A Case of Necrotizing Periodontitis in a Care-Requiring Elderly Person Treated and Managed by Interprofessional Collaboration
Abstract
:1. Introduction
2. Case Presentation
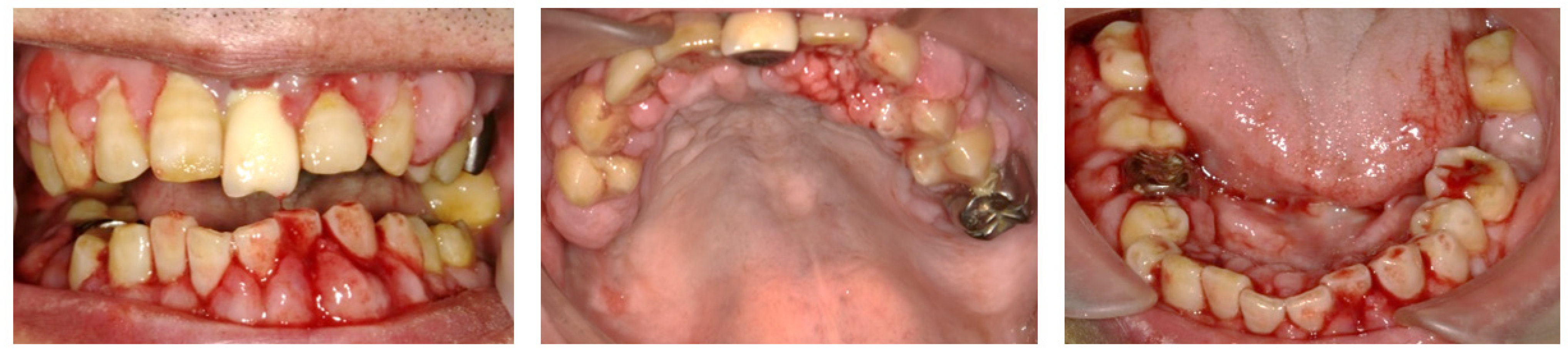
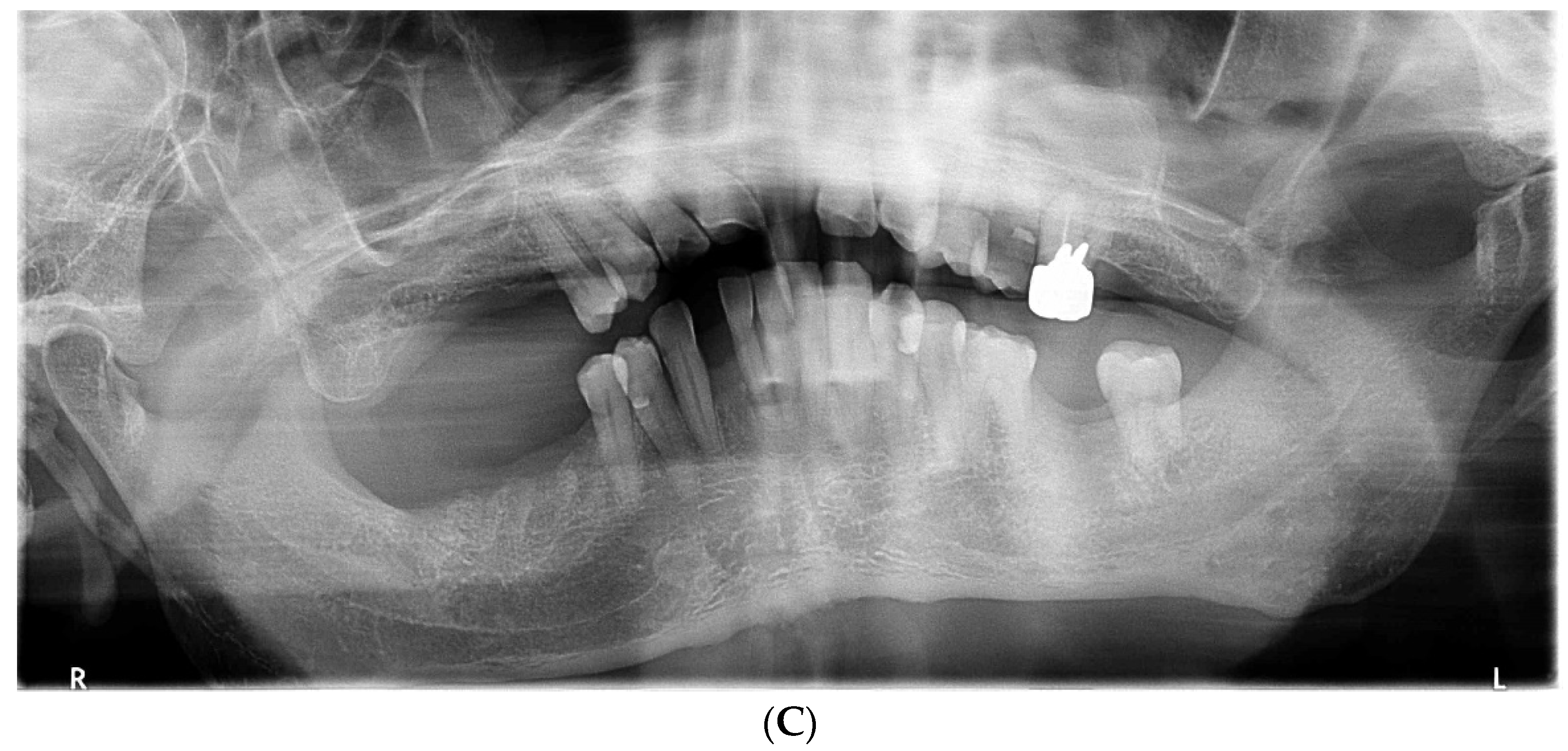
2.1. Day 1: First Examination
2.2. Day 7
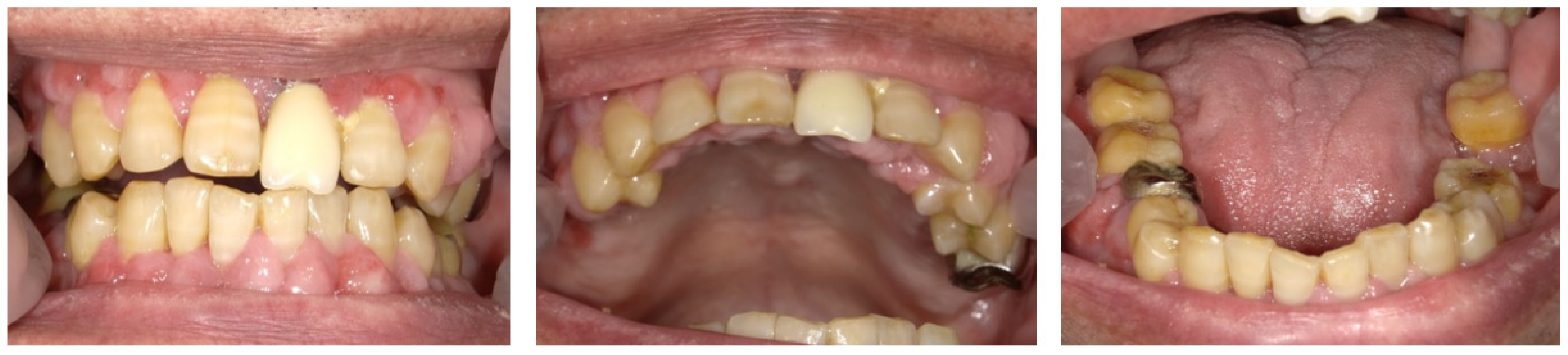
2.3. Day 14 and Later
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Herrera, D.; Retamal-Valdes, B.; Alonso, B.; Feres, M. Acute periodontal lesions (periodontal abscesses and necrotizing periodontal diseases) and endo-periodontal lesions. J. Clin. Periodontol. 2018, 45 (Suppl. 20), S78–S94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, N.; Soskolne, W.; Greenstein, G.; Cochran, D.; Corbet, E.; Meng, H.X.; Newman, M.; Novak, M.J.; Tenenbaum, H. Consensus report: Necrotizing periodontal diseases. Ann. Periodontol. 1999, 4, 78. [Google Scholar] [CrossRef]
- Malek, R.; Gharibi, A.; Khlil, N.; Kissa, J. Necrotizing ulcerative gingivitis. Contemp. Clin. Dent. 2017, 8, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Novak, M.J. Necrotizing ulcerative periodontitis. Ann. Periodontol. 1999, 4, 74–78. [Google Scholar] [CrossRef]
- Rowland, R.W. Necrotizing ulcerative gingivitis. Ann. Periodontol. 1999, 4, 65–73; discussion 78. [Google Scholar] [CrossRef]
- Jepsen, S.; Caton, J.G.; Albandar, J.M.; Bissada, N.F.; Bouchard, P.; Cortellini, P.; Demirel, K.; de Sanctis, M.; Ercoli, C.; Fan, J.; et al. Periodontal manifestations of systemic diseases and developmental and acquired conditions: Consensus report of workgroup 3 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. 1), S237–S248. [Google Scholar] [CrossRef]
- Gowdey, G.; Alijanian, A. Necrotizing ulcerative periodontitis in an HIV patient. J. Calif. Dent. Assoc. 1995, 23, 57–59. [Google Scholar]
- Phiri, R.; Feller, L.; Blignaut, E. The severity, extent and recurrence of necrotizing periodontal disease in relation to HIV status and CD4+ T cell count. J. Int. Acad. Periodontol. 2010, 12, 98–103. [Google Scholar]
- Bermejo-Fenoll, A.; Sanchez-Perez, A. Necrotising periodontal diseases. Med. Oral. Patol. Oral. Cir. Bucal. 2004, 9, 108–119. [Google Scholar]
- Horning, G.M.; Cohen, M.E. Necrotizing ulcerative gingivitis, periodontitis, and stomatitis: Clinical staging and predisposing factors. J. Periodontol. 1995, 66, 990–998. [Google Scholar] [CrossRef]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. 1), S173–S182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevens, A.W., Jr.; Cogen, R.B.; Cohen-Cole, S.; Freeman, A. Demographic and clinical data associated with acute necrotizing ulcerative gingivitis in a dental school population (ANUG-demographic and clinical data). J. Clin. Periodontol. 1984, 11, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Falkler, W.A., Jr.; Martin, S.A.; Vincent, J.W.; Tall, B.D.; Nauman, R.K.; Suzuki, J.B. A clinical, demographic and microbiologic study of ANUG patients in an urban dental school. J. Clin. Periodontol. 1987, 14, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Barnes, G.P.; Bowles, W.F., 3rd; Carter, H.G. Acute necrotizing ulcerative gingivitis: A survey of 218 cases. J. Periodontol. 1973, 44, 35–42. [Google Scholar] [CrossRef]
- Cobb, C.M.; Ferguson, B.L.; Keselyak, N.T.; Holt, L.A.; MacNeill, S.R.; Rapley, J.W. A TEM/SEM study of the microbial plaque overlying the necrotic gingival papillae of HIV-seropositive, necrotizing ulcerative periodontitis. J. Periodontal Res. 2003, 38, 147–155. [Google Scholar] [CrossRef]
- Barr, C.E.; Robbins, M.R. Clinical and radiographic presentations of HIV-1 necrotizing ulcerative periodontitis. Spec. Care Dent. 1996, 16, 237–241. [Google Scholar] [CrossRef]
- Maupin, C.C.; Bell, W.B. The relationship of 17-hydroxycorticosteroid to acute necrotizing ulcerative gingivitis. J. Periodontol. 1975, 46, 721–722. [Google Scholar] [CrossRef]
- Loesche, W.J.; Syed, S.A.; Laughon, B.E.; Stoll, J. The bacteriology of acute necrotizing ulcerative gingivitis. J. Periodontol. 1982, 53, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Johnson, B.D.; Engel, D. Acute necrotizing ulcerative gingivitis. A review of diagnosis, etiology and treatment. J. Periodontol. 1986, 57, 141–150. [Google Scholar] [CrossRef]
- Taiwo, J.O. Oral hygiene status and necrotizing ulcerative gingivitis in Nigerian children. J. Periodontol. 1993, 64, 1071–1074. [Google Scholar] [CrossRef]
- Herrera, D.; Alonso, B.; de Arriba, L.; Santa Cruz, I.; Serrano, C.; Sanz, M. Acute periodontal lesions. Periodontology 2000 2014, 65, 149–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Todescan, S.; Nizar, R. Managing patients with necrotizing ulcerative periodontitis. J. Can. Dent. Assoc. 2013, 79, d44. [Google Scholar] [PubMed]
- Blair, F.M.; Chapple, I.L. Prescribing for periodontal disease. Prim. Dent. J. 2014, 3, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, H.; Yoshida, M.; Yasaka, M.; Yoshida, H.; Murasato, Y.; Fukunaga, D.; Shintani, A.; Okada, Y. Safety of tooth extraction in patients receiving direct oral anticoagulant treatment versus warfarin: A prospective observation study. Int. J. Oral Maxillofac. Surg. 2019, 48, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- DeWald, T.A.; Becker, R.C. The pharmacology of novel oral anticoagulants. J. Thromb. Thrombolysis 2014, 37, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Mauprivez, C.; Khonsari, R.H.; Razouk, O.; Goudot, P.; Lesclous, P.; Descroix, V. Management of dental extraction in patients undergoing anticoagulant oral direct treatment: A pilot study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 122, e146–e155. [Google Scholar] [CrossRef]
- Adcock, D.M.; Gosselin, R. Direct Oral Anticoagulants (DOACs) in the laboratory: 2015 review. Thromb. Res. 2015, 136, 7–12. [Google Scholar] [CrossRef] [Green Version]
- Patel, M.R.; Mahaffey, K.W.; Garg, J.; Pan, G.; Singer, D.E.; Hacke, W.; Breithardt, G.; Halperin, J.L.; Hankey, G.J.; Piccini, J.P.; et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N. Engl. J. Med. 2011, 365, 883–891. [Google Scholar] [CrossRef] [Green Version]
- Zeevi, I.; Allon, D.M.; Rosenfeld, E.; Avishai, G.; Gilman, L.; Nissan, J.; Chaushu, G. Four-year cross-sectional study of bleeding risk in dental patients on direct oral anticoagulants. Quintessence Int. 2017, 48, 503–509. [Google Scholar] [CrossRef]
- Hanken, H.; Grobe, A.; Heiland, M.; Smeets, R.; Kluwe, L.; Wikner, J.; Koehnke, R.; Al-Dam, A.; Eichhorn, W. Postoperative bleeding risk for oral surgery under continued rivaroxaban anticoagulant therapy. Clin. Oral Investig. 2016, 20, 1279–1282. [Google Scholar] [CrossRef]
- Miclotte, I.; Vanhaverbeke, M.; Agbaje, J.O.; Legrand, P.; Vanassche, T.; Verhamme, P.; Politis, C. Pragmatic approach to manage new oral anticoagulants in patients undergoing dental extractions: A prospective case-control study. Clin. Oral Investig. 2017, 21, 2183–2188. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.; Niwa, H.; Minematsu, K. Risk factors affecting postoperative hemorrhage after tooth extraction in patients receiving oral antithrombotic therapy. J. Oral Maxillofac. Surg. 2011, 69, 1550–1556. [Google Scholar] [CrossRef] [PubMed]
- Febbo, A.; Cheng, A.; Stein, B.; Goss, A.; Sambrook, P. Postoperative bleeding following dental extractions in patients anticoagulated with warfarin. J. Oral Maxillofac. Surg. 2016, 74, 1518–1523. [Google Scholar] [CrossRef] [PubMed]
- Wahl, M.J.; Pinto, A.; Kilham, J.; Lalla, R.V. Dental surgery in anticoagulated patients–stop the interruption. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015, 119, 136–157. [Google Scholar] [CrossRef]
- Komatsu, Y.; Kawai, T.; Yamada, H.; Chiba, T. Antithrombotic therapy in dental practice. Dent. J. Iwate Med. Univ. 2021, 45, 105–119. (In Japanese) [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okubo, M.; Kuraji, R.; Kamimura, H.; Numabe, Y.; Ito, K.; Sato, T.; Kokabu, S. A Case of Necrotizing Periodontitis in a Care-Requiring Elderly Person Treated and Managed by Interprofessional Collaboration. Dent. J. 2022, 10, 79. https://doi.org/10.3390/dj10050079
Okubo M, Kuraji R, Kamimura H, Numabe Y, Ito K, Sato T, Kokabu S. A Case of Necrotizing Periodontitis in a Care-Requiring Elderly Person Treated and Managed by Interprofessional Collaboration. Dentistry Journal. 2022; 10(5):79. https://doi.org/10.3390/dj10050079
Chicago/Turabian StyleOkubo, Masahiko, Ryutaro Kuraji, Hideyuki Kamimura, Yukihiro Numabe, Ko Ito, Tsuyoshi Sato, and Shoichiro Kokabu. 2022. "A Case of Necrotizing Periodontitis in a Care-Requiring Elderly Person Treated and Managed by Interprofessional Collaboration" Dentistry Journal 10, no. 5: 79. https://doi.org/10.3390/dj10050079





