Sugar Substitute Stevia Inhibits Biofilm Formation, Exopolysaccharide Production, and Downregulates the Expression of Streptococcal Genes Involved in Exopolysaccharide Synthesis
Abstract
:1. Introduction
2. Methods
2.1. Bacterial Strains and Culture Conditions
2.2. Biofilm Culture
2.3. Quantification of Biofilms
2.4. Acid Production from Biofilms
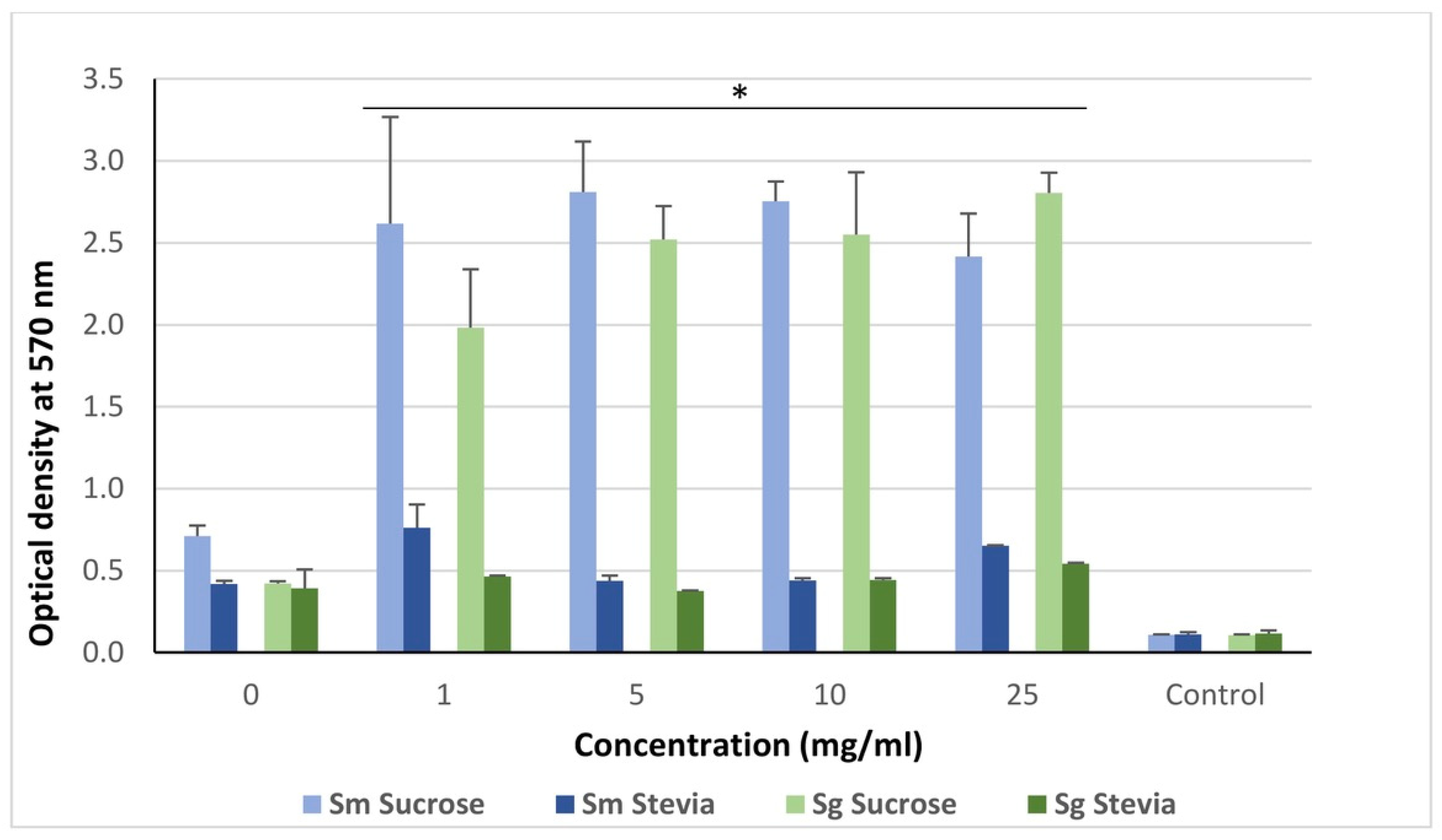
2.5. Exopolysaccharide (EPS) Quantification
2.6. RNA Extraction
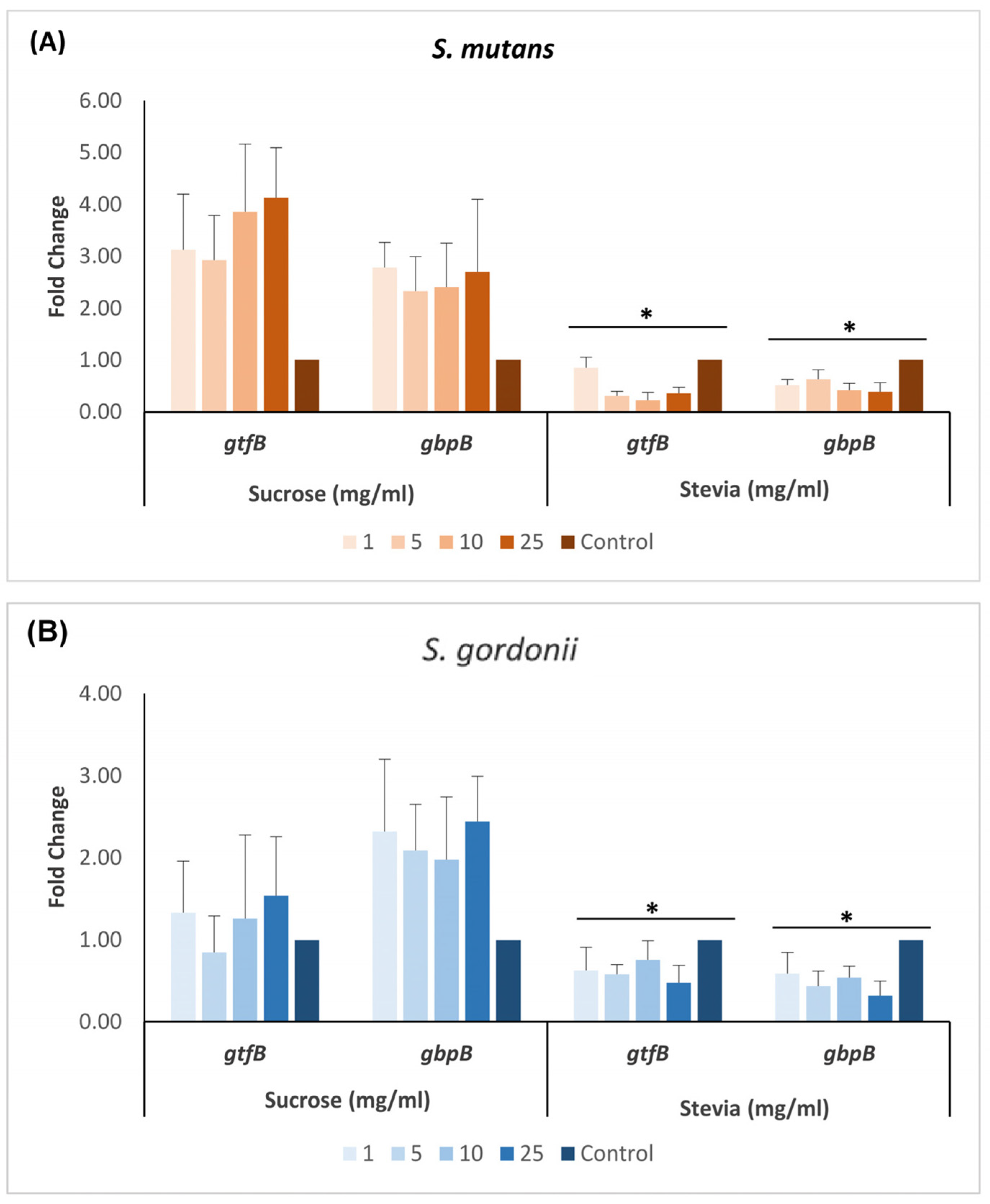
2.7. Reverse Transcription Real-Time PCR
2.8. Statistics
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giacaman, R.A.; Fernandez, C.E.; Munoz-Sandoval, C.; Leon, S.; Garcia-Manriquez, N.; Echeverria, C.; Valdes, S.; Castro, R.J.; Gambetta-Tessini, K. Understanding dental caries as a non-communicable and behavioral disease: Management implications. Front. Oral. Health 2022, 3, 764479. [Google Scholar] [CrossRef]
- Bowen, W.H. Do we need to be concerned about dental caries in the coming millennium? Crit. Rev. Oral. Biol. Med. 2002, 13, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Dennis, D.A.; Gawronski, T.H.; Sudo, S.Z.; Harris, R.S.; Folke, L.E. Variations in microbial and biochemical components of four-day plaque during a four-week controlled diet period. J. Dent. Res. 1975, 54, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Featherstone, J.D. Dental caries: A dynamic disease process. Aust. Dent. J. 2008, 53, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Newbrun, E. Sucrose, the arch criminal of dental caries. Odontol. Revy. 1967, 18, 373–386. [Google Scholar] [PubMed]
- Takahashi, N.; Nyvad, B. The role of bacteria in the caries process: Ecological perspectives. J. Dent. Res. 2011, 90, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.C.; Tabchoury, C.P.; Del Bel Cury, A.A.; Tenuta, L.M.; Rosalen, P.L.; Cury, J.A. Effect of starch on the cariogenic potential of sucrose. Br. J. Nutr. 2005, 94, 44–50. [Google Scholar] [CrossRef]
- Senneby, A.; Davies, J.R.; Svensater, G.; Neilands, J. Acid tolerance properties of dental biofilms in vivo. BMC Microbiol. 2017, 17, 165. [Google Scholar] [CrossRef]
- Minah, G.E.; Lovekin, G.B.; Finney, J.P. Sucrose-induced ecological response of experimental dental plaques from caries-free and caries-susceptible Human volunteers. Infect. Immun. 1981, 34, 662–675. [Google Scholar] [CrossRef] [PubMed]
- Pecharki, G.D.; Cury, J.A.; Paes Leme, A.F.; Tabchoury, C.P.; Del Bel Cury, A.A.; Rosalen, P.L.; Bowen, W.H. Effect of sucrose containing iron (II) on dental biofilm and enamel demineralization in situ. Caries Res. 2005, 39, 123–129. [Google Scholar] [CrossRef]
- Cai, J.N.; Jung, J.E.; Lee, M.H.; Choi, H.M.; Jeon, J.G. Sucrose challenges to Streptococcus mutans biofilms and the curve fitting for the biofilm changes. FEMS Microbiol. Ecol. 2018, 94, fiy091. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Chen, J.; Zhou, X.; Li, Y. Inhibition of Streptococcus mutans biofilm formation by strategies targeting the metabolism of exopolysaccharides. Crit. Rev. Microbiol. 2021, 47, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Ooshima, T.; Matsumura, M.; Hoshino, T.; Kawabata, S.; Sobue, S.; Fujiwara, T. Contributions of three glycosyltransferases to sucrose-dependent adherence of Streptococcus mutans. J. Dent. Res. 2001, 80, 1672–1677. [Google Scholar] [CrossRef] [PubMed]
- Tamesada, M.; Kawabata, S.; Fujiwara, T.; Hamada, S. Synergistic effects of streptococcal glucosyltransferases on adhesive biofilm formation. J. Dent. Res. 2004, 83, 874–879. [Google Scholar] [CrossRef] [PubMed]
- Lynch, D.J.; Fountain, T.L.; Mazurkiewicz, J.E.; Banas, J.A. Glucan-binding proteins are essential for shaping Streptococcus mutans biofilm architecture. FEMS Microbiol. Lett. 2007, 268, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto-Nakano, M.; Fujita, K.; Ooshima, T. Comparison of glucan-binding proteins in cariogenicity of Streptococcus mutans. Oral. Microbiol. Immunol. 2007, 22, 30–35. [Google Scholar] [CrossRef]
- Matsumoto, M.; Fujita, K.; Ooshima, T. Binding of glucan-binding protein C to GTFD-synthesized soluble glucan in sucrose-dependent adhesion of Streptococcus mutans. Oral. Microbiol. Immunol. 2006, 21, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Bowen, W.H.; Koo, H. Biology of Streptococcus mutans-derived glucosyltransferases: Role in extracellular matrix formation of cariogenic biofilms. Caries Res. 2011, 45, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Li, W.; Lin, J.; Chen, Z.; Yu, D. Effect of sucrose concentration on sucrose-dependent adhesion and glucosyltransferase expression of S. mutans in children with severe early-childhood caries (S-ECC). Nutrients 2014, 6, 3572–3586. [Google Scholar] [CrossRef]
- Sun, M.; Kang, Q.; Li, T.; Huang, L.; Jiang, Y.; Xia, W. Effect of high-fructose corn syrup on Streptococcus mutans virulence gene expression and on tooth demineralization. Eur. J. Oral. Sci. 2014, 122, 216–222. [Google Scholar] [CrossRef]
- Lynch, D.J.; Michalek, S.M.; Zhu, M.; Drake, D.; Qian, F.; Banas, J.A. Cariogenicity of Streptococcus mutans glucan-binding protein deletion mutants. Oral. Health Dent. Manag. 2013, 12, 191–199. [Google Scholar] [PubMed]
- Cugini, C.; Shanmugam, M.; Landge, N.; Ramasubbu, N. The Role of Exopolysaccharides in Oral Biofilms. J. Dent. Res. 2019, 98, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Rainey, K.; Michalek, S.M.; Wen, Z.T.; Wu, H. Glycosyltransferase-Mediated Biofilm Matrix Dynamics and Virulence of Streptococcus mutans. Appl. Environ. Microbiol. 2019, 85, e02247-18. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D.; Zaura, E. Dental biofilm: Ecological interactions in health and disease. J. Clin. Periodontol. 2017, 44 (Suppl. S18), S12–S22. [Google Scholar] [CrossRef] [PubMed]
- Sterzenbach, T.; Helbig, R.; Hannig, C.; Hannig, M. Bioadhesion in the oral cavity and approaches for biofilm management by surface modifications. Clin. Oral. Investig. 2020, 24, 4237–4260. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, E.; Cagetti, M.G.; Ionescu, A.; Campus, G.; Lingstrom, P. An in vitro and in vivo comparison of the effect of Stevia rebaudiana extracts on different caries-related variables: A randomized controlled trial pilot study. Caries Res. 2014, 48, 19–23. [Google Scholar] [CrossRef]
- Samuel, P.; Ayoob, K.T.; Magnuson, B.A.; Wolwer-Rieck, U.; Jeppesen, P.B.; Rogers, P.J.; Rowland, I.; Mathews, R. Stevia Leaf to Stevia Sweetener: Exploring Its Science, Benefits, and Future Potential. J. Nutr. 2018, 148, 1186S–1205S. [Google Scholar] [CrossRef]
- Additives, E.P.o.F.; Younes, M.; Aquilina, G.; Engel, K.H.; Fowler, P.; Frutos Fernandez, M.J.; Furst, P.; Gurtler, R.; Gundert-Remy, U. Safety of a proposed amendment of the specifications for steviol glycosides (E 960) as a food additive: To expand the list of steviol glycosides to all those identified in the leaves of Stevia Rebaudiana Bertoni. EFSA J. 2020, 18, e06106. [Google Scholar] [CrossRef]
- Escobar, E.; Piedrahita, M.; Gregory, R.L. Growth and viability of Streptococcus mutans in sucrose with different concentrations of Stevia rebaudiana Bertoni. Clin. Oral. Investig. 2020, 24, 3237–3242. [Google Scholar] [CrossRef]
- Karched, M.; Bhardwaj, R.G.; Inbamani, A.; Asikainen, S. Quantitation of biofilm and planktonic life forms of coexisting periodontal species. Anaerobe 2015, 35, 13–20. [Google Scholar] [CrossRef]
- Packiavathy, I.A.S.V.; Agilandeswari, P.; Musthafa, K.S.; Pandian, S.K.; Ravi, A.V. Antibiofilm and quorum sensing inhibitory potential of Cuminum cyminum and its secondary metabolite methyl eugenol against Gram negative bacterial pathogens. Food Res. Int. 2012, 45, 85–92. [Google Scholar] [CrossRef]
- Gabe, V.; Kacergius, T.; Abu-Lafi, S.; Kalesinskas, P.; Masalha, M.; Falah, M.; Abu-Farich, B.; Melninkaitis, A.; Zeidan, M.; Rayan, A. Inhibitory Effects of Ethyl Gallate on Streptococcus mutans Biofilm Formation by Optical Profilometry and Gene Expression Analysis. Molecules 2019, 24, 529. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Fujimoto, C.; Haruki, Y.; Maeda, T.; Kokeguchi, S.; Petelin, M.; Arai, H.; Tanimoto, I.; Nishimura, F.; Takashiba, S. Quantitative real-time PCR using TaqMan and SYBR Green for Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, Prevotella intermedia, tetQ gene and total bacteria. FEMS Immunol. Med. Microbiol. 2003, 39, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Ajagannanavar, S.L.; Shamarao, S.; Battur, H.; Tikare, S.; Al-Kheraif, A.A.; Al Sayed, M.S. Effect of aqueous and alcoholic Stevia (Stevia rebaudiana) extracts against Streptococcus mutans and Lactobacillus acidophilus in comparison to chlorhexidine: An in vitro study. J. Int. Soc. Prev. Community Dent. 2014, 4, S116–S121. [Google Scholar] [CrossRef] [PubMed]
- Kishta; Derani, M.; Neiva, G.F.; Boynton, J.R.; Kim, Y.E.; Fontana, M. The antimicrobial potential of stevia in an in vitro microbial caries model. Am. J. Dent. 2016, 29, 87–92. [Google Scholar]
- Ganter, J.; Hellwig, E.; Doerken, S.; Al-Ahmad, A. In vitro evaluation of the cariogenic potential of rebaudioside A compared to sucrose and xylitol. Clin. Oral. Investig. 2020, 24, 113–122. [Google Scholar] [CrossRef]
- Theophilus, P.A.; Victoria, M.J.; Socarras, K.M.; Filush, K.R.; Gupta, K.; Luecke, D.F.; Sapi, E. Effectiveness of Stevia Rebaudiana Whole Leaf Extract Against the Various Morphological Forms of Borrelia Burgdorferi in Vitro. Eur. J. Microbiol. Immunol. 2015, 5, 268–280. [Google Scholar] [CrossRef]
- Cerca, N.; Martins, S.; Pier, G.B.; Oliveira, R.; Azeredo, J. The relationship between inhibition of bacterial adhesion to a solid surface by sub-MICs of antibiotics and subsequent development of a biofilm. Res. Microbiol. 2005, 156, 650–655. [Google Scholar] [CrossRef]
- Guo, M.; Yang, K.; Zhou, Z.; Chen, Y.; Zhou, Z.; Chen, P.; Huang, R.; Wang, X. Inhibitory effects of Stevioside on Streptococcus mutans and Candida albicans dual-species biofilm. Front. Microbiol. 2023, 14, 1128668. [Google Scholar] [CrossRef]
- Gupta, E.G.V.; Purwar, S.; Shakyawar, S.; Alok, S.; Sundaram, S. Phytochemical screening and in-vitro studies of antioxidant and antimicrobial activity of extracts of dried stevia rebaudiana leaves. Int. J. Pharm. Sci. Res. 2017, 8, 3354–3360. [Google Scholar] [CrossRef]
- Mujeeb, F.; Bajpai, P.; Pathak, N. Phytochemical evaluation, antimicrobial activity, and determination of bioactive components from leaves of Aegle marmelos. Biomed. Res. Int. 2014, 2014, 497606. [Google Scholar] [CrossRef] [PubMed]
- Wolwer-Rieck, U. The leaves of Stevia rebaudiana (Bertoni), their constituents and the analyses thereof: A review. J. Agric. Food Chem. 2012, 60, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Siraj, E.S.; Pushpanjali, K.; Manoranjitha, B.S. Efficacy of stevioside sweetener on pH of plaque among young adults. Dent. Res. J. 2019, 16, 104–109. [Google Scholar] [CrossRef]
- Das, S.; Das, A.K.; Murphy, R.A.; Punwani, I.C.; Nasution, M.P.; Kinghorn, A.D. Evaluation of the cariogenic potential of the intense natural sweeteners stevioside and rebaudioside A. Caries Res. 1992, 26, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Giacaman, R.A.; Campos, P.; Munoz-Sandoval, C.; Castro, R.J. Cariogenic potential of commercial sweeteners in an experimental biofilm caries model on enamel. Arch. Oral. Biol. 2013, 58, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Augustinho do Nascimento, C.; Kim, R.R.; Ferrari, C.R.; de Souza, B.M.; Braga, A.S.; Magalhaes, A.C. Effect of sweetener containing Stevia on the development of dental caries in enamel and dentin under a microcosm biofilm model. J. Dent. 2021, 115, 103835. [Google Scholar] [CrossRef]
- Gamboa, F.; Chaves, M. Antimicrobial potential of extracts from Stevia rebaudiana leaves against bacteria of importance in dental caries. Acta Odontol. Latinoam 2012, 25, 171–175. [Google Scholar]
- Usha Sathyanarayanan, S.R.; John, B.; Babu, M.E. Anticariogenicity of Stevia rebaudiana Extract when used as a Mouthwash in High Caries Risk Patients: Randomized Controlled Clinical Trial. World J. Dent. 2017, 8, 1–6. [Google Scholar] [CrossRef]
- Koo, H.; Xiao, J.; Klein, M.I.; Jeon, J.G. Exopolysaccharides produced by Streptococcus mutans glucosyltransferases modulate the establishment of microcolonies within multispecies biofilms. J. Bacteriol. 2010, 192, 3024–3032. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, Y.; Han, Q.; Ye, X.; Chen, Y.; Sun, Y.; Liu, Y.; Zou, J.; Qi, G.; Zhou, X.; et al. Synonymous point mutation of gtfB gene caused by therapeutic X-rays exposure reduced the biofilm formation and cariogenic abilities of Streptococcus mutans. Cell. Biosci. 2021, 11, 91. [Google Scholar] [CrossRef]
- Mao, M.Y.; Yang, Y.M.; Li, K.Z.; Lei, L.; Li, M.; Yang, Y.; Tao, X.; Yin, J.X.; Zhang, R.; Ma, X.R.; et al. The rnc Gene Promotes Exopolysaccharide Synthesis and Represses the vicRKX Gene Expressions via MicroRNA-Size Small RNAs in Streptococcus mutans. Front. Microbiol. 2016, 7, 687. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, R.J.; Adams, B.O.; Sandham, H.J.; Abhyankar, S. Cariogenicity of a lactate dehydrogenase-deficient mutant of Streptococcus mutans serotype c in gnotobiotic rats. Infect. Immun. 1989, 57, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Bender, G.R.; Sutton, S.V.; Marquis, R.E. Acid tolerance, proton permeabilities, and membrane ATPases of oral streptococci. Infect. Immun. 1986, 53, 331–338. [Google Scholar] [CrossRef]
- Smith, E.G.; Spatafora, G.A. Gene regulation in S. mutans: Complex control in a complex environment. J. Dent. Res. 2012, 91, 133–141. [Google Scholar] [CrossRef]
- Senadheera, M.D.; Guggenheim, B.; Spatafora, G.A.; Huang, Y.C.; Choi, J.; Hung, D.C.; Treglown, J.S.; Goodman, S.D.; Ellen, R.P.; Cvitkovitch, D.G. A VicRK signal transduction system in Streptococcus mutans affects gtfBCD, gbpB, and ftf expression, biofilm formation, and genetic competence development. J. Bacteriol. 2005, 187, 4064–4076. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.T.; Yates, D.; Ahn, S.J.; Burne, R.A. Biofilm formation and virulence expression by Streptococcus mutans are altered when grown in dual-species model. BMC Microbiol. 2010, 10, 111. [Google Scholar] [CrossRef] [PubMed]
- Lucho, S.R.; do Amaral, M.; Bianchi, V.; Almagro, L.; Ferrer, M.; Calderon, A.; jacira Braga, E. Sequencing Analysis and Enzyme Activity Assay of SrUGT76G1 Revealed the Mechanism Toward on/off Production of Rebaudioside—A in Stevia Plants. Res. Sq. 2021, 1–21. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlKanderi, S.; AlFreeh, M.; Bhardwaj, R.G.; Karched, M. Sugar Substitute Stevia Inhibits Biofilm Formation, Exopolysaccharide Production, and Downregulates the Expression of Streptococcal Genes Involved in Exopolysaccharide Synthesis. Dent. J. 2023, 11, 267. https://doi.org/10.3390/dj11120267
AlKanderi S, AlFreeh M, Bhardwaj RG, Karched M. Sugar Substitute Stevia Inhibits Biofilm Formation, Exopolysaccharide Production, and Downregulates the Expression of Streptococcal Genes Involved in Exopolysaccharide Synthesis. Dentistry Journal. 2023; 11(12):267. https://doi.org/10.3390/dj11120267
Chicago/Turabian StyleAlKanderi, Sara, Monerah AlFreeh, Radhika G. Bhardwaj, and Maribasappa Karched. 2023. "Sugar Substitute Stevia Inhibits Biofilm Formation, Exopolysaccharide Production, and Downregulates the Expression of Streptococcal Genes Involved in Exopolysaccharide Synthesis" Dentistry Journal 11, no. 12: 267. https://doi.org/10.3390/dj11120267
APA StyleAlKanderi, S., AlFreeh, M., Bhardwaj, R. G., & Karched, M. (2023). Sugar Substitute Stevia Inhibits Biofilm Formation, Exopolysaccharide Production, and Downregulates the Expression of Streptococcal Genes Involved in Exopolysaccharide Synthesis. Dentistry Journal, 11(12), 267. https://doi.org/10.3390/dj11120267




