Mechanistic Effects of E-Liquids on Biofilm Formation and Growth of Oral Commensal Streptococcal Communities: Effect of Flavoring Agents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Supplies
2.2. Bacterial Strains
2.3. Stock E-Liquid
2.4. Saliva Preparation
2.5. Biofilm Assays and Confocal Analysis
2.6. Colony Forming Unit Assay
2.7. Death Curves
2.8. Statistical Analysis
3. Results
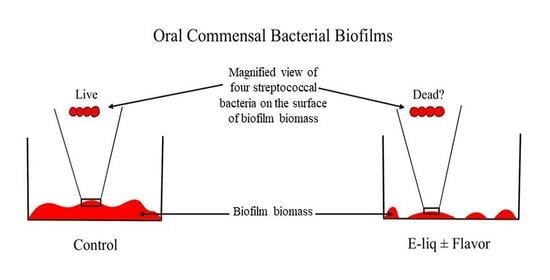
3.1. Biofilm Assays
3.2. Colony Forming Unit Assay
3.3. Death Curves
3.4. Bacterial Mass Pre- and Post-Treatments
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palazzolo, D.L. Electronic Cigarettes and Vaping: A New Challenge in Clinical Medicine and Public Health. A Literature Review. Front. Public Health 2013, 1, 56. [Google Scholar] [CrossRef] [PubMed]
- CDC Tobacco Free Youth and Tobacco Use. Available online: https://www.cdc.gov/tobacco/data_statistics/fact_sheets/youth_data/tobacco_use/index.htm (accessed on 9 November 2021).
- E-Cigarettes: Facts, Stats and Regulations. Available online: https://truthinitiative.org/research-resources/emerging-tobacco-products/e-cigarettes-facts-stats-and-regulations (accessed on 15 February 2022).
- Get the Facts on E-Cigarettes|Know the Risks: E-Cigarettes & Young People|U.S. Surgeon General’s Report. Available online: https://e-cigarettes.surgeongeneral.gov/getthefacts.html (accessed on 15 February 2022).
- St. Helen, G.; Liakoni, E.; Nardone, N.; Addo, N.; Jacob, P.; Benowitz, N.L. Comparison of Systemic Exposure to Toxic and/or Carcinogenic Volatile Organic Compounds (VOC) during Vaping, Smoking, and Abstention. Cancer Prev. Res. 2020, 13, 153–162. [Google Scholar] [CrossRef] [PubMed]
- McRobbie, H.; Bullen, C.; Hartmann-Boyce, J.; Hajek, P. Electronic Cigarettes for Smoking Cessation and Reduction. In Cochrane Database of Systematic Reviews; The Cochrane Collaboration, Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2014; p. CD010216.pub2. [Google Scholar]
- Nicotine without Smoke: Tobacco Harm Reduction. Available online: https://www.rcplondon.ac.uk/projects/outputs/nicotine-without-smoke-tobacco-harm-reduction (accessed on 2 January 2022).
- Polosa, R.; Caponnetto, P.; Morjaria, J.B.; Papale, G.; Campagna, D.; Russo, C. Effect of an Electronic Nicotine Delivery Device (e-Cigarette) on Smoking Reduction and Cessation: A Prospective 6-Month Pilot Study. BMC Public Health 2011, 11, 786. [Google Scholar] [CrossRef] [PubMed]
- Stratton, K.; Kwan, L.; Eaton, D. Public Health Consequences of E-Cigarettes; The National Academies Press: Washington, DC, USA, 2018; ISBN 978-0-309-46834-3. [Google Scholar]
- Cox, S.; Leigh, N.J.; Vanderbush, T.S.; Choo, E.; Goniewicz, M.L.; Dawkins, L. An Exploration into “Do-It-Yourself” (DIY) e-Liquid Mixing: Users’ Motivations, Practices and Product Laboratory Analysis. Addict. Behav. Rep. 2019, 9, 100151. [Google Scholar] [CrossRef] [PubMed]
- Youth Vaping, Smoking & Nicotine Use. Available online: https://truthinitiative.org/our-top-issues/vaping-issue (accessed on 6 December 2021).
- Chun, L.F.; Moazed, F.; Calfee, C.S.; Matthay, M.A.; Gotts, J.E. Pulmonary Toxicity of E-Cigarettes. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2017, 313, L193–L206. [Google Scholar] [CrossRef]
- Traboulsi, H.; Cherian, M.; Abou Rjeili, M.; Preteroti, M.; Bourbeau, J.; Smith, B.M.; Eidelman, D.H.; Baglole, C.J. Inhalation Toxicology of Vaping Products and Implications for Pulmonary Health. Int. J. Mol. Sci. 2020, 21, 3495. [Google Scholar] [CrossRef]
- Leigh, N.J.; Lawton, R.I.; Hershberger, P.A.; Goniewicz, M.L. Flavourings Significantly Affect Inhalation Toxicity of Aerosol Generated from Electronic Nicotine Delivery Systems (ENDS). Tob. Control 2016, 25, ii81–ii87. [Google Scholar] [CrossRef]
- Leigh, N.J.; Tran, P.L.; O’Connor, R.J.; Goniewicz, M.L. Cytotoxic Effects of Heated Tobacco Products (HTP) on Human Bronchial Epithelial Cells. Tob. Control 2018, 27, s26–s29. [Google Scholar] [CrossRef]
- Aug, A.; Altraja, S.; Kilk, K.; Porosk, R.; Soomets, U.; Altraja, A. E-Cigarette Affects the Metabolome of Primary Normal Human Bronchial Epithelial Cells. PLoS ONE 2015, 10, e0142053. [Google Scholar] [CrossRef]
- Lee, H.-W.; Park, S.-H.; Weng, M.; Wang, H.-T.; Huang, W.C.; Lepor, H.; Wu, X.-R.; Chen, L.-C.; Tang, M. E-Cigarette Smoke Damages DNA and Reduces Repair Activity in Mouse Lung, Heart, and Bladder as Well as in Human Lung and Bladder Cells. Proc. Natl. Acad. Sci. USA 2018, 115, E1560–E1569. [Google Scholar] [CrossRef]
- Schweitzer, K.S.; Chen, S.X.; Law, S.; Van Demark, M.; Poirier, C.; Justice, M.J.; Hubbard, W.C.; Kim, E.S.; Lai, X.; Wang, M.; et al. Endothelial Disruptive Proinflammatory Effects of Nicotine and E-Cigarette Vapor Exposures. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2015, 309, L175–L187. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Jiang, D.; Minor, M.; Chu, H.W. Electronic Cigarette Liquid Increases Inflammation and Virus Infection in Primary Human Airway Epithelial Cells. PLoS ONE 2014, 9, e108342. [Google Scholar] [CrossRef] [PubMed]
- Kalininskiy, A.; Bach, C.T.; Nacca, N.E.; Ginsberg, G.; Marraffa, J.; Navarette, K.A.; McGraw, M.D.; Croft, D.P. E-Cigarette, or Vaping, Product Use Associated Lung Injury (EVALI): Case Series and Diagnostic Approach. Lancet Respir. Med. 2019, 7, 1017–1026. [Google Scholar] [CrossRef]
- Wolf, M.; Rock, L.K. EVALI: New Information on Vaping-Induced Lung Injury. Available online: https://www.health.harvard.edu/blog/evali-new-information-on-vaping-induced-lung-injury-2020040319359 (accessed on 31 December 2021).
- E-Cigarette, or Vaping Product, Use Associated Lung Injury (EVALI) > Fact Sheets > Yale Medicine. Available online: https://www.yalemedicine.org/conditions/evali (accessed on 9 November 2021).
- Socransky, S.S.; Haffajee, A.D. Periodontal Microbial Ecology. Periodontol 2000 2005, 38, 135–187. [Google Scholar] [CrossRef]
- Burne, R.A. Oral Streptococci… Products of Their Environment. J. Dent. Res. 1998, 77, 445–452. [Google Scholar] [CrossRef]
- Huang, R.; Li, M.; Gregory, R.L. Bacterial Interactions in Dental Biofilm. Virulence 2011, 2, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Berger, D.; Rakhamimova, A.; Pollack, A.; Loewy, Z. Oral Biofilms: Development, Control, and Analysis. High Throughput 2018, 7, 24. [Google Scholar] [CrossRef]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The Oral Microbiota: Dynamic Communities and Host Interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef]
- Gross, E.L.; Beall, C.J.; Kutsch, S.R.; Firestone, N.D.; Leys, E.J.; Griffen, A.L. Beyond Streptococcus Mutans: Dental Caries Onset Linked to Multiple Species by 16S RRNA Community Analysis. PLoS ONE 2012, 7, e47722. [Google Scholar] [CrossRef]
- Herrero, E.R.; Slomka, V.; Bernaerts, K.; Boon, N.; Hernandez-Sanabria, E.; Passoni, B.B.; Quirynen, M.; Teughels, W. Antimicrobial Effects of Commensal Oral Species Are Regulated by Environmental Factors. J. Dent. 2016, 47, 23–33. [Google Scholar] [CrossRef]
- Huang, X.; Browngardt, C.M.; Jiang, M.; Ahn, S.-J.; Burne, R.A.; Nascimento, M.M. Diversity in Antagonistic Interactions between Commensal Oral Streptococci and Streptococcus Mutans. Caries Res. 2018, 52, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Hanel, A.N.; Herzog, H.M.; James, M.G.; Cuadra, G.A. Effects of Oral Commensal Streptococci on Porphyromonas Gingivalis Invasion into Oral Epithelial Cells. Dent. J. 2020, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Joshi, V.; Matthews, C.; Aspiras, M.; de Jager, M.; Ward, M.; Kumar, P. Smoking Decreases Structural and Functional Resilience in the Subgingival Ecosystem. J. Clin. Periodontol. 2014, 41, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- Mason, M.R.; Preshaw, P.M.; Nagaraja, H.N.; Dabdoub, S.M.; Rahman, A.; Kumar, P.S. The Subgingival Microbiome of Clinically Healthy Current and Never Smokers. ISME J. 2015, 9, 268–272. [Google Scholar] [CrossRef]
- Cuadra, G.A.; Smith, M.T.; Nelson, J.M.; Loh, E.K.; Palazzolo, D.L. A Comparison of Flavorless Electronic Cigarette-Generated Aerosol and Conventional Cigarette Smoke on the Survival and Growth of Common Oral Commensal Streptococci. Int. J. Environ. Res. Public Health 2019, 16, 1669. [Google Scholar] [CrossRef]
- Nelson, J.M.; Cuadra, G.A.; Palazzolo, D.L. A Comparison of Flavorless Electronic Cigarette-Generated Aerosol and Conventional Cigarette Smoke on the Planktonic Growth of Common Oral Commensal Streptococci. Int. J. Environ. Res. Public Health 2019, 16, 5004. [Google Scholar] [CrossRef]
- Fischman, J.S.; Sista, S.; Lee, D.; Cuadra, G.A.; Palazzolo, D.L. Flavorless vs. Flavored Electronic Cigarette-Generated Aerosol and E-Liquid on the Growth of Common Oral Commensal Streptococci. Front. Physiol. 2020, 11, 585416. [Google Scholar] [CrossRef]
- Cichońska, D.; Kusiak, A.; Kochańska, B.; Ochocińska, J.; Świetlik, D. Influence of Electronic Cigarettes on Selected Antibacterial Properties of Saliva. Int. J. Environ. Res. Public Health 2019, 16, 4433. [Google Scholar] [CrossRef]
- Chopyk, J.; Bojanowski, C.M.; Shin, J.; Moshensky, A.; Fuentes, A.L.; Bonde, S.S.; Chuki, D.; Pride, D.T.; Crotty Alexander, L.E. Compositional Differences in the Oral Microbiome of E-Cigarette Users. Front. Microbiol. 2021, 12, 1250. [Google Scholar] [CrossRef]
- Pushalkar, S.; Paul, B.; Li, Q.; Yang, J.; Vasconcelos, R.; Makwana, S.; González, J.M.; Shah, S.; Xie, C.; Janal, M.N.; et al. Electronic Cigarette Aerosol Modulates the Oral Microbiome and Increases Risk of Infection. iScience 2020, 23, 100884. [Google Scholar] [CrossRef]
- He, J.; Li, Y.; Cao, Y.; Xue, J.; Zhou, X. The Oral Microbiome Diversity and Its Relation to Human Diseases. Folia Microbiol. 2015, 60, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Palazzolo, D.; Nelson, J.M.; Hudson, Z. The Use of HPLC-PDA in Determining Nicotine and Nicotine-Related Alkaloids from E-Liquids: A Comparison of Five E-Liquid Brands Purchased Locally. Int. J. Environ. Res. Public Health 2019, 16, 3015. [Google Scholar] [CrossRef] [PubMed]
- Cuadra-Saenz, G.; Rao, D.L.; Underwood, A.J.; Belapure, S.A.; Campagna, S.R.; Sun, Z.; Tammariello, S.; Rickard, A.H. Autoinducer-2 Influences Interactions amongst Pioneer Colonizing Streptococci in Oral Biofilms. Microbiology 2012, 158, 1783–1795. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.S.; Kolenbrander, P.E. Development of a Multispecies Oral Bacterial Community in a Saliva-Conditioned Flow Cell. Appl. Environ. Microbiol. 2004, 70, 4340–4348. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, G.A. Microtiter Dish Biofilm Formation Assay. JoVE 2011, 2437. [Google Scholar] [CrossRef]
- Kolenbrander, P.E.; Palmer, R.J.; Rickard, A.H.; Jakubovics, N.S.; Chalmers, N.I.; Diaz, P.I. Bacterial Interactions and Successions during Plaque Development. Periodontol. 2000 2006, 42, 47–79. [Google Scholar] [CrossRef]
- Yoshida, Y.; Palmer, R.J.; Yang, J.; Kolenbrander, P.E.; Cisar, J.O. Streptococcal Receptor Polysaccharides: Recognition Molecules for Oral Biofilm Formation. BMC Oral Health 2006, 6 (Suppl. 1), S12. [Google Scholar] [CrossRef]
- Willcox, M.D.; Drucker, D.B. Surface Structures, Co-Aggregation and Adherence Phenomena of Streptococcus Oralis and Related Species. Microbios 1989, 59, 19–29. [Google Scholar]
- Yang, J.; Yoshida, Y.; Cisar, J.O. Genetic Basis of Coaggregation Receptor Polysaccharide Biosynthesis in Streptococcus Sanguinis and Related Species. Mol. Oral Microbiol. 2014, 29, 24–31. [Google Scholar] [CrossRef]
- Thomas, S.C.; Xu, F.; Pushalkar, S.; Lin, Z.; Thakor, N.; Vardhan, M.; Flaminio, Z.; Khodadadi-Jamayran, A.; Vasconcelos, R.; Akapo, A.; et al. Electronic Cigarette Use Promotes a Unique Periodontal Microbiome. mBio 2022, 13, e00075-22. [Google Scholar] [CrossRef]
- Ebersole, J.; Samburova, V.; Son, Y.; Cappelli, D.; Demopoulos, C.; Capurro, A.; Pinto, A.; Chrzan, B.; Kingsley, K.; Howard, K.; et al. Harmful Chemicals Emitted from Electronic Cigarettes Andpotential Deleterious Effects in the Oral Cavity. Tob. Induc. Dis. 2020, 18, 41. [Google Scholar] [CrossRef] [PubMed]
- Isik Andrikopoulos, G.; Farsalinos, K.; Poulas, K. Electronic Nicotine Delivery Systems (ENDS) and Their Relevance in Oral Health. Toxics 2019, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.S.; Clark, P.; Brinkman, M.C.; Saxena, D. Novel Nicotine Delivery Systems. Adv. Dent. Res. 2019, 30, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.G.; Flanigan, S.S.; LeBlanc, M.; Vallarino, J.; MacNaughton, P.; Stewart, J.H.; Christiani, D.C. Flavoring Chemicals in E-Cigarettes: Diacetyl, 2,3-Pentanedione, and Acetoin in a Sample of 51 Products, Including Fruit-, Candy-, and Cocktail-Flavored E-Cigarettes. Environ. Health Perspect. 2016, 124, 733–739. [Google Scholar] [CrossRef]
- Vape Flavors and Vape Juice: What You Need to Know. Available online: https://www.hopkinsmedicine.org/health/wellness-and-prevention/vape-flavors-and-vape-juice-what-you-need-to-know (accessed on 9 December 2021).
- Material Safety Data Sheet: Trans-Cinnamaldehyde. Available online: https://fscimage.fishersci.com/msds/96752.htm (accessed on 5 March 2022).
- Material Safety Data Sheet: Menthol. Available online: https://fscimage.fishersci.com/msds/23818.htm (accessed on 5 March 2022).
- Shen, S.; Zhang, T.; Yuan, Y.; Lin, S.; Xu, J.; Ye, H. Effects of Cinnamaldehyde on Escherichia Coli and Staphylococcus Aureus Membrane. Food Control 2015, 47, 196–202. [Google Scholar] [CrossRef]
- Silva, W.M.F.; Bona, N.P.; Pedra, N.S.; Cunha, K.F.D.; Fiorentini, A.M.; Stefanello, F.M.; Zavareze, E.R.; Dias, A.R.G. Risk Assessment of In Vitro Cytotoxicity, Antioxidant and Antimicrobial Activities of Mentha Piperita L. Essential Oil. J. Toxicol. Environ. Health A 2022, 85, 230–242. [Google Scholar] [CrossRef]
- An, S.-J.; Namkung, J.-U.; Ha, K.-W.; Jun, H.-K.; Kim, H.Y.; Choi, B.-K. Inhibitory Effect of D-Arabinose on Oral Bacteria Biofilm Formation on Titanium Discs. Anaerobe, 2022; in press. [Google Scholar] [CrossRef]
- Balasubramanian, A.R.; Vasudevan, S.; Shanmugam, K.; Lévesque, C.M.; Solomon, A.P.; Neelakantan, P. Combinatorial Effects of Trans-Cinnamaldehyde with Fluoride and Chlorhexidine on Streptococcus Mutans. J. Appl. Microbiol. 2021, 130, 382–393. [Google Scholar] [CrossRef]
- Kreth, J.; Merritt, J.; Qi, F. Bacterial and Host Interactions of Oral Streptococci. DNA Cell Biol. 2009, 28, 397–403. [Google Scholar] [CrossRef]
- Scannapieco, F.A. Saliva-Bacterium Interactions in Oral Microbial Ecology. Crit. Rev. Oral Biol. Med. 1994, 5, 203–248. [Google Scholar] [CrossRef]
- Rosan, B.; Lamont, R.J. Dental Plaque Formation. Microbes Infect. 2000, 2, 1599–1607. [Google Scholar] [CrossRef]
- Diaz, P.I.; Chalmers, N.I.; Rickard, A.H.; Kong, C.; Milburn, C.L.; Palmer, R.J.; Kolenbrander, P.E. Molecular Characterization of Subject-Specific Oral Microflora during Initial Colonization of Enamel. Appl. Environ. Microbiol. 2006, 72, 2837–2848. [Google Scholar] [CrossRef] [PubMed]
- Jakubovics, N.S.; Kolenbrander, P.E. The Road to Ruin: The Formation of Disease-Associated Oral Biofilms. Oral Dis. 2010, 16, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.P.; Luo, W.; Pankow, J.F.; Strongin, R.M.; Peyton, D.H. Hidden Formaldehyde in E-Cigarette Aerosols. N. Engl. J. Med. 2015, 372, 392–394. [Google Scholar] [CrossRef]
- Kaufman, J.W.; Farahmand, K. In Vivo Measurements of Human Oral Cavity Heat and Water Vapor Transport. Respir. Physiol. Neurobiol. 2006, 150, 261–277. [Google Scholar] [CrossRef] [PubMed]
- Sosnowski, T.R.; Odziomek, M. Particle Size Dynamics: Toward a Better Understanding of Electronic Cigarette Aerosol Interactions with the Respiratory System. Front. Physiol. 2018, 9, 853. [Google Scholar] [CrossRef]
- Olmedo, P.; Goessler, W.; Tanda, S.; Grau-Perez, M.; Jarmul, S.; Aherrera, A.; Chen, R.; Hilpert, M.; Cohen, J.E.; Navas-Acien, A.; et al. Metal Concentrations in E-Cigarette Liquid and Aerosol Samples: The Contribution of Metallic Coils. Environ. Health Perspect. 2018, 126, 027010. [Google Scholar] [CrossRef]
- Palazzolo, D.L.; Crow, A.P.; Nelson, J.M.; Johnson, R.A. Trace Metals Derived from Electronic Cigarette (ECIG) Generated Aerosol: Potential Problem of ECIG Devices That Contain Nickel. Front. Physiol. 2016, 7, 663. [Google Scholar] [CrossRef]
- Marsh, P.D.; Head, D.A.; Devine, D.A. Dental Plaque as a Biofilm and a Microbial Community—Implications for Treatment. J. Oral Biosci. 2015, 57, 185–191. [Google Scholar] [CrossRef]
- Cichońska, D.; Kusiak, A.; Piechowicz, L.; Świetlik, D. A Pilot Investigation into the Influence of Electronic Cigarettes on Oral Bacteria. Pdia 2021, 38, 1092–1098. [Google Scholar] [CrossRef]






| E-Liquid Constituents | Percent Stock E-Liquid added to BHI * | ||||||
|---|---|---|---|---|---|---|---|
| Stock E-Liquid | Propylene Glycol | Vegetable Glycerine | Stock Flavor | Nicotine (mg/mL) | 5% | 3% | 1% |
| Flavorless | 50% | 50% | 0% | 20 | 0% | 0% | 0% |
| Flavored | 37.5% | 37.5% | 25% | 20 | 1.25% | 0.75% | 0.25% |
| S. gordonii | Flavorless | Tobacco | Menthol | Cinnamon | Strawberry | Blueberry |
|---|---|---|---|---|---|---|
| 1% E-liquid | 0.77 ± 0.07 * | 0.76 ± 0.07 | 0.84 ± 0.07 | 0.65 ± 0.03 | 0.81 ± 0.07 | 0.81 ± 0.07 |
| 3% E-liquid | 0.55 ± 0.03 | 0.57 ± 0.05 | 0.40 ± 0.05 | 0.25 ± 0.05 p < 0.001 | 0.50 ± 0.05 | 0.60 ± 0.05 |
| 5% E-liquid | 0.30 ± 0.03 | 0.33 ± 0.04 | 0.12 ± 0.02 p < 0.01 | 0.04 ± 0.03 p < 0.001 | 0.29 ± 0.03 | 0.31 ± 0.03 |
| S. intermedius | Flavorless | Tobacco | Menthol | Cinnamon | Strawberry | Blueberry |
| 1% E-liquid | 1.16 ± 0.04 | 1.23 ± 0.03 | 1.15 ± 0.04 | 0.38 ± 0.08 p < 0.001 | 1.16 ± 0.04 | 1.21 ± 0.03 |
| 3% E-liquid | 1.04 ± 0.04 | 1.00 ± 0.04 | 0.89 ± 0.06 | 0.07 ± 0.03 p < 0.001 | 0.96 ± 0.06 | 1.05 ± 0.03 |
| 5% E-liquid | 0.70 ± 0.04 | 0.63 ± 0.07 | 0.46 ± 0.04 | 0.07 ± 0.04 p < 0.001 | 0.55 ± 0.10 | 0.69 ± 0.05 |
| S. mitis | Flavorless | Tobacco | Menthol | Cinnamon | Strawberry | Blueberry |
| 1% E-liquid | 0.48 ± 0.03 | 0.54 ± 0.05 | 0.49 ± 0.03 | 0.37 ± 0.06 | 0.54 ± 0.05 | 0.52 ± 0.05 |
| 3% E-liquid | 0.43 ± 0.03 | 0.39 ± 0.01 | 0..33 ± 0.02 p < 0.05 | 0.14 ± 0.03 p < 0.001 | 0.39 ± 0.02 | 0.40 ± 0.02 |
| 5% E-liquid | 0.28 ± 0.03 | 0.25 ± 0.03 | 0.09 ± 0.02 p < 0.001 | 0.05 ± 0.03 p < 0.001 | 0.26 ± 0.03 | 0.27 ± 0.02 |
| S. oralis | Flavorless | Tobacco | Menthol | Cinnamon | Strawberry | Blueberry |
| 1% E-liquid | 0.68 ± 0.02 | 0.72 ± 0.02 | 0.58 ± 0.02 | 0.35 ± 0.03 p < 0.001 | 0.74 ± 0.03 | 0.65 ± 0.05 |
| 3% E-liquid | 0.44 ± 0.02 | 0.47± 0.02 | 0.33 ± 0.02 p < 0.01 | 0.15 ± 0.02 p < 0.001 | 0.52 ± 0.03 | 0.49 ± 0.02 |
| 5% E-liquid | 0.32 ± 0.03 | 0.30 ± 0.03 | 0.11 ± 0.03 p < 0.001 | 0.04 ± 0.03 p < 0.001 | 0.20 ± 0.01 | 0.33 ± 0.04 |
| Mixed Species # | Flavorless | Tobacco | Menthol | Cinnamon | Strawberry | Blueberry |
| 1% E-liquid | 0.67 ± 0.03 | 0.71 ± 0.04 | 0.61 ± 0.04 | 0.45 ± 0.04 p < 0.001 | 0.70 ± 0.03 | 0.75 ± 0.02 |
| 3% E-liquid | 0.50 ± 0.03 | 0.43 ± 0.02 | 0.27 ± 0.03 p < 0.001 | 0.15 ± 0.03 p < 0.001 | 0.44 ± 0.03 | 0.51 ± 0.03 |
| 5% E-liquid | 0.36 ± 0.04 | 0.25 ± 0.03 | 0.31 ± 0.02 p < 0.001 | −0.14 ± 0.01 p < 0.001 | 0.21 ± 0.03 p < 0.01 | 0.30 ± 0.02 |
| S. gordonii | S. intermedius | S. mitis | S. oralis | |
|---|---|---|---|---|
| PBS | +++ | +++ | +++ | +++ |
| Flavorless E-liquid | ++ | +++ | +++ | ++ |
| Tobacco | + | + | ++ | ++ |
| Menthol | − | − | − | − |
| Cinnamon | − | − | − | − |
| Strawberry | − | − | − | − |
| Blueberry | − | − | − | − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, C.P.; Palazzolo, D.L.; Cuadra, G.A. Mechanistic Effects of E-Liquids on Biofilm Formation and Growth of Oral Commensal Streptococcal Communities: Effect of Flavoring Agents. Dent. J. 2022, 10, 85. https://doi.org/10.3390/dj10050085
Xu CP, Palazzolo DL, Cuadra GA. Mechanistic Effects of E-Liquids on Biofilm Formation and Growth of Oral Commensal Streptococcal Communities: Effect of Flavoring Agents. Dentistry Journal. 2022; 10(5):85. https://doi.org/10.3390/dj10050085
Chicago/Turabian StyleXu, Christina P., Dominic L. Palazzolo, and Giancarlo A. Cuadra. 2022. "Mechanistic Effects of E-Liquids on Biofilm Formation and Growth of Oral Commensal Streptococcal Communities: Effect of Flavoring Agents" Dentistry Journal 10, no. 5: 85. https://doi.org/10.3390/dj10050085
APA StyleXu, C. P., Palazzolo, D. L., & Cuadra, G. A. (2022). Mechanistic Effects of E-Liquids on Biofilm Formation and Growth of Oral Commensal Streptococcal Communities: Effect of Flavoring Agents. Dentistry Journal, 10(5), 85. https://doi.org/10.3390/dj10050085