Oral Health and Liver Disease: Bidirectional Associations—A Narrative Review
Abstract
:1. Introduction
2. Methods
3. Oral Manifestations in Chronic Liver Diseases
3.1. Oral Mucosa
3.2. Xerostomia
3.3. Teeth
3.4. Periodontal Infections
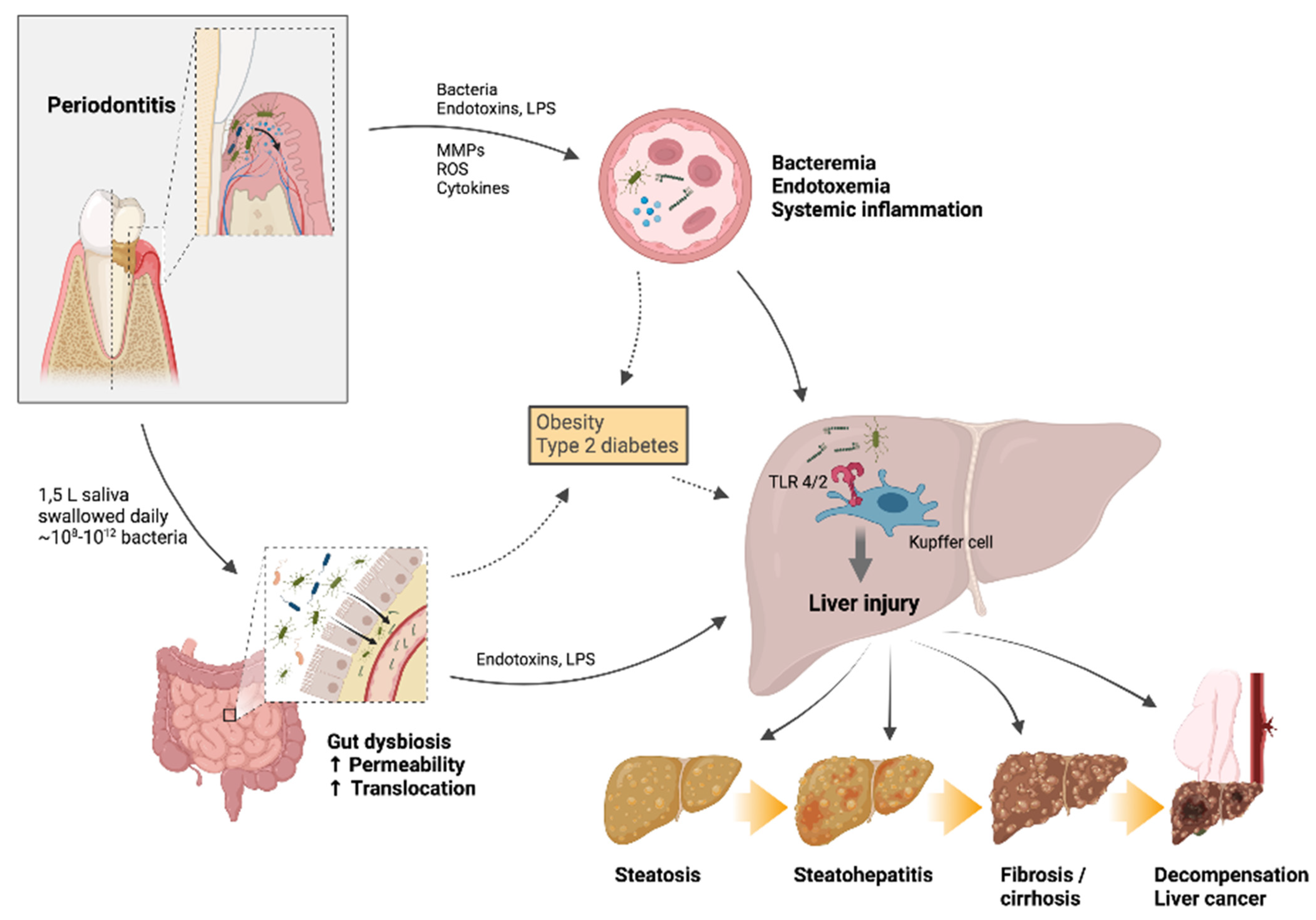
4. Oral-Gut-Liver Axis
4.1. Links between Periodontal and Systemic Disease
4.2. Role of the Gut in Liver Disease
4.3. Experimental Periodontitis: Effects on the Liver
4.4. Associations between Periodontal Disease and Liver Disease in Human Studies
4.5. Systemic and Liver Effects of Periodontal Treatment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hajishengallis, G.; Chavakis, T. Local and Systemic Mechanisms Linking Periodontal Disease and Inflammatory Comorbidities. Nat. Rev. Immunol. 2021, 21, 426–440. [Google Scholar] [CrossRef]
- Kuraji, R.; Sekino, S.; Kapila, Y.; Numabe, Y. Periodontal Disease-Related Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis: An Emerging Concept of Oral-Liver Axis. Periodontol 2000 2021, 87, 204–240. [Google Scholar] [CrossRef]
- Acharya, C.; Sahingur, S.E.; Bajaj, J.S. Microbiota, Cirrhosis, and the Emerging Oral-Gut-Liver Axis. JCI Insight 2017, 2, 94416. [Google Scholar] [CrossRef] [PubMed]
- Hatasa, M.; Yoshida, S.; Takahashi, H.; Tanaka, K.; Kubotsu, Y.; Ohsugi, Y.; Katagiri, T.; Iwata, T.; Katagiri, S. Relationship between NAFLD and Periodontal Disease from the View of Clinical and Basic Research, and Immunological Response. Int. J. Mol. Sci. 2021, 22, 3728. [Google Scholar] [CrossRef]
- Selmi, C.; Meroni, P.L.; Gershwin, M.E. Primary Biliary Cirrhosis and Sjögren’s Syndrome: Autoimmune Epithelitis. J. Autoimmun. 2012, 39, 34–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vieira, C.L.Z.; Caramelli, B. The History of Dentistry and Medicine Relationship: Could the Mouth Finally Return to the Body? Oral. Dis. 2009, 15, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Price, W.A. Dental Infections and Related Degenerative Diseases: Some Structural and Biochemical Factors. J. Am. Med. Assoc. 1925, 84, 254–261. [Google Scholar] [CrossRef]
- Mattila, K.J.; Nieminen, M.S.; Valtonen, V.V.; Rasi, V.P.; Kesäniemi, Y.A.; Syrjälä, S.L.; Jungell, P.S.; Isoluoma, M.; Hietaniemi, K.; Jokinen, M.J. Association between Dental Health and Acute Myocardial Infarction. BMJ 1989, 298, 779–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsen, I.; Yamazaki, K. Can Oral Bacteria Affect the Microbiome of the Gut? J. Oral Microbiol. 2019, 11, 1586422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arimatsu, K.; Yamada, H.; Miyazawa, H.; Minagawa, T.; Nakajima, M.; Ryder, M.I.; Gotoh, K.; Motooka, D.; Nakamura, S.; Iida, T.; et al. Oral Pathobiont Induces Systemic Inflammation and Metabolic Changes Associated with Alteration of Gut Microbiota. Sci. Rep. 2014, 4, 4828. [Google Scholar] [CrossRef] [Green Version]
- Kitamoto, S.; Nagao-Kitamoto, H.; Jiao, Y.; Gillilland, M.G.; Hayashi, A.; Imai, J.; Sugihara, K.; Miyoshi, M.; Brazil, J.C.; Kuffa, P.; et al. The Intermucosal Connection between the Mouth and Gut in Commensal Pathobiont-Driven Colitis. Cell 2020, 182, 447–462.e14. [Google Scholar] [CrossRef] [PubMed]
- Albillos, A.; de Gottardi, A.; Rescigno, M. The Gut-Liver Axis in Liver Disease: Pathophysiological Basis for Therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, A.; Debelius, J.; Brenner, D.A.; Karin, M.; Loomba, R.; Schnabl, B.; Knight, R. The Gut-Liver Axis and the Intersection with the Microbiome. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Chopyk, D.M.; Grakoui, A. Contribution of the Intestinal Microbiome and Gut Barrier to Hepatic Disorders. Gastroenterology 2020, 159, 849–863. [Google Scholar] [CrossRef]
- Trebicka, J.; Macnaughtan, J.; Schnabl, B.; Shawcross, D.L.; Bajaj, J.S. The Microbiota in Cirrhosis and Its Role in Hepatic Decompensation. J. Hepatol. 2021, 75 (Suppl. 1), S67–S81. [Google Scholar] [CrossRef] [PubMed]
- Helenius-Hietala, J.; Suominen, A.L.; Ruokonen, H.; Knuuttila, M.; Puukka, P.; Jula, A.; Meurman, J.H.; Åberg, F. Periodontitis Is Associated with Incident Chronic Liver Disease-A Population-Based Cohort Study. Liver Int. 2019, 39, 583–591. [Google Scholar] [CrossRef] [Green Version]
- Åberg, F.; Helenius-Hietala, J. Oro-Hepatic Link, Endotoxemia, and Systemic Inflammation: The Role of Chronic Periodontitis. Hepatology 2016, 63, 1736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajaj, J.S.; Matin, P.; White, M.B.; Fagan, A.; Deeb, J.G.; Acharya, C.; Dalmet, S.S.; Sikaroodi, M.; Gillevet, P.M.; Sahingur, S.E. Periodontal Therapy Favorably Modulates the Oral-Gut-Hepatic Axis in Cirrhosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G824–G837. [Google Scholar] [CrossRef] [Green Version]
- Kamata, Y.; Kessoku, T.; Shimizu, T.; Kobayashi, T.; Kurihashi, T.; Sato, S.; Kuraji, S.; Aoyama, N.; Iwasaki, T.; Takashiba, S.; et al. Efficacy and Safety of PERIOdontal Treatment versus Usual Care for Nonalcoholic Liver Disease: Protocol of the PERION Multicenter, Two-Arm, Open-Label, Randomized Trial. Trials 2020, 21, 291. [Google Scholar] [CrossRef] [Green Version]
- Guggenheimer, J.; Close, J.M.; Eghtesad, B.; Shay, C. Characteristics of Oral Abnormalities in Liver Transplant Candidates. Int. J. Organ Transplant. Med. 2010, 1, 107–113. [Google Scholar]
- de Oliveira Rech, B.; Rocha Tenório, J.; Bertoldi Franco, J.; Medina, J.B.; Gallottini, M.; Pérez-Sayáns, M.; Ortega, K.L. Risk of Bleeding during Oral Surgery in Patients with Liver Cirrhosis: A Systematic Review. J. Am. Dent. Assoc. 2021, 152, 46–54.e2. [Google Scholar] [CrossRef]
- Helenius-Hietala, J.; Åberg, F.; Meurman, J.H.; Nordin, A.; Isoniemi, H. Oral Surgery in Liver Transplant Candidates: A Retrospective Study on Delayed Bleeding and Other Complications. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 121, 490–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sommer, S.; Magagnin, K.; Kramer, P.F.; Tovo, M.F.; Bervian, J. Green Teeth Associated with Neonatal Hyperbilirubinemia Caused by Biliary Atresia: Review and Case Report. J. Clin. Pediatr. Dent. 2010, 35, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Olczak-Kowalczyk, D.; Gozdowski, D.; Pawłowska, J.; Grenda, R. The Status of Dental and Jaw Bones in Children and Adolescents after Kidney and Liver Transplantation. Ann. Transpl. 2012, 17, 72–81. [Google Scholar] [CrossRef]
- Hosey, M.T.; Gordon, G.; Kelly, D.A.; Shaw, L. Oral Findings in Children with Liver Transplants. Int. J. Paediatr. Dent. 1995, 5, 29–34. [Google Scholar] [CrossRef]
- Guggenheimer, J.; Moore, P.A. Xerostomia: Etiology, Recognition and Treatment. J. Am. Dent. Assoc. 2003, 134, 61–69. [Google Scholar] [CrossRef]
- Lins, L.; Bittencourt, P.L.; Evangelista, M.A.; Lins, R.; Codes, L.; Cavalcanti, A.R.; Paraná, R.; Bastos, J. Oral Health Profile of Cirrhotic Patients Awaiting Liver Transplantation in the Brazilian Northeast. Transpl. Proc. 2011, 43, 1319–1321. [Google Scholar] [CrossRef]
- Ebert, E.C. Gastrointestinal and Hepatic Manifestations of Sjogren Syndrome. J. Clin. Gastroenterol. 2012, 46, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Helenius-Hietala, J.; Ruokonen, H.; Grönroos, L.; Rissanen, H.; Suominen, L.; Isoniemi, H.; Meurman, J.H. Self-Reported Oral Symptoms and Signs in Liver Transplant Recipients and a Control Population. Liver Transpl. 2013, 19, 155–163. [Google Scholar] [CrossRef]
- Jensen, A.; Ladegaard Grønkjær, L.; Holmstrup, P.; Vilstrup, H.; Kilian, M. Unique Subgingival Microbiota Associated with Periodontitis in Cirrhosis Patients. Sci. Rep. 2018, 8, 10718. [Google Scholar] [CrossRef] [Green Version]
- Grønkjær, L.L. Periodontal Disease and Liver Cirrhosis: A Systematic Review. SAGE Open Med. 2015, 3. [Google Scholar] [CrossRef] [Green Version]
- da Silva Santos, P.S.; Fernandes, K.S.; Gallottini, M.H.C. Assessment and Management of Oral Health in Liver Transplant Candidates. J. Appl. Oral Sci. 2012, 20, 241–245. [Google Scholar] [CrossRef] [Green Version]
- Helenius-Hietala, J.; Meurman, J.H.; Höckerstedt, K.; Lindqvist, C.; Isoniemi, H. Effect of the Aetiology and Severity of Liver Disease on Oral Health and Dental Treatment Prior to Transplantation. Transpl. Int. 2012, 25, 158–165. [Google Scholar] [CrossRef]
- Dukić, W.; Dobrijević, T.T.; Katunarić, M.; Milardović, S.; Segović, S. Erosive Lesions in Patients with Alcoholism. J. Am. Dent. Assoc. 2010, 141, 1452–1458. [Google Scholar] [CrossRef]
- Lodi, G.; Pellicano, R.; Carrozzo, M. Hepatitis C Virus Infection and Lichen Planus: A Systematic Review with Meta-Analysis. Oral Dis. 2010, 16, 601–612. [Google Scholar] [CrossRef]
- Scully, C.; Carrozzo, M. Oral Mucosal Disease: Lichen Planus. Br. J. Oral Maxillofac. Surg. 2008, 46, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Helenius-Hietala, J.; Ruokonen, H.; Grönroos, L.; Rissanen, H.; Vehkalahti, M.M.; Suominen, L.; Isoniemi, H.; Meurman, J.H. Oral Mucosal Health in Liver Transplant Recipients and Controls. Liver Transpl. 2014, 20, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, S.d.M.C.; Teixeira, R.; de Aguiar, M.C.F.; de Moura, M.D.G.; do Carmo, M.A.V. Oral Mucosal Conditions in Chronic Hepatitis C Brazilian Patients: A Cross-Sectional Study. J. Public Health Dent. 2009, 69, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Elahi, M.; Telkabadi, M.; Samadi, V.; Vakili, H. Association of Oral Manifestations with Ulcerative Colitis. Gastroenterol. Hepatol. Bed Bench 2012, 5, 155–160. [Google Scholar]
- Zbar, A.P.; Ben-Horin, S.; Beer-Gabel, M.; Eliakim, R. Oral Crohn’s Disease: Is It a Separable Disease from Orofacial Granulomatosis? A Review. J. Crohns Colitis 2012, 6, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Nagao, Y.; Hashimoto, K.; Sata, M. Candidiasis and Other Oral Mucosal Lesions during and after Interferon Therapy for HCV-Related Chronic Liver Diseases. BMC Gastroenterol. 2012, 12, 155. [Google Scholar] [CrossRef] [Green Version]
- da Cunha, S.F.d.C.; de Melo, D.A.; Braga, C.B.M.; Vannucchi, H.; da Cunha, D.F. Papillary Atrophy of the Tongue and Nutritional Status of Hospitalized Alcoholics. An. Bras. Dermatol. 2012, 87, 84–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díaz-Ortiz, M.L.; Micó-Llorens, J.M.; Gargallo-Albiol, J.; Baliellas-Comellas, C.; Berini-Aytés, L.; Gay-Escoda, C. Dental Health in Liver Transplant Patients. Med. Oral Patol. Oral Cir. Bucal. 2005, 10, 72–76. [Google Scholar]
- Guggenheimer, J.; Close, J.M.; Eghtesad, B. Sialadenosis in Patients with Advanced Liver Disease. Head Neck Pathol. 2009, 3, 100–105. [Google Scholar] [CrossRef] [Green Version]
- Vissink, A.; Visser, A.; Spijkervet, F.K.L. Oral medicine 1. Causes and clinical symptoms of dry mouth. Ned. Tijdschr. Tandheelkd. 2012, 119, 493–498. [Google Scholar] [CrossRef] [Green Version]
- Aguiar, I.; Lins-Kusterer, L.; Lins, L.-S.-S.; Paraná, R.; Bastos, J.; Carvalho, F.-M. Quality of Life, Work Ability and Oral Health among Patients with Chronic Liver Diseases. Med. Oral Patol. Oral Cir. Bucal. 2019, 24, e392–e397. [Google Scholar] [CrossRef] [PubMed]
- von Bültzingslöwen, I.; Sollecito, T.P.; Fox, P.C.; Daniels, T.; Jonsson, R.; Lockhart, P.B.; Wray, D.; Brennan, M.T.; Carrozzo, M.; Gandera, B.; et al. Salivary Dysfunction Associated with Systemic Diseases: Systematic Review and Clinical Management Recommendations. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007, 103, S57.e1–S57.e15. [Google Scholar] [CrossRef]
- Rangé, H.; Camy, S.; Cohen, J.; Colon, P.; Bouchard, P. Dental Treatment of an Adult Patient with a History of Biliary Atresia. Quintessence Int. 2012, 43, 337–341. [Google Scholar]
- Lussi, A.; Jaeggi, T. Erosion—Diagnosis and Risk Factors. Clin. Oral Investig. 2008, 12 (Suppl. 1), S5–S13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guggenheimer, J.; Eghtesad, B.; Stock, D.J. Dental Management of the (Solid) Organ Transplant Patient. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2003, 95, 383–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peres, M.A.; Macpherson, L.M.D.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreño, C.C.; Kearns, C.; et al. Oral Diseases: A Global Public Health Challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef]
- Verma, D.; Garg, P.K.; Dubey, A.K. Insights into the Human Oral Microbiome. Arch. Microbiol. 2018, 200, 525–540. [Google Scholar] [CrossRef]
- Pérez-Chaparro, P.J.; Gonçalves, C.; Figueiredo, L.C.; Faveri, M.; Lobão, E.; Tamashiro, N.; Duarte, P.; Feres, M. Newly Identified Pathogens Associated with Periodontitis: A Systematic Review. J. Dent. Res. 2014, 93, 846–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eke, P.I.; Dye, B.A.; Wei, L.; Thornton-Evans, G.O.; Genco, R.J. CDC Periodontal Disease Surveillance Workgroup: James Beck (University of North Carolina, Chapel Hill, USA), Gordon Douglass (Past President, American Academy of Periodontology), Roy Page (University of Washin Prevalence of Periodontitis in Adults in the United States: 2009 and 2010. J. Dent. Res. 2012, 91, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Liljestrand, J.M.; Paju, S.; Buhlin, K.; Persson, G.R.; Sarna, S.; Nieminen, M.S.; Sinisalo, J.; Mäntylä, P.; Pussinen, P.J. Lipopolysaccharide, a Possible Molecular Mediator between Periodontitis and Coronary Artery Disease. J. Clin. Periodontol. 2017, 44, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Crasta, K.; Daly, C.G.; Mitchell, D.; Curtis, B.; Stewart, D.; Heitz-Mayfield, L.J.A. Bacteraemia Due to Dental Flossing. J. Clin. Periodontol. 2009, 36, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Daly, C.G.; Mitchell, D.; Curtis, B. Incidence and Magnitude of Bacteraemia Caused by Flossing and by Scaling and Root Planing. J. Clin. Periodontol. 2013, 40, 41–52. [Google Scholar] [CrossRef]
- Horliana, A.C.R.T.; Chambrone, L.; Foz, A.M.; Artese, H.P.C.; Rabelo, M.d.S.; Pannuti, C.M.; Romito, G.A. Dissemination of Periodontal Pathogens in the Bloodstream after Periodontal Procedures: A Systematic Review. PLoS ONE 2014, 9, e98271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomás, I.; Diz, P.; Tobías, A.; Scully, C.; Donos, N. Periodontal Health Status and Bacteraemia from Daily Oral Activities: Systematic Review/Meta-Analysis. J. Clin. Periodontol. 2012, 39, 213–228. [Google Scholar] [CrossRef]
- Forner, L.; Larsen, T.; Kilian, M.; Holmstrup, P. Incidence of Bacteremia after Chewing, Tooth Brushing and Scaling in Individuals with Periodontal Inflammation. J. Clin. Periodontol. 2006, 33, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Geerts, S.O.; Nys, M.; De, M.P.; Charpentier, J.; Albert, A.; Legrand, V.; Rompen, E.H. Systemic Release of Endotoxins Induced by Gentle Mastication: Association with Periodontitis Severity. J. Periodontol. 2002, 73, 73–78. [Google Scholar] [CrossRef]
- von Troil-Lindén, B.; Torkko, H.; Alaluusua, S.; Jousimies-Somer, H.; Asikainen, S. Salivary Levels of Suspected Periodontal Pathogens in Relation to Periodontal Status and Treatment. J. Dent. Res. 1995, 74, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.S.; Hayward, M.R.; Coelho, L.P.; Li, S.S.; Costea, P.I.; Voigt, A.Y.; Wirbel, J.; Maistrenko, O.M.; Alves, R.J.; Bergsten, E.; et al. Extensive Transmission of Microbes along the Gastrointestinal Tract. Elife 2019, 8, e42693. [Google Scholar] [CrossRef]
- Rao, R. Endotoxemia and Gut Barrier Dysfunction in Alcoholic Liver Disease. Hepatology 2009, 50, 638–644. [Google Scholar] [CrossRef]
- Seki, E.; De Minicis, S.; Osterreicher, C.H.; Kluwe, J.; Osawa, Y.; Brenner, D.A.; Schwabe, R.F. TLR4 Enhances TGF-Beta Signaling and Hepatic Fibrosis. Nat. Med. 2007, 13, 1324–1332. [Google Scholar] [CrossRef]
- Männistö, V.; Färkkilä, M.; Pussinen, P.; Jula, A.; Männistö, S.; Lundqvist, A.; Valsta, L.; Salomaa, V.; Perola, M.; Åberg, F. Serum Lipopolysaccharides Predict Advanced Liver Disease in the General Population. JHEP Rep. 2019, 1, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Trebicka, J.; Bork, P.; Krag, A.; Arumugam, M. Utilizing the Gut Microbiome in Decompensated Cirrhosis and Acute-on-Chronic Liver Failure. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 167–180. [Google Scholar] [CrossRef]
- Qin, N.; Yang, F.; Li, A.; Prifti, E.; Chen, Y.; Shao, L.; Guo, J.; Le Chatelier, E.; Yao, J.; Wu, L.; et al. Alterations of the Human Gut Microbiome in Liver Cirrhosis. Nature 2014, 513, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, M.; Naka, S.; Nakano, K.; Wada, K.; Endo, H.; Mawatari, H.; Imajo, K.; Nomura, R.; Hokamura, K.; Ono, M.; et al. Involvement of a Periodontal Pathogen, Porphyromonas Gingivalis on the Pathogenesis of Non-Alcoholic Fatty Liver Disease. BMC Gastroenterol. 2012, 12, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furusho, H.; Miyauchi, M.; Hyogo, H.; Inubushi, T.; Ao, M.; Ouhara, K.; Hisatune, J.; Kurihara, H.; Sugai, M.; Hayes, C.N.; et al. Dental Infection of Porphyromonas Gingivalis Exacerbates High Fat Diet-Induced Steatohepatitis in Mice. J. Gastroenterol. 2013, 48, 1259–1270. [Google Scholar] [CrossRef] [PubMed]
- Kuraji, R.; Ito, H.; Fujita, M.; Ishiguro, H.; Hashimoto, S.; Numabe, Y. Porphyromonas Gingivalis Induced Periodontitis Exacerbates Progression of Non-Alcoholic Steatohepatitis in Rats. Clin. Exp. Dent. Res. 2016, 2, 216–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakahara, T.; Hyogo, H.; Ono, A.; Nagaoki, Y.; Kawaoka, T.; Miki, D.; Tsuge, M.; Hiraga, N.; Hayes, C.N.; Hiramatsu, A.; et al. Involvement of Porphyromonas Gingivalis in the Progression of Non-Alcoholic Fatty Liver Disease. J. Gastroenterol. 2018, 53, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Nagasaki, A.; Sakamoto, S.; Chea, C.; Ishida, E.; Furusho, H.; Fujii, M.; Takata, T.; Miyauchi, M. Odontogenic Infection by Porphyromonas Gingivalis Exacerbates Fibrosis in NASH via Hepatic Stellate Cell Activation. Sci. Rep. 2020, 10, 4134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsukasaki, M.; Komatsu, N.; Nagashima, K.; Nitta, T.; Pluemsakunthai, W.; Shukunami, C.; Iwakura, Y.; Nakashima, T.; Okamoto, K.; Takayanagi, H. Host Defense against Oral Microbiota by Bone-Damaging T Cells. Nat. Commun. 2018, 9, 701. [Google Scholar] [CrossRef]
- Nakajima, M.; Arimatsu, K.; Kato, T.; Matsuda, Y.; Minagawa, T.; Takahashi, N.; Ohno, H.; Yamazaki, K. Oral Administration of P. Gingivalis Induces Dysbiosis of Gut Microbiota and Impaired Barrier Function Leading to Dissemination of Enterobacteria to the Liver. PLoS ONE 2015, 10, e0134234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komazaki, R.; Katagiri, S.; Takahashi, H.; Maekawa, S.; Shiba, T.; Takeuchi, Y.; Kitajima, Y.; Ohtsu, A.; Udagawa, S.; Sasaki, N.; et al. Periodontal Pathogenic Bacteria, Aggregatibacter Actinomycetemcomitans Affect Non-Alcoholic Fatty Liver Disease by Altering Gut Microbiota and Glucose Metabolism. Sci. Rep. 2017, 7, 13950. [Google Scholar] [CrossRef]
- Ishikawa, M.; Yoshida, K.; Okamura, H.; Ochiai, K.; Takamura, H.; Fujiwara, N.; Ozaki, K. Oral Porphyromonas Gingivalis Translocates to the Liver and Regulates Hepatic Glycogen Synthesis through the Akt/GSK-3β Signaling Pathway. Biochim. Biophys. Acta 2013, 1832, 2035–2043. [Google Scholar] [CrossRef] [Green Version]
- Fujita, M.; Kuraji, R.; Ito, H.; Hashimoto, S.; Toen, T.; Fukada, T.; Numabe, Y. Histological Effects and Pharmacokinetics of Lipopolysaccharide Derived from Porphyromonas Gingivalis on Rat Maxilla and Liver Concerning with Progression into Non-Alcoholic Steatohepatitis. J. Periodontol. 2018, 89, 1101–1111. [Google Scholar] [CrossRef]
- Ding, L.-Y.; Liang, L.-Z.; Zhao, Y.-X.; Yang, Y.-N.; Liu, F.; Ding, Q.-R.; Luo, L.-J. Porphyromonas Gingivalis-Derived Lipopolysaccharide Causes Excessive Hepatic Lipid Accumulation via Activating NF-ΚB and JNK Signaling Pathways. Oral Dis. 2019, 25, 1789–1797. [Google Scholar] [CrossRef]
- Matsuda, Y.; Kato, T.; Takahashi, N.; Nakajima, M.; Arimatsu, K.; Minagawa, T.; Sato, K.; Ohno, H.; Yamazaki, K. Ligature-Induced Periodontitis in Mice Induces Elevated Levels of Circulating Interleukin-6 but Shows Only Weak Effects on Adipose and Liver Tissues. J. Periodontal. Res. 2016, 51, 639–646. [Google Scholar] [CrossRef]
- Tomofuji, T.; Sanbe, T.; Ekuni, D.; Azuma, T.; Irie, K.; Maruyama, T.; Tamaki, N.; Yamamoto, T. Oxidative Damage of Rat Liver Induced by Ligature-Induced Periodontitis and Chronic Ethanol Consumption. Arch. Oral Biol. 2008, 53, 1113–1118. [Google Scholar] [CrossRef]
- Rivera, C.A.; Adegboyega, P.; van Rooijen, N.; Tagalicud, A.; Allman, M.; Wallace, M. Toll-Like Receptor-4 Signaling and Kupffer Cells Play Pivotal Roles in the Pathogenesis of Non-Alcoholic Steatohepatitis. J. Hepatol. 2007, 47, 571–579. [Google Scholar] [CrossRef] [Green Version]
- Engelmann, C.; Sheikh, M.; Sharma, S.; Kondo, T.; Loeffler-Wirth, H.; Zheng, Y.B.; Novelli, S.; Hall, A.; Kerbert, A.J.C.; Macnaughtan, J.; et al. Toll-Like Receptor 4 Is a Therapeutic Target for Prevention and Treatment of Liver Failure. J. Hepatol. 2020, 73, 102–112. [Google Scholar] [CrossRef]
- Tomofuji, T.; Ekuni, D.; Sanbe, T.; Azuma, T.; Tamaki, N.; Irie, K.; Maruyama, T.; Yamamoto, T.; Watanabe, T.; Miyauchi, M.; et al. Effects of Improvement in Periodontal Inflammation by Toothbrushing on Serum Lipopolysaccharide Concentration and Liver Injury in Rats. Acta Odontol. Scand. 2009, 67, 200–205. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Y.-C.; Zhu, B.-L.; Wu, C.-C.; Lin, R.-F.; Zhang, X. Association between Periodontal Disease, Tooth Loss and Liver Diseases Risk. J. Clin. Periodontol. 2020, 47, 1053–1063. [Google Scholar] [CrossRef]
- Wijarnpreecha, K.; Panjawatanan, P.; Cheungpasitporn, W.; Lukens, F.J.; Harnois, D.M.; Pungpapong, S.; Ungprasert, P. The Association between Periodontitis and Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. J. Gastrointestin. Liver Dis. 2020, 29, 211–217. [Google Scholar] [CrossRef]
- Weintraub, J.A.; Lopez Mitnik, G.; Dye, B.A. Oral Diseases Associated with Nonalcoholic Fatty Liver Disease in the United States. J. Dent. Res. 2019, 98, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Alazawi, W.; Bernabe, E.; Tai, D.; Janicki, T.; Kemos, P.; Samsuddin, S.; Syn, W.-K.; Gillam, D.; Turner, W. Periodontitis Is Associated with Significant Hepatic Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease. PLoS ONE 2017, 12, e0185902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Widita, E.; Yoshihara, A.; Hanindriyo, L.; Miyazaki, H. Relationship between Clinical Periodontal Parameters and Changes in Liver Enzymes Levels over an 8-Year Period in an Elderly Japanese Population. J. Clin. Periodontol. 2018, 45, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Akinkugbe, A.A.; Slade, G.D.; Barritt, A.S.; Cole, S.R.; Offenbacher, S.; Petersmann, A.; Kocher, T.; Lerch, M.M.; Mayerle, J.; Völzke, H.; et al. Periodontitis and Non-Alcoholic Fatty Liver Disease, a Population-Based Cohort Investigation in the Study of Health in Pomerania. J. Clin. Periodontol. 2017, 44, 1077–1087. [Google Scholar] [CrossRef]
- Jordão, H.W.; McKenna, G.; McMenamin, Ú.C.; Kunzmann, A.T.; Murray, L.J.; Coleman, H.G. The Association between Self-Reported Poor Oral Health and Gastrointestinal Cancer Risk in the UK Biobank: A Large Prospective Cohort Study. United Eur. Gastroenterol. J. 2019, 7, 1241–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michaud, D.S.; Kelsey, K.T.; Papathanasiou, E.; Genco, C.A.; Giovannucci, E. Periodontal Disease and Risk of All Cancers among Male Never Smokers: An Updated Analysis of the Health Professionals Follow-up Study. Ann. Oncol. 2016, 27, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Petrick, J.L.; Abnet, C.C.; Graubard, B.I.; Murphy, G.; Weinstein, S.J.; Männistö, S.; Albanes, D.; McGlynn, K.A. Tooth Loss and Liver Cancer Incidence in a Finnish Cohort. Cancer Causes Control 2017, 28, 899–904. [Google Scholar] [CrossRef]
- Thistle, J.E.; Yang, B.; Petrick, J.L.; Fan, J.-H.; Qiao, Y.-L.; Abnet, C.C.; Taylor, P.R.; McGlynn, K.A. Association of Tooth Loss with Liver Cancer Incidence and Chronic Liver Disease Mortality in a Rural Chinese Population. PLoS ONE 2018, 13, e0203926. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, N.; Takaki, A.; Tomofuji, T.; Endo, Y.; Kasuyama, K.; Ekuni, D.; Yasunaka, T.; Yamamoto, K.; Morita, M. Stage of Hepatocellular Carcinoma Is Associated with Periodontitis. J. Clin. Periodontol. 2011, 38, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Aberg, F.; Helenius-Hietala, J.; Meurman, J.; Isoniemi, H. Association between Dental Infections and the Clinical Course of Chronic Liver Disease. Hepatol. Res. 2014, 44, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Grønkjær, L.L.; Holmstrup, P.; Schou, S.; Schwartz, K.; Kongstad, J.; Jepsen, P.; Vilstrup, H. Presence and Consequence of Tooth Periapical Radiolucency in Patients with Cirrhosis. Hepat. Med. 2016, 8, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.-H.; Lee, C.-Y.; Chang, W.-T.; Wu, P.-H.; Chen, L.-A.; Huang, J.-W.; Su, W.-L.; Kuo, K.-K. The Association between Oral Health Status and the Clinical Outcome of Cirrhotic Patients on the Waiting List for Liver Transplantation. Kaohsiung J. Med. Sci. 2021, 37, 910–917. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Betrapally, N.S.; Hylemon, P.B.; Heuman, D.M.; Daita, K.; White, M.B.; Unser, A.; Thacker, L.R.; Sanyal, A.J.; Kang, D.J.; et al. Salivary Microbiota Reflects Changes in Gut Microbiota in Cirrhosis with Hepatic Encephalopathy. Hepatology 2015, 62, 1260–1271. [Google Scholar] [CrossRef] [Green Version]
- Pietiäinen, M.; Liljestrand, J.M.; Kopra, E.; Pussinen, P.J. Mediators between Oral Dysbiosis and Cardiovascular Diseases. Eur. J. Oral Sci. 2018, 126 (Suppl. 1), 26–36. [Google Scholar] [CrossRef] [Green Version]
- Teeuw, W.J.; Slot, D.E.; Susanto, H.; Gerdes, V.E.A.; Abbas, F.; D’Aiuto, F.; Kastelein, J.J.P.; Loos, B.G. Treatment of Periodontitis Improves the Atherosclerotic Profile: A Systematic Review and Meta-Analysis. J. Clin. Periodontol. 2014, 41, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Pussinen, P.J.; Vilkuna-Rautiainen, T.; Alfthan, G.; Palosuo, T.; Jauhiainen, M.; Sundvall, J.; Vesanen, M.; Mattila, K.; Asikainen, S. Severe Periodontitis Enhances Macrophage Activation via Increased Serum Lipopolysaccharide. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 2174–2180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Önder, C.; Kurgan, Ş.; Altıngöz, S.M.; Bağış, N.; Uyanık, M.; Serdar, M.A.; Kantarcı, A.; Günhan, M. Impact of Non-Surgical Periodontal Therapy on Saliva and Serum Levels of Markers of Oxidative Stress. Clin. Oral Investig. 2017, 21, 1961–1969. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Herrera, M.; Abad-Jiménez, Z.; Silvestre, F.J.; López-Domènech, S.; Márquez-Arrico, C.F.; Silvestre-Rangil, J.; Víctor, V.M.; Rocha, M. Effect of Non-Surgical Periodontal Treatment on Oxidative Stress Markers in Leukocytes and Their Interaction with the Endothelium in Obese Subjects with Periodontitis: A Pilot Study. J. Clin. Med. 2020, 9, 2117. [Google Scholar] [CrossRef]
- Tonetti, M.S.; D’Aiuto, F.; Nibali, L.; Donald, A.; Storry, C.; Parkar, M.; Suvan, J.; Hingorani, A.D.; Vallance, P.; Deanfield, J. Treatment of Periodontitis and Endothelial Function. N. Engl. J. Med. 2007, 356, 911–920. [Google Scholar] [CrossRef] [Green Version]
- D’Aiuto, F.; Gkranias, N.; Bhowruth, D.; Khan, T.; Orlandi, M.; Suvan, J.; Masi, S.; Tsakos, G.; Hurel, S.; Hingorani, A.D.; et al. Systemic Effects of Periodontitis Treatment in Patients with Type 2 Diabetes: A 12 Month, Single-Centre, Investigator-Masked, Randomised Trial. Lancet Diabetes Endocrinol. 2018, 6, 954–965. [Google Scholar] [CrossRef]


| Oral Manifestation | Relation to Liver Disease | Reference |
|---|---|---|
| Petechiae, telangiectasia, hematoma, gingival bleeding, reduced wound healing | Coagulopathy | de Oliveira Rech 2021 [21] Helenius-Hietala 2016 [22] |
| Discolorations of teeth, enamel hypoplasia, delayed eruption of teeth | Biliary atresia, malnutrition | Sommer 2010 [23] Olczak-Kowalczyk 2012 [24] Hosey 1995 [25] |
| Xerostomia Hyposalivation | HCV, PBC, IBD/PSC | Guggenheimer 2003 [26] Lins 2011 [27] Ebert 2012 [28] Helenius-Hietala 2013 [29] |
| Periodontal disease | Cytopenia, dysbiosis, compromised immune system | Kuraji 2021 [2] Acharya 2017 [3] Jensen 2018 [30] |
| Tooth decay (caries) | Hyposalivation/xerostomia, alcohol-related liver disease | Gronkjaer 2015 [31] Lins 2011 [27] Silva Santos 2012 [32] Helenius-Hietala 2012 [33] |
| Erosion | Alcohol-related liver disease, gastric reflux | Dukic 2010 [34] Helenius-Hietala 2012 [33] |
| Oral lichen planus, lichenoid lesions | HCV, PBC | Lodi 2010 [35] Scully 2008 [36] Helenius-Hietala 2014 [37] |
| Leukoplakia | HCV | Grossman 2009 [38] |
| Mucosal ulcers | IBD/PSC, PBC | Elahi 2012 [39] Zbar 2012 [40] |
| Candidiasis, angular cheilitis | Compromised immune system | Nagao 2012 [41] Helenius-Hietala 2012 [33] Helenius-Hietala 2014 [37] |
| Glossitis, atrophic tongue | Alcohol-related liver disease, nutritional deficiencies | Cunha 2012 [42] |
| Fissured tongue | HCV, IBD/PSC | Elahi 2012 [39] Diaz-Ortiz 2005 [43] Helenius-Hietala 2012 [33] Guggenheimer 2010 [20] |
| Parotid gland enlargement/ sialadenitis | Alcohol-related liver disease, HCV | Guggenheimer 2009 [44] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Åberg, F.; Helenius-Hietala, J. Oral Health and Liver Disease: Bidirectional Associations—A Narrative Review. Dent. J. 2022, 10, 16. https://doi.org/10.3390/dj10020016
Åberg F, Helenius-Hietala J. Oral Health and Liver Disease: Bidirectional Associations—A Narrative Review. Dentistry Journal. 2022; 10(2):16. https://doi.org/10.3390/dj10020016
Chicago/Turabian StyleÅberg, Fredrik, and Jaana Helenius-Hietala. 2022. "Oral Health and Liver Disease: Bidirectional Associations—A Narrative Review" Dentistry Journal 10, no. 2: 16. https://doi.org/10.3390/dj10020016
APA StyleÅberg, F., & Helenius-Hietala, J. (2022). Oral Health and Liver Disease: Bidirectional Associations—A Narrative Review. Dentistry Journal, 10(2), 16. https://doi.org/10.3390/dj10020016






