRef]
- Hlina, J.A.; Wells, J.A.L.; Pankhurst, J.R.; Love, J.B.; Arnold, P.L. Uranium rhodium bonding in heterometallic complexes. Dalton Trans. 2017, 46, 5540–5545. [Google Scholar] [CrossRef]
- Geng, G.; Zhang, M.; Wang, P.; Wang, S.; Maron, L.; Zhu, C. Identification of a uranium-rhodium triple bond in a heterometallic cluster. Proc. Natl. Acad. Sci. USA 2019, 116, 17654–17658. [Google Scholar]
- Ritchey, J.M.; Zozulin, A.J.; Wrobleski, D.A.; Ryan, R.R.; Wasserman, H.J.; Moody, D.C.; Paine, R.T. An organothorium-nickel phosphido complex with a short thorium-nickel distance. The structure of Th(η5-C5Me5)2(μ-PPh2)2Ni(C)(CO)2. J. Am. Chem. Soc. 1985, 107, 501–503. [Google Scholar] [CrossRef]
- Hlina, J.A.; Pankhurst, J.R.; Kaltsoyannis, N.; Arnold, P.L. Metal−Metal Bonding in Uranium−Group 10 Complexes. J. Am. Chem. Soc. 2016, 138, 3333–3345. [Google Scholar] [CrossRef] [PubMed]
- Camp, C.; Toniolo, D.; Andrez, J.; Pecaut, J.; Mazzanti, M. A versatile route to homo- and hetero-bimetallic 5f-5f and 3d-5f complexes supported by a redox active ligand framework. Dalton Trans. 2017, 46, 11145–11148. [Google Scholar] [CrossRef]
- Kozimor, S.A.; Bartlett, B.M.; Rinehart, J.D.; Long, J.R. Magnetic exchange coupling in chloride-bridged 5f-3d heterometallic complexes generated via insertion into a Uranium(IV) dimethylpyrazolate dimer. J. Am. Chem. Soc. 2007, 129, 10672–10674. [Google Scholar] [CrossRef]
- Feng, G.; Zhang, M.; Shao, S.; Wang, X.; Wang, S.; Maron, L.; Zhu, C. Transition-metal-bridged bimetallic clusters with multiple uranium-metal bonds. Nat. Chem. 2019, 11, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; McCabe, K.N.; Wang, S.; Maron, L.; Zhu, C. Construction of heterometallic clusters with multiple uranium-metal bonds by using dianionic nitrogen-phosphorus ligands. Chem. Sci. 2020, 11, 7585–7592. [Google Scholar] [CrossRef]
- Hay, P.J.; Ryan, R.R.; Salazar, K.V.; Wrobleski, D.A.; Sattelberger, A.P. Synthesis and x-ray structure of (C5Me5)2Th(μ-PPh2)2Pt(PMe3): A complex with a thorium-platinum bond. J. Am. Chem. Soc. 1986, 108, 313–315. [Google Scholar] [CrossRef]
- Leverd, P.C.; Lance, M.; Nierlich, M.; Vigner, J.; Ephritikhine, M. Synthesis and crystal structure of homoleptic uranium hexathiolates: [NEt2H2]2[U(SPh)6] and [(Ph3P)Cu(μ-SPh)3-U(μ-SPh)3Cu(PPh3)]. J. Chem. Soc. Dalton Trans. 1994, 3563–3567. [Google Scholar] [CrossRef]
- Yang, P.; Zhou, E.; Hou, G.; Zi, G.; Ding, W.; Walter, M.D. Experimental and Computational Studies on the Formation of Thorium-Copper Heterobimetallics. Chem. Eur. J. 2016, 22, 13845–13849. [Google Scholar] [CrossRef]
- Fortier, S.; Walensky, J.R.; Wu, G.; Hayton, T.W. High-Valent Uranium Alkyls: Evidence for the Formation of UVI(CH2SiMe3)6. J. Am. Chem. Soc. 2011, 133, 11732–11743. [Google Scholar] [CrossRef]
- Minasian, S.G.; Krinsky, J.L.; Williams, V.A.; Arnold, J. A heterobimetallic complex with an unsupported Uranium(III)-Aluminum(I) bond: (CpSiMe3)3U-AlCp* (Cp* = C5Me5). J. Am. Chem. Soc. 2008, 130, 10086–10087. [Google Scholar] [CrossRef]
- Minasian, S.G.; Krinsky, J.L.; Rinehart, J.D.; Copping, R.; Tyliszczak, T.; Janousch, M.; Shuh, D.K.; Arnold, J. A Comparison of 4f vs 5f Metal-Metal Bonds in (CpSiMe3)3M-ECp* (M = Nd, U; E = Al, Ga; Cp* = C5Me5): Synthesis, Thermodynamics, Magnetism, and Electronic Structure. J. Am. Chem. Soc. 2009, 131, 13767–13783. [Google Scholar] [CrossRef]
- Liddle, S.T.; McMaster, J.; Mills, D.P.; Blake, A.J.; Jones, C.; Woodul, W.D. σ and π Donation in an Unsupported Uranium-Gallium Bond. Angew. Chem. Int. Ed. 2009, 48, 1077–1080. [Google Scholar] [CrossRef]
- Diaconescu, P.L.; Odum, A.L.; Agapie, T.; Cummins, C.C. Uranium-Group 14 Element Single Bonds: Isolation and Characterization of a Uranium(IV) Silyl Species. Organometallics 2001, 20, 4993–4995. [Google Scholar] [CrossRef]
- Porchia, M.; Brianese, N.; Casellato, U.; Ossola, F.; Rossetto, G.; Zanella, P. Tri(η-cyclopentadienyl)uranium(IV) silyl and siloxide compounds: Crystal structure of [U(η5-C5H5)3(OSiPh3)]: Insertion of Isocyanide into a uranium-silicon bond. J. Chem. Soc. Dalton Trans. 1989, 677–681. [Google Scholar] [CrossRef]
- Brackbill, I.J.; Douair, I.; Lussier, D.J.; Boreen, M.A.; Maron, L.; Arnold, J. Synthesis and Structure of Uranium-Silylene Complexes. Chem. Eur. J. 2020, 26, 2360–2364. [Google Scholar] [CrossRef]
- Réant, B.L.L.; Berryman, V.E.J.; Seed, J.A.; Basford, A.R.; Formanuik, A.; Wooles, A.J.; Kaltsoyannis, N.; Liddle, S.T.; Mills, D.P. Polarised Covalent Thorium(IV)- and Uranium(IV)-Silicon Bonds. Chem. Commun. 2020, 56, 12620–12623. [Google Scholar] [CrossRef]
- Porchia, M.; Ossola, F.; Rossetto, G.; Zanella, P.; Brianese, N. Synthesis of triscyclopentadienyl(triphenylgermyl)uranium and facile isonitrile insertion into the uranium-germanium bond. J. Chem. Soc. Chem. Commun. 1987, 550–551. [Google Scholar] [CrossRef]
- Porchia, M.; Casellato, U.; Ossola, F.; Rossetto, G.; Zanella, P.; Graziani, R. Synthesis and crystal structure of triscyclopentadienyl-(triphenyltin)uranium: The first example of a uranium-tin bond. J. Chem. Soc. Chem. Commun. 1986, 1034–1035. [Google Scholar] [CrossRef]
- Winston, M.S.; Batista, E.R.; Yang, P.; Tondreau, A.M.; Boncella, J.M. Extending Stannyl Anion Chemistry to the Actinides: Synthesis and Characterization of a Uranium−Tin Bond. Inorg. Chem. 2016, 55, 5534–5539. [Google Scholar] [CrossRef]
- Rookes, T.M.; Wildman, E.P.; Balazs, G.; Gardner, B.M.; Wooles, A.J.; Gregson, M.; Tuna, F.; Scheer, M.; Liddle, S.T. Actinide-Pnictide (An-Pn) Bonds Spanning Non-Metal, Metalloid, and Metal Combinations (An = U, Th; Pn = P, As, Sb, Bi). Angew. Chem. Int. Ed. 2018, 57, 1332–1336. [Google Scholar] [CrossRef]
- Lichtenberger, N.; Wilson, R.J.; Eulenstein, A.R.; Massa, W.; Clérac, R.; Weigend, F.; Dehnen, S. Main Group Metal-Actinide Magnetic Coupling and Structural Response Upon U4+ Inclusion into Bi, Tl/Bi, or Pb/Bi Cages. J. Am. Chem. Soc. 2016, 138, 9033–9036. [Google Scholar] [CrossRef]
- Eulenstein, A.R.; Franzke, Y.J.; Lichtenberger, N.; Wilson, R.J.; Deubner, H.L.; Kraus, F.; Clérac, R.; Weigand, F.; Dehnen, S. Substantial π-aromaticity in the anionic heavy-metal cluster [Th@Bi12]4−. Nat. Chem. 2021, 13, 149–155. [Google Scholar] [CrossRef]
- Gardner, B.M.; Lewis, W.; Blake, A.J.; Liddle, S.T. Thorium Triamidoamine Complexes: Synthesis of an Unusual Dinuclear Tuck-In-Tuck-Over Thorium Metallacycle Featuring the Longest Known Thorium-σ-Alkyl Bond. Organometallics 2015, 34, 2386–2394. [Google Scholar] [CrossRef]
- Green, M.L.H.; Pratt, L.; Wilkinson, G. Biscyclopentadienylrhenium hydride. J. Chem. Soc. 1958, 3916–3922. [Google Scholar] [CrossRef]
- Karunananda, M.K.; Mankad, N.P. Heterobimetalluc H2 Addition and Alkene/Alkane Elimination Reactions Related to the Mechanism of E-Selective Alkyne Semihydrogenation. Organometallics 2017, 36, 220–227. [Google Scholar] [CrossRef]
- Pyykkö, P. Additive Covalent Radii for Single-, Double-, and Triple-Bonded Molecules and Tetrahedrally Bonded Crystals: A Summary. J. Phys. Chem. A 2015, 119, 2326–2337. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, J.P.A.; Atkinson, B.E.; Doyle, L.R.; Wooles, A.J.; Kaltsoyannis, N.; Liddle, S.T. The Ditungsten Decacarbonyl Dianion. Dalton Trans. 2020, 49, 9330–9355. [Google Scholar] [CrossRef]
- Oxford Diffraction/Agilent Technologies UK Ltd. CrysAlisPRO Version 39.46; Oxford Diffraction/Agilent Technologies UK Ltd.: Yarnton, UK, 2013. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal structure determination. Acta Cryst. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Farugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Persistence of Vision Pty Ltd. Persistence of Vision (TM) Raytracer; Persistence of Vision Pty. Ltd.: Williamstown, Melbourne, VIC, Australia, 2020. [Google Scholar]
- Fonseca Guerra, C.; Snijders, J.G.; Te Velde, G.; Baerends, E.J. Towards an order-N DFT Method. Theor. Chem. Acc. 1998, 99, 391–403. [Google Scholar] [CrossRef]
- Te Velde, G.; Bickelhaupt, F.M.; Van Gisbergen, S.J.; Fonseca Guerra, A.C.; Baerends, E.J.; Snijders, J.G.; Ziegler, T. Chemistry with ADF. J. Comput. Chem. 2001, 22, 931–967. [Google Scholar] [CrossRef]
- Van Lenthe, E.; Baerends, E.J.; Snijders, J.G. Relativistic regular two-component Hamiltonians. J. Chem. Phys. 1993, 99, 4597–4610. [Google Scholar] [CrossRef]
- Van Lenthe, E.; Baerends, E.J.; Snijders, J.G. Relativistic total energy using regular approximations. J. Chem. Phys. 1994, 101, 9783–9792. [Google Scholar] [CrossRef]
- Van Lenthe, E.; Ehlers, A.E.; Baerends, E.J. Geometry optimization in the Zero Order Regular Approximation for relativistic effects. J. Chem. Phys. 1999, 110, 8943–8953. [Google Scholar] [CrossRef]
- Vosko, S.H.; Wilk, L.; Nusair, M. Accurate spin-dependent electron liquid correlation energies for local spin density calculations: A critical analysis. Can. J. Phys. 1980, 58, 1200–1211. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behaviour. Phys. Rev. A 1988, 38, 3098. [Google Scholar] [CrossRef]
- Perdew, J.P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822. [Google Scholar] [CrossRef]
- Portmann, S.; Luthi, H.P. MOLEKEL: An interactive molecular graphics tool. Chimia 2000, 54, 766–770. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostrowski, J.P.A.; Wooles, A.J.; Liddle, S.T. Synthesis and Characterisation of Molecular Polarised-Covalent Thorium-Rhenium and -Ruthenium Bonds. Inorganics 2021, 9, 30. https://doi.org/10.3390/inorganics9050030
Ostrowski JPA, Wooles AJ, Liddle ST. Synthesis and Characterisation of Molecular Polarised-Covalent Thorium-Rhenium and -Ruthenium Bonds. Inorganics. 2021; 9(5):30. https://doi.org/10.3390/inorganics9050030
Chicago/Turabian StyleOstrowski, Joseph P. A., Ashley J. Wooles, and Stephen T. Liddle. 2021. "Synthesis and Characterisation of Molecular Polarised-Covalent Thorium-Rhenium and -Ruthenium Bonds" Inorganics 9, no. 5: 30. https://doi.org/10.3390/inorganics9050030
APA StyleOstrowski, J. P. A., Wooles, A. J., & Liddle, S. T. (2021). Synthesis and Characterisation of Molecular Polarised-Covalent Thorium-Rhenium and -Ruthenium Bonds. Inorganics, 9(5), 30. https://doi.org/10.3390/inorganics9050030






