Effect of Extending Corticotomy Depth to Trabecular Bone on Accelerating Orthodontic Tooth Movement in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample
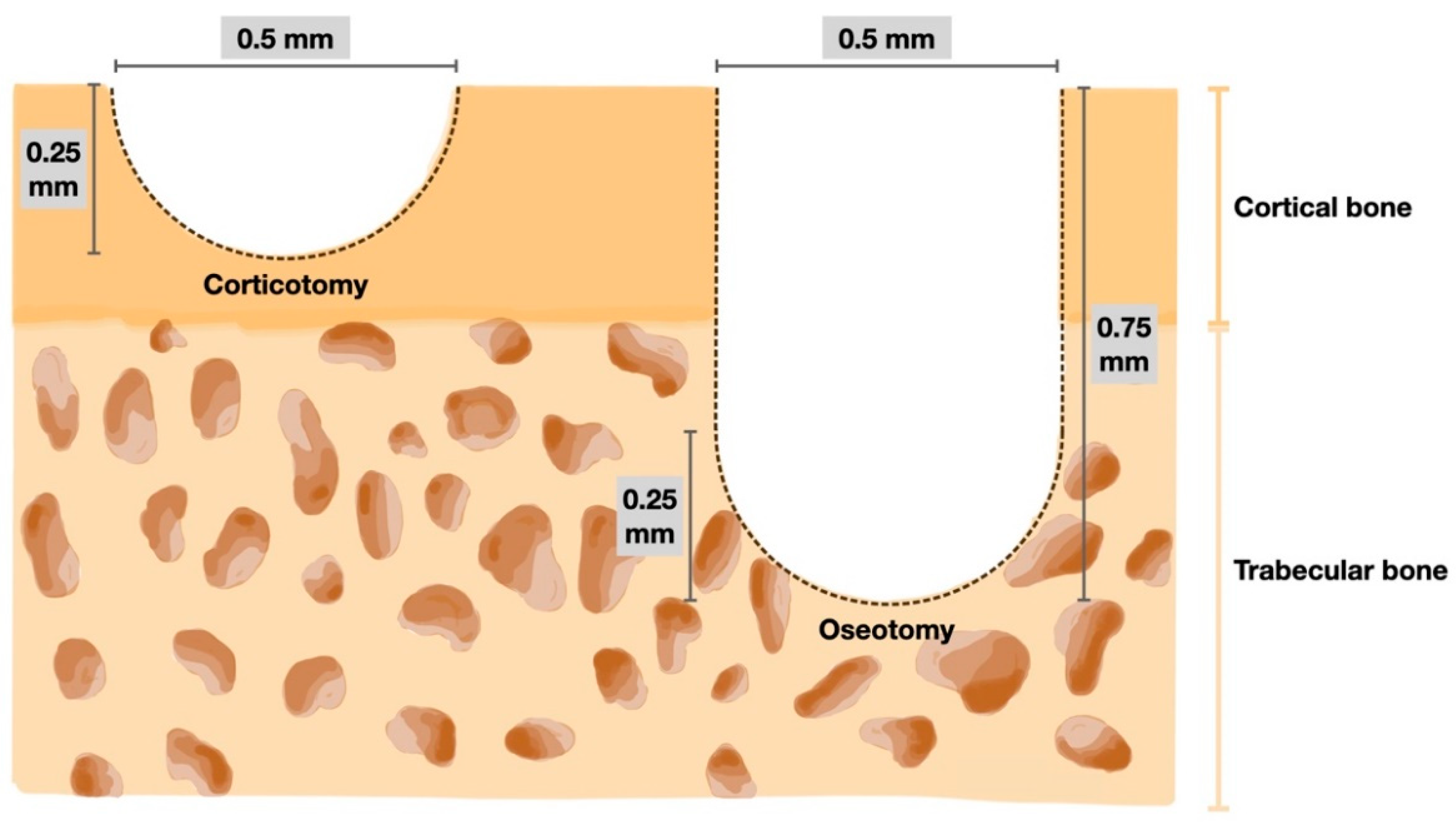
2.2. Surgical Procedure
= (π (0.25)2 (0.5) + 2/3 π (0.25)3) × 4
= 0.5238 mm3
= (2/3 π (0.25)3) × 16
= 0.5238 mm3
2.3. Orthodontic Spring Placement
2.4. Tooth Movement Measurements and Micro-CT Analysis
2.5. Statistical Analysis
3. Results
3.1. Amount of Tooth Movement
3.2. Rate of Tooth Movement
3.3. Alveolar Bone Changes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Richter, A.E.; Arruda, A.O.; Peters, M.C.; Sohn, W. Incidence of caries lesions among patients treated with comprehensive orthodontics. Am. J. Orthod. Dentofac. Orthop. 2011, 139, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.S.; Alves, L.S.; Zenkner, J.E.D.A.; Zanatta, F.B.; Maltz, M. Gingival enlargement in orthodontic patients: Effect of treatment duration. Am. J. Orthod. Dentofac. Orthop. 2017, 152, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Weltman, B.; Vig, K.W.L.; Fields, H.W.; Shanker, S.; Kaizar, E.E. Root resorption associated with orthodontic tooth movement: A systematic review. Am. J. Orthod. Dentofac. Orthop. 2010, 137, 462–476. [Google Scholar] [CrossRef] [PubMed]
- Frost, H.M. The regional acceleratory phenomenon: A review. Henry Hosp. Med. J. 1983, 31, 3–9. [Google Scholar]
- Wilcko, M.T.; Wilcko, W.M.; Pulver, J.J.; Bissada, N.F.; Bouquot, J.E. Accelerated osteogenic orthodontics technique: A 1-stage surgically facilitated rapid orthodontic technique with alveolar augmentation. J. Oral Maxillofac. Surg. 2009, 67, 2149–2159. [Google Scholar] [CrossRef]
- Wilcko, W.M.; Wilcko, T.; Bouquot, J.E.; Ferguson, D.J. Rapid orthodontics with alveolar reshaping: Two case reports of decrowding. Int. J. Periodontics Restor. Dent. 2001, 21, 9–19. [Google Scholar]
- Fleming, P.S.; Fedorowicz, Z.; Johal, A.; El-Angbawi, A.; Pandis, N. Surgical adjunctive procedures for accelerating orthodontic treatment. Cochrane Database Syst. Rev. 2015, 6, CD010572. [Google Scholar] [CrossRef]
- Tizini, M.; Ibrahim, G. Retraction of the upper maxillary incisors with corticotomy-facilitated orthodontics and mini-implants. Saudi J. Dent. Res. 2014, 5, 146–151. [Google Scholar] [CrossRef]
- Iino, S.; Sakoda, S.; Ito, G.; Nishimori, T.; Ikeda, T.; Miyawaki, S. Acceleration of orthodontic tooth movement by alveolar corticotomy in the dog. Am. J. Orthod. Dentofac. Orthop. 2007, 131, 448.e1–448.e8. [Google Scholar] [CrossRef]
- Hassan, A.H.; Al-Saeed, S.H.; Al-Maghlouth, B.A.; Bahammam, M.A.; Linjawi, A.I.; El-Bialy, T.H. Corticotomy-assisted orthodontic treatment. A systematic review of the biological basis and clinical effect- iveness. Saudi Med. J. 2015, 36, 794–801. [Google Scholar] [CrossRef]
- Sebaoun, J.D.; Kantarci, A.; Turner, J.W.; Carvalho, R.S.; Van Dyke, T.E.; Ferguson, D.J. Modeling of trabecular bone and lamina dura following selective alveolar decortication in rats. J. Periodontol. 2008, 79, 1679–1688. [Google Scholar] [CrossRef] [PubMed]
- Teng, G.; Liou, E. Interdental Osteotomies Induce Regional Acceleratory Phenomenon and Accelerate Orthodontic Tooth Movement. J. Oral. Maxillofac. Surg. 2014, 72, 19–29. [Google Scholar] [PubMed]
- Cohen, G.; Campbell, P.; Rossouw, P.; Buschang, P. Effects of increased surgical trauma on rates of tooth movement and apical root resorption in foxhound dogs. Orthod. Craniofac. Res. 2010, 13, 179–190. [Google Scholar] [PubMed]
- Ozkan, T.H.; Arici, S. The effect of different micro-osteoperforation depths on the rate of orthodontic tooth movement: A single-center, single-blind, randomized clinical trial. Korean J. Orthod. 2021, 51, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Zhu, X.; Lu, J.; Dai, J.; Fang, B.; Shen, S. Accelerated Orthodontic Tooth Movement Following Le Fort I Osteotomy in a Rodent Model. J. Oral. Maxillofac. Surg. 2014, 72, 764–772. [Google Scholar] [CrossRef]
- Leethanakul, C.; Kanokkulchai, S.; Pongpanich, S.; Leepong, N.; Charoemratrote, C. Interseptal bone reduction on the rate of maxillary canine retraction. Angle Orthod. 2014, 84, 839–845. [Google Scholar] [CrossRef]
- Gong, J.K.; Arnold, J.S.; Cohn, S.H. Composition of trabecular and cortical bone. Anat. Rec. 1964, 149, 325–331. [Google Scholar]
- Seeman, E.; Delmas, P.D. Bone Quality—The Material and Structural Basis of Bone Strength and Fragility. N. Engl. J. Med. 2006, 354, 2250–2261. [Google Scholar]
- Buckwalter, J.A.; Glimcher, M.J.; Cooper, R.R.; Recker, R. Bone biology. I: Structure, blood supply, cells, matrix, and mineralization. Instr. Course Lect. 1996, 45, 371–386. [Google Scholar]
- Verna, C. The rate and the type of orthodontic tooth movement is influenced by bone turnover in a rat model. Eur. J. Orthod. 2000, 22, 343–352. [Google Scholar]
- Wilcko, M.T.; Wilcko, W.M.; Bissada, N.F. An Evidence-Based Analysis of Periodontally Accelerated Orthodontic and Osteogenic Techniques: A Synthesis of Scientific Perspectives. Semin. Orthod. 2008, 14, 305–316. [Google Scholar]
- Chang, J.; Chen, P.J.; Dutra, E.H.; Nanda, R.; Yadav, S. The effect of the extent of surgical insult on orthodontic tooth movement. Eur. J. Orthod. 2019, 41, 601–608. [Google Scholar] [PubMed]
- Abbas, N.H.; Sabet, N.E.; Hassan, I.T. Evaluation of corticotomy-facilitated orthodontics and piezocision in rapid canine retraction. Am. J. Orthod. Dentofac. Orthop. 2016, 149, 473–480. [Google Scholar]
- Schindeler, A.; McDonald, M.M.; Bokko, P.; Little, D.G. Bone remodeling during fracture repair: The cellular picture. Semin. Cell Dev. Biol. 2008, 19, 459–466. [Google Scholar]
- Ji, Y.; Tang, Y.; Wu, Q.; Huang, D.; Zhu, J.; Kang, F. The effects of mandibular osteotomy on maxillary orthodontic tooth movement and bone remodelling in a rat model. Eur. J. Orthod. 2021, 43, 467–472. [Google Scholar]
- Uhthoff, H.K.; Rahn, B.A. Healing patterns of metaphyseal fractures. Clin. Orthop. Relat. Res. 1981, 160, 295–303. [Google Scholar]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pathomkulmai, T.; Chanmanee, P.; Samruajbenjakun, B. Effect of Extending Corticotomy Depth to Trabecular Bone on Accelerating Orthodontic Tooth Movement in Rats. Dent. J. 2022, 10, 158. https://doi.org/10.3390/dj10090158
Pathomkulmai T, Chanmanee P, Samruajbenjakun B. Effect of Extending Corticotomy Depth to Trabecular Bone on Accelerating Orthodontic Tooth Movement in Rats. Dentistry Journal. 2022; 10(9):158. https://doi.org/10.3390/dj10090158
Chicago/Turabian StylePathomkulmai, Thanapat, Pannapat Chanmanee, and Bancha Samruajbenjakun. 2022. "Effect of Extending Corticotomy Depth to Trabecular Bone on Accelerating Orthodontic Tooth Movement in Rats" Dentistry Journal 10, no. 9: 158. https://doi.org/10.3390/dj10090158
APA StylePathomkulmai, T., Chanmanee, P., & Samruajbenjakun, B. (2022). Effect of Extending Corticotomy Depth to Trabecular Bone on Accelerating Orthodontic Tooth Movement in Rats. Dentistry Journal, 10(9), 158. https://doi.org/10.3390/dj10090158





