Analysis of the Osseointegration Process of Dental Implants by Electron Paramagnetic Resonance: An In Vivo Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Investigated Materials
2.2. Research Methods
2.2.1. Theoretical Background
2.2.2. Experimental Setup
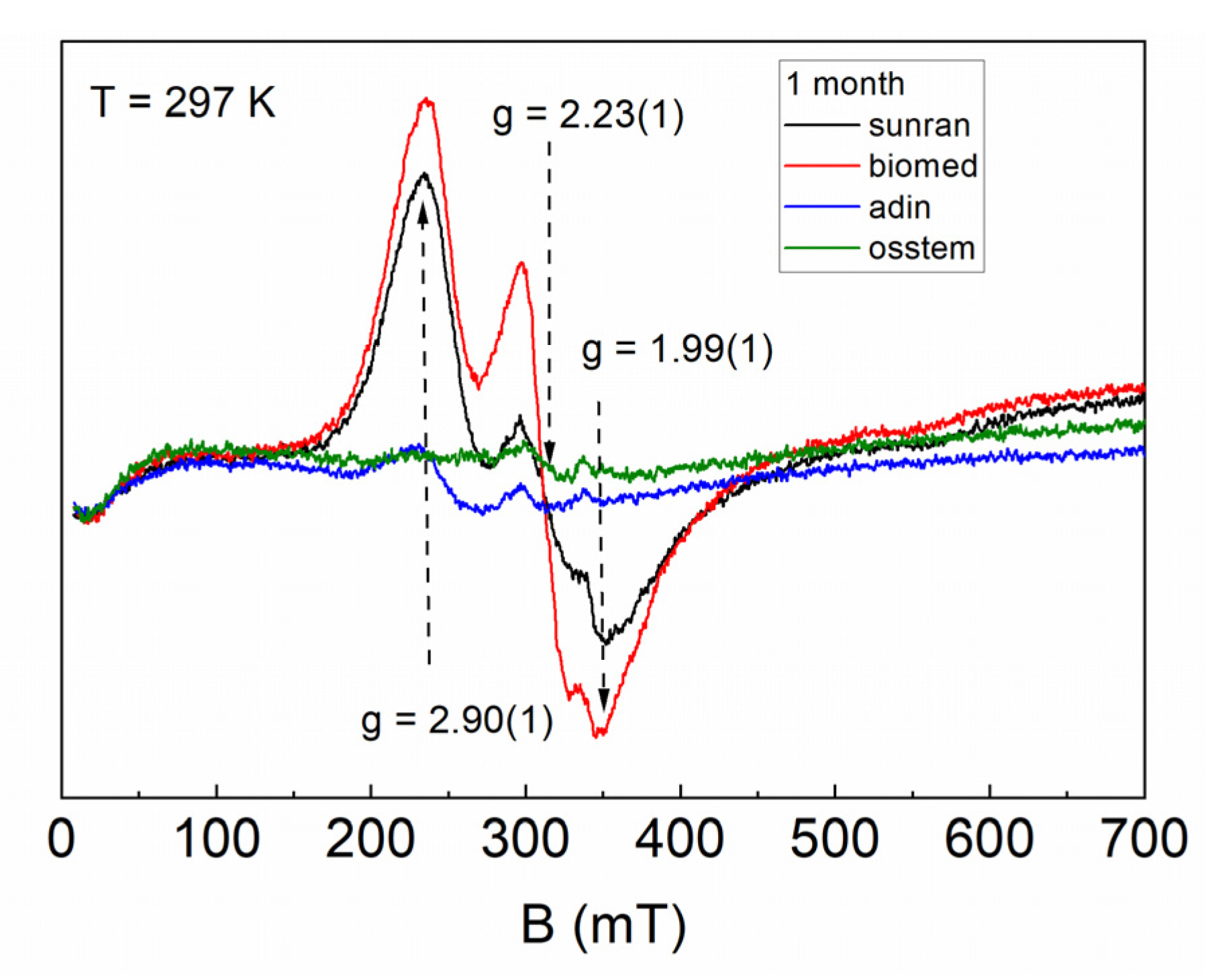
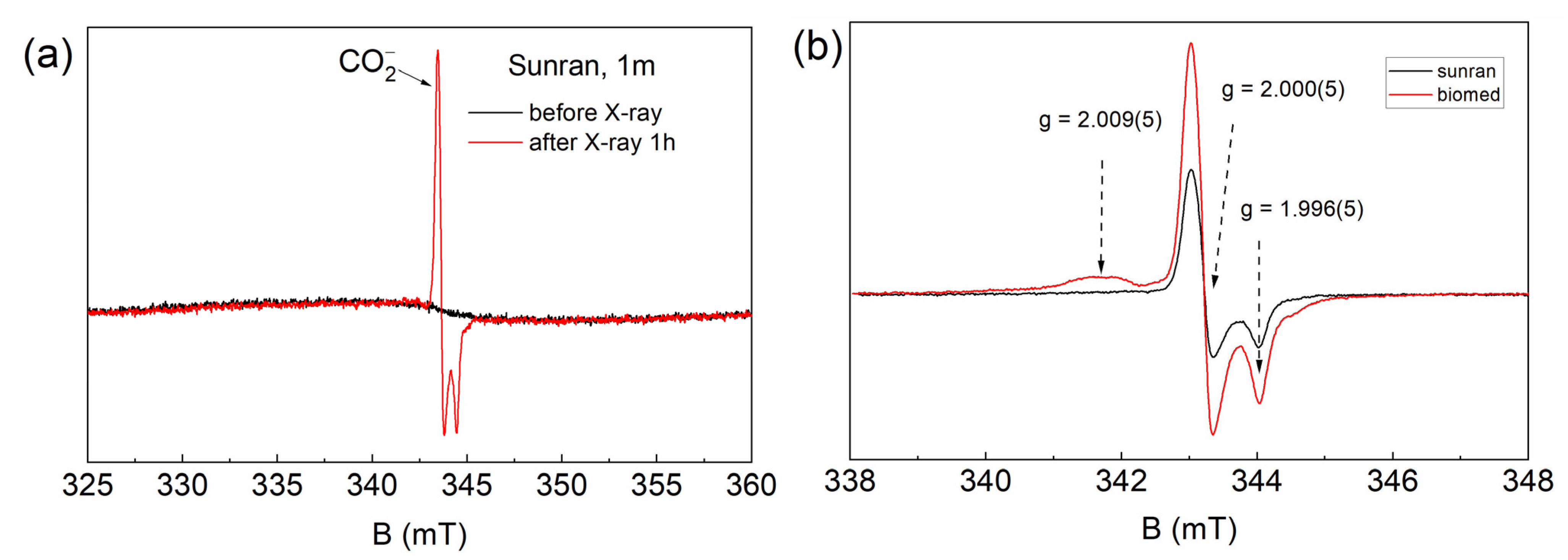
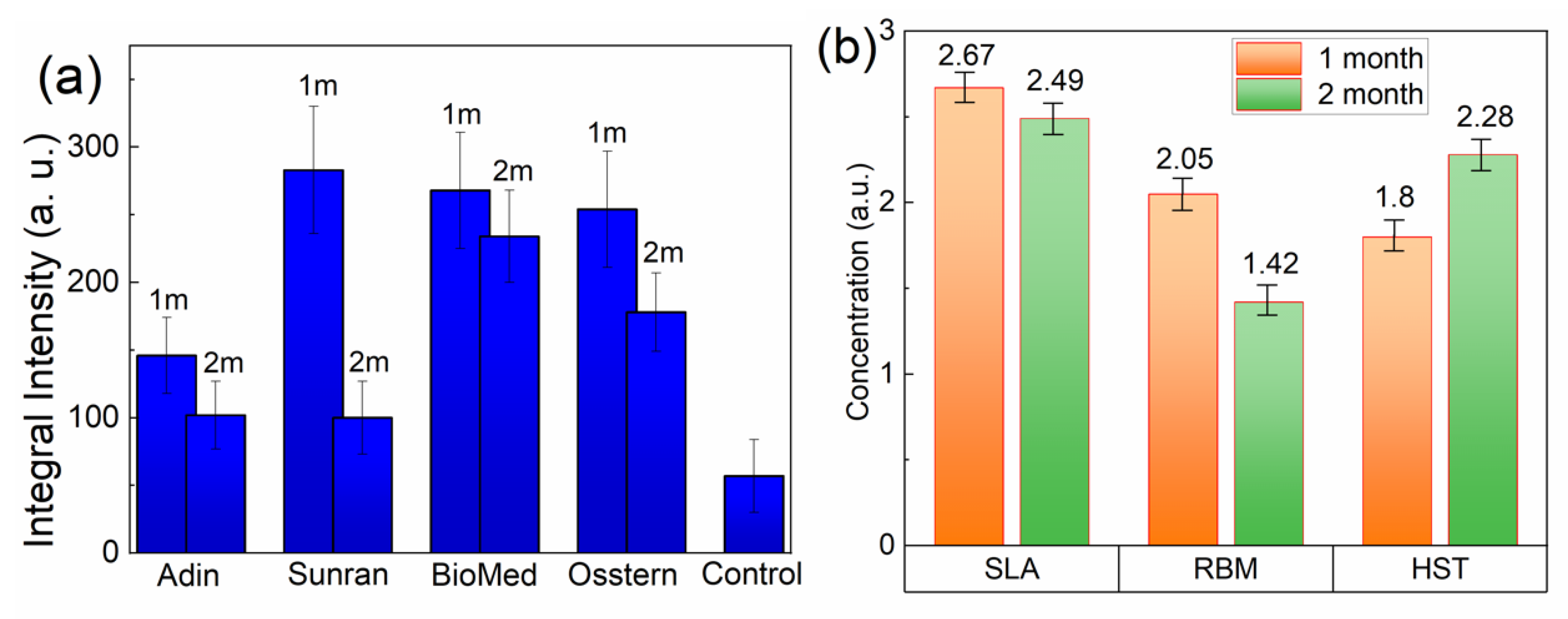
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2017, 3, 278–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef] [PubMed]
- Branemark, P.I.; Zarb, G.A.; Albrektsson, T. Tissue-Integrated Prostheses: Osseointegration in Clinical Dentistry; Quintessence Publishing Co.: Chicago, IL, USA, 1985; pp. 11–76. [Google Scholar]
- Brånemark, P.-I.; Breine, U.; Adell, R.; Hansson, B.O.; Lindström, J.; Ohlsson, Å. Intra-Osseous Anchorage of Dental Prostheses: I. Experimental Studies. Scand. J. Plast. Reconstr. Surg. 1969, 3, 81–100. [Google Scholar] [CrossRef]
- Del Fabbro, M.; Taschieri, S.; Canciani, E.; Addis, A.; Musto, F.; Weinstein, R.; Dellavia, C. Osseointegration of Titanium Implants With Different Rough Surfaces. Implant Dent. 2017, 26, 357–366. [Google Scholar] [CrossRef]
- Rittel, D.; Dorogoy, A.; Shemtov-Yona, K. Modeling the effect of osseointegration on dental implant pullout and torque removal tests. Clin. Implant Dent. Relat. Res. 2018, 20, 683–691. [Google Scholar] [CrossRef]
- Baqain, Z.H.; Moqbel, W.Y.; Sawair, F.A. Early dental implant failure: Risk factors. Br. J. Oral Maxillofac. Surg. 2012, 50, 239–243. [Google Scholar] [CrossRef]
- Jin, W.; Chu, P.K. Orthopedic Implants. Encycl. Biomed. Eng. 2019, 1, 3. [Google Scholar] [CrossRef]
- Filho, L.C.D.C.; Marcello-Machado, R.M.; De Castilhos, E.D.; Cury, A.A.D.B.; Faot, F. Can implant surfaces affect implant stability during osseointegration? A randomized clinical trial. Braz. Oral Res. 2018, 32, e110. [Google Scholar] [CrossRef]
- Elias, C.N.; Rocha, F.A.; Nascimento, A.L.; Coelho, P.G. Influence of implant shape, surface morphology, surgical technique and bone quality on the primary stability of dental implants. J. Mech. Behav. Biomed. Mater. 2012, 16, 169–180. [Google Scholar] [CrossRef]
- Herrero-Climent, M.; Ruizª, M.M.R.; Calvo, P.L.; Santos, J.V.R.; Perez, R.A.; Gil Mur, F.J. Effectiveness of a new dental implant bioactive surface: Histological and histomorphometric comparative study in minipigs. Clin. Oral Investig. 2017, 22, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.; Thomsen, P.; Palmquist, A. A Review of the Impact of Implant Biomaterials on Osteocytes. J. Dent. Res. 2018, 97, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Salerno, M.; Itri, A.; Frezzato, M.; Rebaudi, A. Surface Microstructure of Dental Implants before and After Insertion. Implant Dent. 2015, 24, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Coelho, P.G.; Takayama, T.; Yoo, D.; Jimbo, R.; Karunagaran, S.; Tovar, N.; Janal, M.N.; Yamano, S. Nanometer-scale features on micrometer-scale surface texturing: A bone histological, gene expression, and nanomechanical study. Bone 2014, 65, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, C.; Moon, B.-S.; Kim, H.-E.; Jang, T.-S. Enhancement of osseointegration by direct coating of rhBMP-2 on target-ion induced plasma sputtering treated SLA surface for dental application. J. Biomater. Appl. 2016, 31, 807–818. [Google Scholar] [CrossRef]
- Shiba, K.; Taneichi, H.; Namikawa, T.; Inami, S.; Takeuchi, D.; Nohara, Y. Osseointegration improves bone–implant interface of pedicle screws in the growing spine: A biomechanical and histological study using an in vivo immature porcine model. Eur. Spine J. 2017, 26, 2754–2762. [Google Scholar] [CrossRef]
- Gehrke, S.A.; Júnior, J.A.; Treichel, T.L.E.; Dedavid, B.A. Biomechanical and histological evaluation of four different implant macrogeometries in the early osseointegration process: An in vivo animal study. J. Mech. Behav. Biomed. Mater. 2021, 125, 104935. [Google Scholar] [CrossRef]
- Xu, D.; Crocombe, A.; Xu, W. Numerical evaluation of bone remodelling associated with trans-femoral osseointegration implant—A 68 month follow-up study. J. Biomech. 2015, 49, 488–492. [Google Scholar] [CrossRef] [Green Version]
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium phosphates in biomedical applications: Materials for the future? Mater. Today 2015, 19, 69–87. [Google Scholar] [CrossRef]
- Epple, M.; Ganesan, K.; Heumann, R.; Klesing, J.; Kovtun, A.; Neumann, S.; Sokolova, V. Application of calcium phosphatenanoparticles in biomedicine. J. Mater. Chem. 2009, 20, 18–23. [Google Scholar] [CrossRef]
- Gabbasov, B.; Gafurov, M.; Starshova, A.; Shurtakova, D.; Murzakhanov, F.; Mamin, G.; Orlinskii, S. Conventional, pulsed and high-field electron paramagnetic resonance for studying metal impurities in calcium phosphates of biogenic and synthetic origins. J. Magn. Magn. Mater. 2019, 470, 109–117. [Google Scholar] [CrossRef]
- Murzakhanov, F.; Gabbasov, B.; Iskhakova, K.; Voloshin, A.; Mamin, G.; Putlyaev, V.; Klimashina, E.; Fadeeva, I.; Fom-in, A.; Barinov, S.; et al. Conventional electron paramagnetic resonance for studying synthetic calcium phosphates with metal impurities (Mn2+, Cu2+, Fe3+). Magn. Reson. Solids 2017, 19, 17207–17210. [Google Scholar]
- Goldberg, M.; Gafurov, M.R.; Makshakova, O.N.; Smirnov, V.; Komlev, V.S.; Barinov, S.M.; Kudryavtsev, E.; Sergeeva, N.; Achmedova, S.; Mamin, G.V.; et al. Influence of Al on the Structure and in Vitro Behavior of Hydroxyapatite Nanopowders. J. Phys. Chem. B 2019, 123, 9143–9154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gafurov, M.; Biktagirov, T.; Mamin, G.; Orlinskii, S. A DFT, X- and W-band EPR and ENDOR Study of Nitrogen-Centered Species in (Nano)Hydroxyapatite. Appl. Magn. Reson. 2014, 45, 1189–1203. [Google Scholar] [CrossRef]
- Gafurov, M.; Biktagirov, T.; Yavkin, B.; Mamin, G.; Filippov, Y.; Klimashina, E.; Putlayev, V.; Orlinskii, S. Nitrogen-containing species in the structure of the synthesized nano-hydroxyapatite. J. Exp. Theor. Phys. Lett. 2014, 99, 196–203. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, M.A.; Akopyan, A.V.; Gafurov, M.R.; Makshakova, O.N.; Donskaya, N.O.; Fomin, A.S.; Polikarpova, P.P.; Anisimov, A.V.; Murzakhanov, F.F.; Leonov, A.V.; et al. Iron-Doped Mesoporous Powders of Hydroxyapatite as Molybdenum-Impregnated Catalysts for Deep Oxidative Desulfurization of Model Fuel: Synthesis and Experimental and Theoretical Studies. J. Phys. Chem. C 2021, 125, 11604–11619. [Google Scholar] [CrossRef]
- Goldberg, M.; Gafurov, M.; Murzakhanov, F.; Fomin, A.; Antonova, O.; Khairutdinova, D.; Pyataev, A.; Makshakova, O.; Konovalov, A.; Leonov, A.; et al. Mesoporous Iron(III)-Doped Hydroxyapatite Nanopowders Obtained via Iron Oxalate. Nanomaterials 2021, 11, 811. [Google Scholar] [CrossRef]
- Gustafsson, H.; Hallbeck, M.; Lindgren, M.; Kolbun, N.; Jonson, M.; Engström, M.; de Muinck, E.; Zachrisson, H. Visualization of oxidative stress in ex vivo biopsies using electron paramagnetic resonance imaging. Magn. Reson. Med. 2014, 73, 1682–1691. [Google Scholar] [CrossRef] [Green Version]
- Murzakhanov, F.F.; Grishin, P.O.; Goldberg, M.A.; Yavkin, B.V.; Mamin, G.V.; Orlinskii, S.B.; Fedotov, A.Y.; Petrakova, N.V.; Antuzevics, A.; Gafurov, M.R.; et al. Radiation-Induced Stable Radicals in Calcium Phosphates: Results of Multifrequency EPR, EDNMR, ESEEM, and ENDOR Studies. Appl. Sci. 2021, 11, 7727. [Google Scholar] [CrossRef]
- Murzakhanov, F.; Mamin, G.V.; Orlinskii, S.; Goldberg, M.; Petrakova, N.V.; Fedotov, A.Y.; Grishin, P.; Gafurov, M.R.; Komlev, V.S. Study of Electron–Nuclear Interactions in Doped Calcium Phosphates by Various Pulsed EPR Spectroscopy Techniques. ACS Omega 2021, 6, 25338–25349. [Google Scholar] [CrossRef]
- Brik, A.B.; Dubok, V.A.; Rosenfeld, L.G.; Klimenko, A.P.; Kalinichenko, A.M. The Use of EPR to Study the Processes of Assimilation of Implants by Living Bone Tissue. Actual Probl. Mod. Med. Bull. Ukr. Med. Dent. Acad. 2007, 7, 262–266, (In Russian. Poltava, Ukraine). [Google Scholar]
- Levêque, P.; Leprince, J.G.; Bebelman, S.; Devaux, J.; Leloup, G.; Gallez, B. Spectral spatial electron paramagnetic resonance imaging as a tool to study photoactive dimethacrylate-based dental resins. J. Magn. Reson. 2012, 220, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Esquivel-Chirino, C.; Gómez-Landeros, J.C.; Carabantes-Campos, E.P.; Carmona-Ruiz, D.; Valero-Princet, Y.; Márquez-Correa, C.; Morales-González, J.A. The Impact of Oxidative Stress on Dental Implants. Eur. J. Dent. Oral Health 2021, 2, 1–8. [Google Scholar] [CrossRef]
- Leprince, J.; Lamblin, G.; Truffier-Boutry, D.; Demoustier-Champagne, S.; Devaux, J.; Mestdagh, M.; Leloup, G. Kinetic study of free radicals trapped in dental resins stored in different environments. Acta Biomater. 2009, 5, 2518–2524. [Google Scholar] [CrossRef]
- Gilinskaya, L.G. Organic radicals in natural apatites according to EPR data: Potential genetic and paleoclimatic indicators. J. Struct. Chem. 2010, 51, 471–481. [Google Scholar] [CrossRef]
- Fisher, B.V.; Morgan, R.E.; Phillips, G.O.; Wardale, H.W. Radiation Damage in Calcium Phosphates and Collagen: An Interpretation of ESR Spectra. Radiat. Res. 1971, 46, 229. [Google Scholar] [CrossRef]
- Madi, M.; Zakaria, O.; Ichinose, S.; Kasugai, S. Effect of Induced Periimplantitis on Dental Implants with and Without Ultrathin Hydroxyapatite Coating. Implant Dent. 2016, 25, 39–46. [Google Scholar] [CrossRef]
- Fattibene, P.; Callens, F. EPR dosimetry with tooth enamel: A review. Appl. Radiat. Isot. 2010, 68, 2033–2116. [Google Scholar] [CrossRef] [Green Version]
- Wencka, M.; Hoffmann, S.; Hercman, H. EPR Dating of Hydroxyapatite from Fossil Bones. Transient Effects after γ and UV Irradiation. Acta Phys. Pol. A 2005, 108, 331–337. [Google Scholar] [CrossRef]
- Abdul’Yanov, V.A.; Galiullina, L.; Galyavich, A.; Izotov, V.G.; Mamin, G.; Orlinskii, S.; Rodionov, A.A.; Salakhov, M.K.; Silkin, N.I.; Sitdikova, L.M.; et al. Stationary and high-frequency pulsed electron paramagnetic resonance of a calcified atherosclerotic plaque. J. Exp. Theor. Phys. Lett. 2008, 88, 69–73. [Google Scholar] [CrossRef]
- Erceg, I.; Maltar-Strmečki, N.; Jurašin, D.; Strasser, V.; Ćurlin, M.; Lyons, D.; Radatović, B.; Mlinarić, N.; Kralj, D.; Sikirić, M. Comparison of the Effect of the Amino Acids on Spontaneous Formation and Transformation of Calcium Phosphates. Crystals 2021, 11, 792. [Google Scholar] [CrossRef]
- Grishin, P.; Kalinnikova, E. Clinical studies of the stability and process of osteointegration of dental implants during immediate and delayed implantation. Actual Probl. Dent. 2021, 16, 97–103. [Google Scholar] [CrossRef]
- Taniguchi, Y.; Aoki, A.; Mizutani, K.; Takeuchi, Y.; Ichinose, S.; Takasaki, A.A.; Schwarz, F.; Izumi, Y. Optimal Er:YAG laser irradiation parameters for debridement of microstructured fixture surfaces of titanium dental implants. Lasers Med. Sci. 2012, 28, 1057–1068. [Google Scholar] [CrossRef]
- Strutynska, N.; Livitska, O.; Prylutska, S.; Yumyna, Y.; Zelena, P.; Skivka, L.; Malyshenko, A.; Vovchenko, L.; Strelchuk, V.; Prylutskyy, Y.; et al. New nanostructured apatite-type (Na+,Zn2+,CO32−)-doped calcium phosphates: Preparation, mechanical properties and antibacterial activity. J. Mol. Struct. 2020, 1222, 128932. [Google Scholar] [CrossRef]
- Schramm, D.U.; Rossi, A.M. EPR and ENDOR studies on CO2− radicals in γ-irradiated B-type carbonated apatites. Phys. Chem. Chem. Phys. 2000, 2, 1339–1343. [Google Scholar] [CrossRef]
- Murzakhanov, F.F.; Mamin, G.V.; Goldberg, M.A.; Knotko, A.V.; Gafurov, M.R.; Orlinskii, S.B. EPR of Radiation-Induced Nitrogen Centers in Hydroxyapatite: New Approaches to the Study of Electron-Nuclear Interactions. Russ. J. Co-Ord. Chem. 2020, 46, 729–737. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalinnikova, E.; Sadovnikova, M.; Rodionov, A.; Murzakhanov, F.; Grishin, P. Analysis of the Osseointegration Process of Dental Implants by Electron Paramagnetic Resonance: An In Vivo Study. Dent. J. 2022, 10, 28. https://doi.org/10.3390/dj10020028
Kalinnikova E, Sadovnikova M, Rodionov A, Murzakhanov F, Grishin P. Analysis of the Osseointegration Process of Dental Implants by Electron Paramagnetic Resonance: An In Vivo Study. Dentistry Journal. 2022; 10(2):28. https://doi.org/10.3390/dj10020028
Chicago/Turabian StyleKalinnikova, Elena, Margarita Sadovnikova, Alexander Rodionov, Fadis Murzakhanov, and Peter Grishin. 2022. "Analysis of the Osseointegration Process of Dental Implants by Electron Paramagnetic Resonance: An In Vivo Study" Dentistry Journal 10, no. 2: 28. https://doi.org/10.3390/dj10020028
APA StyleKalinnikova, E., Sadovnikova, M., Rodionov, A., Murzakhanov, F., & Grishin, P. (2022). Analysis of the Osseointegration Process of Dental Implants by Electron Paramagnetic Resonance: An In Vivo Study. Dentistry Journal, 10(2), 28. https://doi.org/10.3390/dj10020028





