Clear Cell Odontogenic Carcinoma: A Series of Three Cases
Abstract
:1. Introduction
2. Materials and Methods
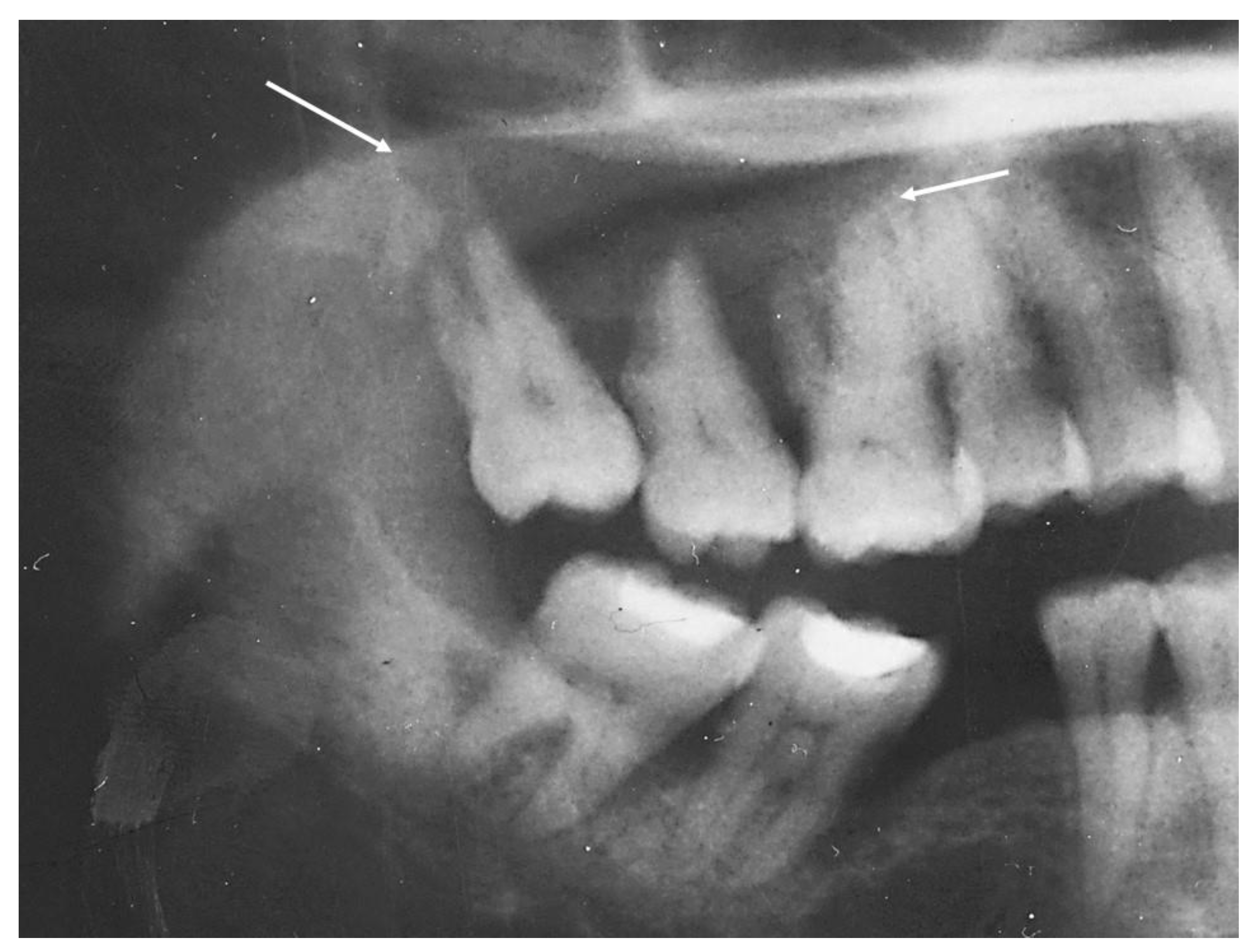
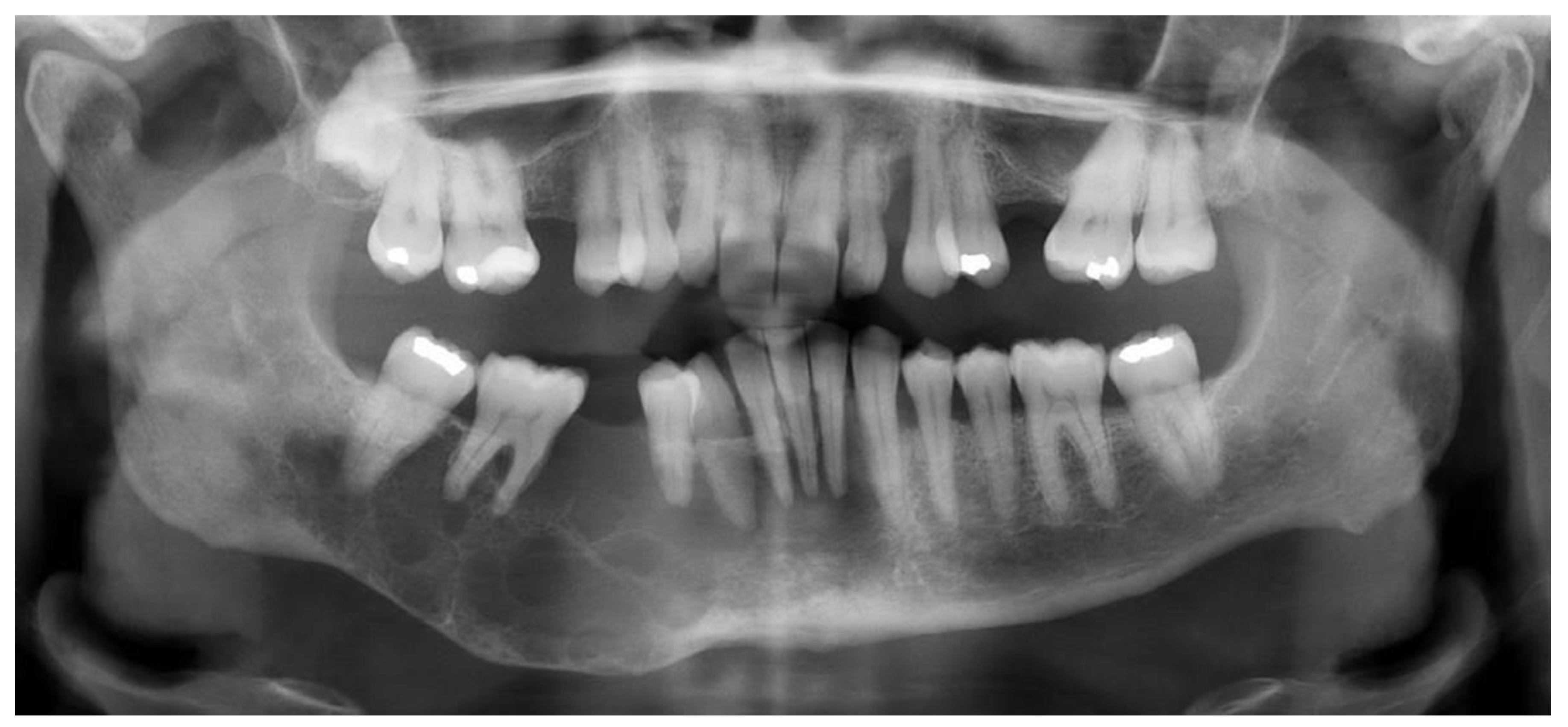
2.1. Case 1
2.2. Case 2
2.3. Case 3
3. Summary of Cases
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guastaldi, F.; Faquin, W.; Gootkind, F.; Hashemi, S.; August, M.; Iafrate, A.; Rivera, M.; Kaban, L.; Jaquinet, A.; Troulis, M. Clear cell odontogenic carcinoma: A rare jaw tumor. A summary of 107 reported cases. Int. J. Oral Maxillofac. Surg. 2019, 48, 1405–1410. [Google Scholar] [CrossRef] [PubMed]
- Datar, U.V.; Kamat, M.S.; Kanitkar, S.S.; Byakodi, S.S. Clear cell odontogenic carcinoma: A rare case report with emphasis on differential diagnosis. J. Can. Res. Ther. 2017, 13, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-C.; Kim, S.-W.; Baek, Y.-J.; Lee, H.-G.; Ryu, M.-H.; Hwang, D.-S.; Kim, U.-K. Misdiagnosis of ameloblastoma in a patient with clear cell odontogenic carcinoma: A case report. J. Korean Assoc. Oral Maxillofac. Surg. 2019, 45, 116–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, S.; Faverani, L.P.; Dos Santos, G.M.; Martins, E.P.; Garcia, I.R. Clear cell odontogenic carcinoma of the mandible: A treatment strategy. J. Appl. Oral 2018, 26, e20160645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhariwal, R.; Ray, J.G.; Swain, N. Clear cell odontogenic carcinoma of maxilla: A case report and mini review. J. Oral Maxillofac. Pathol. 2013, 17, 89–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loyola, A.M.; Cardoso, S.V.; de Faria, P.R.; Servato, J.P.S.; de Paulo, L.F.B.; Eisenberg, A.L.A.; Dias, F.L.; Gomes, C.; Gomez, R.S. Clear cell odontogenic carcinoma: Report of 7 new cases and systematic review of the current knowledge. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015, 120, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Kalsi, A.S.; Williams, S.P.; Shah, K.A.; Fasanmade, A. Clear Cell Odontogenic Carcinoma: A Rare Neoplasm of the Maxillary Bone. J. Oral Maxillofac. Surg. 2014, 72, 935–938. [Google Scholar] [CrossRef] [PubMed]
- Eversole, L.R. Malignant epithelial odontogenic tumors. Semin. Diagn. Pathol. 1999, 16, 317–324. [Google Scholar] [PubMed]
- Neville, B.W.; Damm, D.D.; Allen, C.P.; Chi, A.C. Oral and Maxillofacial Pathology, 4th ed.; Elsevier: Saint Louis, MO, USA, 2016. [Google Scholar]
- Mosqueda-Taylor, A.; Neville, B.W.; Tatemoto, Y.; Ogawa, I.; Takata, T. Odontogenic Carcinoma with Dentinoid: A New Odontogenic Carcinoma. Head Neck Pathol. 2014, 8, 421–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariano, F.V.; Gondak, R.O.; Scarini, J.F.; Da Silva, E.C.A.; Caravina, G.; Scapulatempo-Neto, C.; Almeida, O.P.; Altemani, A.; Taylor, A.M. Odontogenic Carcinoma with Dentinoid in Long-Term Follow-up with 2 Recurrences. Int. J. Surg. Pathol. 2019, 28, 181–187. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, A.K.; Chan, J.K.C.; Grandis, J.R.; Takata, T.; Slootweg, P.J. Odontogenic and maxillofacial bone tumours. In WHO Classification of Head and Neck Tumours; Feger, J., Ed.; IARC: Lyon, France, 2017. [Google Scholar]
- Liu, L.; Zhang, J.W.; Zhu, N.S.; Zhu, Y.; Guo, B.; Yang, X.H. Clear Cell Odontogenic Carcinoma: A Clinicopathological and Immunocytochemical Analysis. Pathol. Oncol. Res. 2020, 26, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
- Bilodeau, E.A.; Weinreb, I.; Antonescu, C.R.; Zhang, L.; Dacic, S.; Muller, S.; Barker, B.; Seethala, R.R. Clear Cell Odontogenic Carcinomas Show EWSR1 Rearrangements: A Novel Finding and a Biological Link to Salivary Clear Cell Carcinomas. Am. J. Surg. Pathol. 2013, 37, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Gondak, R.O.; Mariano, F.V.; de Sousa, S.F.; de Siqueira, E.C.; Díaz, K.P.; Martins, L.A.L.; Altemani, A.; Mosqueda-Taylor, A.; Gomez, R.S.; Gomes, C.C. CTNNB1 and APC mutations in odontogenic carcinoma with dentinoid. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 129, e249–e256. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.; Bhavthankar, J.D.; Mandale, M.S.; Barewad, B. Clear cell odontogenic carcinoma: Case report of a deceptive pathology. J. Oral Maxillofac. Pathol. 2019, 23, 140–143. [Google Scholar] [PubMed]
- Bilodeau, E.A.; Hoschar, A.P.; Barnes, E.L.; Hunt, J.L.; Seethala, R.R. Clear Cell Carcinoma and Clear Cell Odontogenic Carcinoma: A Comparative Clinicopathologic and Immunohistochemical Study. Head Neck Pathol. 2011, 5, 101–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Age/Sex | 54-year-old female | 49-year-old female | 40-year-old male |
| Presentation | Painful mass | Painful mass | Mass recurrence |
| Location | Right maxilla | Right mandible | Right maxilla |
| Staining/IHC | PAS (+) D-labile Mucicarmine (−), p63 (+) CK5/6 (+), CK19 (+) | PAS (+) D-labile Mucicarmine (−) CK5/6 (+) | H&E only |
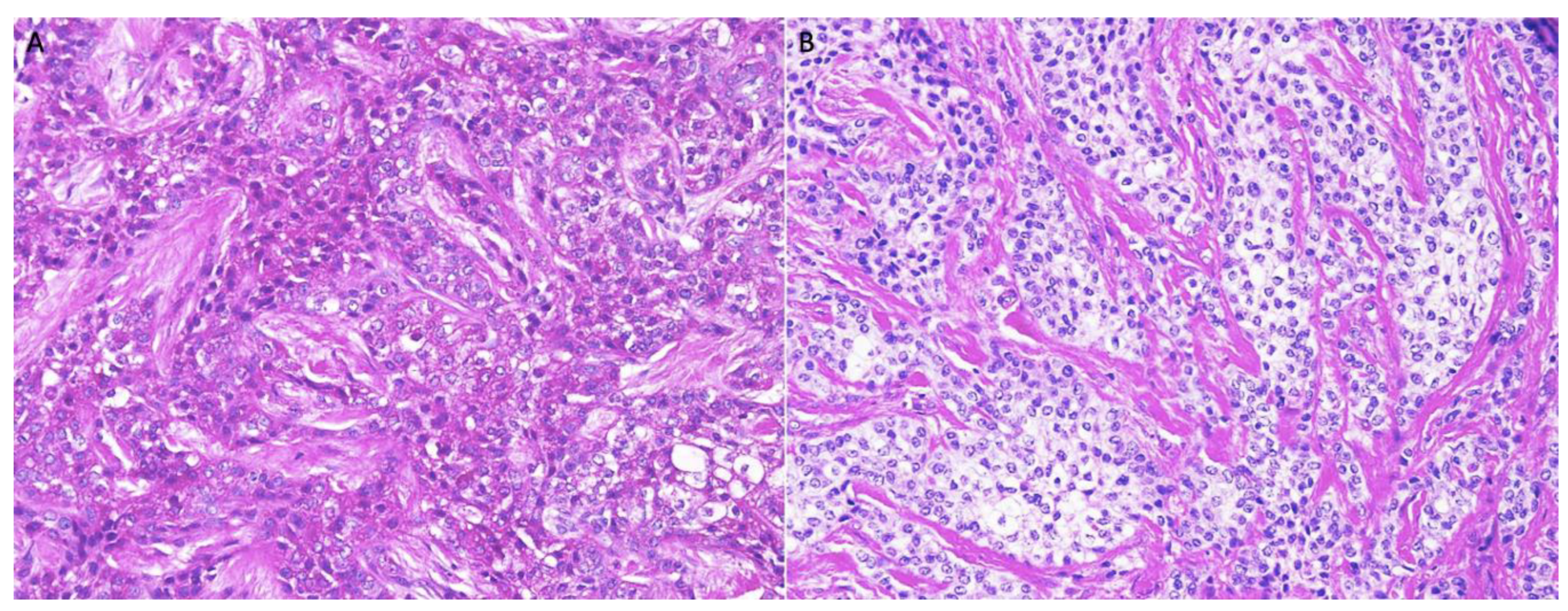
| Morphology | Infiltrative, non-encapsulated malignant neoplastic odontogenic epithelial proliferation with clear cell component in a dense fibrous connective tissue stroma. Neoplastic cells arranged in anastomosing trabeculae. Nuclear hyperchromasia and pleomorphism surrounded by clear, vacuolated cytoplasm. Stroma was hyalinized, densely collagenized, hypocellular, and hypovascular. | Neoplastic odontogenic epithelial proliferation diffusely infiltrated the connective tissue stroma. Neoplastic cells arranged in islands of variable size, trabeculae, and nests. Nuclei were hyperchromatic, central, and pleomorphic surrounded by clear cytoplasm. | Infiltrative odontogenic epithelial neoplasm intermixed with eosinophilic dentinoid matrix. Neoplastic cells arranged in infiltrative, non-encapsulated sheets, cords, and nests of polygonal cells with central hyperchromatic and slightly pleomorphic nuclei surrounded by clear cytoplasm and occasional pale eosinophilic cytoplasm. Occasional mitotic figures were noted. Neoplastic epithelial sheets and cords blended with eosinophilic cellular matrix without cellular rimming consistent with dentinoid deposits. |
| Procedure | Right maxillectomy with negative margins | Left mandibulectomy Right temporomandibular joint arthroplasty Left fibula free flap | Right maxillectomy without orbital exenteration Right selective neck dissection Forearm free flap Four months adjuvant radiation |
| Differential Diagnosis | Distinguishing Features |
|---|---|
| Odontogenic origin | |
| Clear cell ameloblastoma | Palisading clear cells and columnar cells with ameloblast-like differentiation on histology Immunopositive for calretinin, CK8, CK13, CK19 |
| Clear cell calcifying epithelial odontogenic tumor | Liesgang’s calcifications and amyloid deposits on histology with apple-green birefringence on Congo red stain |
| Salivary origin | |
| Mucoepidermoid carcinoma | Pale basophilic foamy cytoplasm on histology Positive alcian blue stain, mucicarmine, and mucin Immunopositive for CK19 |
| Myoepithelial carcinoma | Immunopositive for S100, alpha SMA, calponin, vimentin |
| Acinic cell carcinoma | Interspersed basophilic granules on histology Positive for PTAH stain Immunopositive for S100, pancytokeratin, vimentin, CEA, GFAP |
| Hyalinizing clear cell carcinoma | Peripheral palisading and hyalinization of stroma on histology Immunopositive for pancytokeratin and EMA |
| Metastatic origin | |
| Renal cell carcinoma | Immunopositive for CD10 and PAX8 |
| Thyroid carcinoma | Immunopositive for TTF-1, Thyroglobulin |
| Prostatic carcinoma | Increased serum PSA |
| Melanotic origin | |
| Amelanotic melanoma | Immunopositive for Masson-Fontana stain, melan A, and HMB-45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, A.; Cullen, C.; Mattox, S.N.; Kozman, D.; Patel, N.; Sharma, S.; Abdelsayed, R. Clear Cell Odontogenic Carcinoma: A Series of Three Cases. Dent. J. 2022, 10, 34. https://doi.org/10.3390/dj10030034
Ullah A, Cullen C, Mattox SN, Kozman D, Patel N, Sharma S, Abdelsayed R. Clear Cell Odontogenic Carcinoma: A Series of Three Cases. Dentistry Journal. 2022; 10(3):34. https://doi.org/10.3390/dj10030034
Chicago/Turabian StyleUllah, Asad, Christian Cullen, Samantha N. Mattox, Diana Kozman, Nikhil Patel, Suash Sharma, and Rafik Abdelsayed. 2022. "Clear Cell Odontogenic Carcinoma: A Series of Three Cases" Dentistry Journal 10, no. 3: 34. https://doi.org/10.3390/dj10030034
APA StyleUllah, A., Cullen, C., Mattox, S. N., Kozman, D., Patel, N., Sharma, S., & Abdelsayed, R. (2022). Clear Cell Odontogenic Carcinoma: A Series of Three Cases. Dentistry Journal, 10(3), 34. https://doi.org/10.3390/dj10030034








