Abstract
The aim of this systematic review is to report the treatment options (Intervention) and outcomes (O) for primary teeth affected by periodontitis (Population) and if the treatment of primary teeth can prevent the spread of periodontitis to permanent teeth (Outcomes). The following databases were searched for papers published before December 2022: PubMed, Embase, Web of Science, and Ebscohost. Studies on children affected by periodontitis involving the primary teeth were included and those on children who presented with periodontitis as a manifestation of systemic disease were excluded. Narrative synthesis and methodological quality assessments were performed for the included studies. Three interventional studies (without a control group) that evaluated treatments involving scaling and root planing (SRP with antibiotics) and extraction were included (total n = 60 patients). Additionally, twelve case reports/case series articles (n = 19 patients) were identified. The diagnoses ranged from aggressive periodontitis to juvenile periodontitis and pre-pubertal periodontitis. Based on a limited number of published studies, it was found that the early treatment of periodontitis affecting the primary teeth using SRP and systemic antibiotics resulted in favorable improvements in PD and CAL. Limited evidence suggests that SRP and the extraction of the primary teeth involved have the potential to prevent periodontitis affecting permanent teeth. Future trials are required to standardize the treatment protocols and to confirm these findings.
1. Introduction
Periodontitis is an inflammatory condition that is induced by microbial plaque and results in attachment loss and bone loss [1]. A form of periodontitis called Grade C molar-incisor periodontitis (formerly known as aggressive periodontitis) is a rapidly progressing form of periodontitis that is characterized by early onset and rapid attachment loss in a healthy individual [2]. In general, this form of periodontitis is reported to have a circum-pubertal onset and can affect permanent teeth. The involvement of primary teeth in this pattern of periodontitis can occur in systemically healthy children [3,4,5,6,7]. In addition, evidence has shown that patients presenting with Grade C molar-incisor pattern periodontitis affecting permanent dentition showed attachment loss that affected the primary teeth as well [3,8].
Before the release of the 2018 classification of periodontal and peri-implant diseases [9], periodontitis affecting the primary teeth was diagnosed using different terminologies, including juvenile periodontitis (localized and generalized), aggressive periodontitis (AP—both localized and generalized), rapidly progressive periodontitis, and pre-pubertal periodontitis [10]. However, the 2018 classification of periodontal and peri-implant diseases did not include a separate category for periodontitis affecting the primary teeth [9]. Treating patients with periodontitis that affects the primary teeth can be challenging, due to rapid disease progression and a lower periodontal attachment area compared to permanent teeth, which can lead to early tooth loss [11]. Tooth loss in growing patients has long-term consequences regarding the development of the jaws and the permanent teeth [12]. Since periodontitis can be treated predictably in its early stages, it is important to recognize this condition as soon as it manifests in the primary teeth [13]. The aim of treatment in such cases is to establish a healthy periodontium that can be maintained to minimize primary tooth loss and the spread of periodontitis to permanent teeth [11]. Currently, the available treatment modalities for periodontitis that affects the primary teeth include scaling and root planing (SRP) with or without adjunct antibiotics and the extraction of the affected teeth [5,6,8,14].
Several systematic reviews [15,16,17,18] have evaluated the various treatment modalities that are adopted for periodontitis that affects permanent dentition. However, to date, no systematic review has been published that reports the management of periodontitis affecting the primary teeth. Hence, the aim of this systematic review is to report the treatment options and outcomes for primary teeth that have been affected by periodontitis. The secondary aim of this review is to report if the treatment of primary teeth that have been affected by periodontitis can prevent the spread of periodontitis to permanent teeth.
2. Materials and Methods
The manuscript has been reported according to the PRISMA 2020 guidelines for reporting systematic reviews [19]. The review protocol has been published in PROSPERO ([20]) (Ref. No: CRD42019127469; version: January 2023).
2.1. Inclusion and Exclusion Criteria
Original research articles that reported the treatment outcomes of periodontitis affecting the primary teeth were considered for inclusion in the current review. Since the new 2018 classification for periodontal diseases does not include a separate category for the primary teeth [9], we used the term “periodontitis affecting primary teeth” to encompass previous definitions, including juvenile periodontitis (localized and generalized), AP (localized and generalized), rapidly progressive periodontitis, and pre-pubertal periodontitis involving the primary teeth. The population (P) studied comprised children with periodontitis affecting the primary teeth. The interventions (I) that were included were the use of SRP with or without antibiotics and extraction. The primary outcomes (O) of interest were an improvement in probing depth (PD) and clinical attachment loss (CAL), with a follow-up of at least 4–6 weeks. The secondary outcome (O) of interest was the appearance of periodontitis in permanent teeth after the treatment of primary teeth, recorded during the follow-up, as evaluated by clinical measurements with or without a radiographic evaluation. Studies performed on children with medical conditions (immune deficiencies or conditions that would be considered to fall under “periodontitis as a manifestation of systemic disease”) were excluded. The treatment of periodontitis that affected mixed dentition was included if separate data for the primary teeth were available. Case reports where no treatment was provided were excluded (Table 1).

Table 1.
Inclusion and exclusion criteria.
2.2. Types of Included Studies
Initially, case-control, cohort studies, non-randomized studies of interventions, and clinical trials (with or without control groups) were included. However, an initial search revealed only three studies that met the inclusion criteria. The inclusion criteria were then broadened to encompass case series and case reports since several of these were identified during the search. Publications in languages other than English were excluded. Whenever missing information was encountered, the authors of that paper were contacted to elicit further information.
2.3. Search Strategy
The electronic search was performed in the following registries for papers published up to December 2022: PubMed, Embase, Web of Science, and Ebscohost. For the database searches, keywords and Boolean operators that were specific to each database were used to form the search strategy (the detailed search strategy is presented in the Supplementary Materials). The title and abstracts of all articles from the database searches were reviewed independently by two reviewers (EK, AM). The full texts were retrieved for all the included articles that were subsequently reviewed, to arrive at a final list of studies based on the criteria mentioned above. Any disagreements were noted and resolved in a discussion between the two reviewers and a third reviewer was available for consultation when necessary.
Apart from the database searches, an independent hand-search was performed in the European Journal of Paediatric Dentistry, Clinical Oral Investigations, and the European Archives of Paediatric Dentistry up to December 2022. Additional publications were searched by screening the bibliographies and reference lists from the included studies. Studies excluded after obtaining the full text were documented separately, along with the relevant reasons for exclusion (see the Supplementary Materials).
2.4. Data Extraction and Quality Assessment
Data were extracted from the included studies after the full-text screening. The data representing the primary outcomes (PD and CAL improvement) and secondary outcomes (permanent tooth involvement) were summarized using a narrative review and descriptive statistics.
The National Institutes of Health (NIH) tool [21], which is used for intervention studies with no control group, was utilized to assess the risk of bias in the included interventional studies. This tool was chosen since the interventional studies included in this paper had no control group. This tool evaluates the following criteria: objectives, eligibility criteria, representative population, sample size, intervention delivery, outcome measurement, follow-up, statistical methods, and multiple time point measurements [21]. An assessment of the risk of bias in the included studies was performed for the included case reports using the CARE [22] guidelines.
3. Results
3.1. Details of the Included Studies
The details of the search results are presented in the PRISMA flowchart shown in Figure 1. A total of 1505 articles were identified, with 1493 selected from the databases (PubMed: 596; Ebsco: 112; Embase: 438; Web of Science: 347). The treatment results for the patient population in one interventional study [23] were included in a subsequent follow-up paper [4] examining more patients and with a longer follow-up. However, the secondary aim of assessing the involvement of permanent teeth was only addressed in the former paper [23]; hence, both papers were included [4,23] for narrative synthesis. Additionally, two [24,25] case series reported the same case using different follow-up periods; hence, the paper with the longer follow-up [24] was included. In total, 11 case reports, 1 case series [5,6,7,14,24,26,27,28,29,30,31,32], and 3 interventional studies with no control group [4,23,33] were identified and included in the review (Figure 1). The characteristics of the included studies are described in Table 2 (interventional studies with no control group) including the number of cases, the treatment provided, follow-up, PD, and CAL at different time points, as well as the involvement of permanent teeth. Table 3 contains information regarding the included case reports, including patient age/sex, diagnosis, treatment provided, PD reduction, duration of follow-up, and the involvement of permanent teeth. The kappa value at the full-text screening stage was 0.53 (moderate agreement). A meta-analysis could not be performed since there was heterogeneity in the treatment protocols used in the interventional studies.
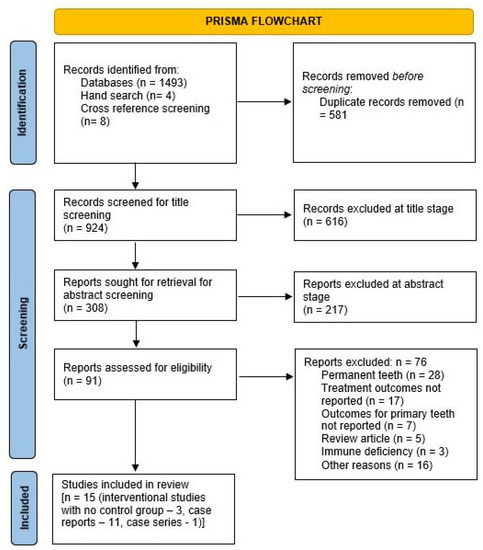
Figure 1.
PRISMA flowchart for screening the articles.

Table 2.
Data from the included interventional studies with no control group. Periodontal involvement of the permanent teeth was measured using clinical measurements (with or without radiographic data).

Table 3.
Data from the included case reports/case series.
3.2. Description of the Included Interventional Studies
A total of 3 interventional studies with no control group were included in the present systematic review (Table 2). Miller et al. (2017) [4] reported the treatment outcomes in 27 systemically healthy patients with localized aggressive periodontitis (LAP) affecting the primary teeth. The affected teeth were treated using SRP, with adjunct systemic antibiotics (500 mg amoxicillin and 250 mg metronidazole three times a day for 7 days). A significant reduction in PD and gain in CAL was seen following treatment. While comparing the treatment outcomes in the primary and permanent teeth, a greater improvement in CAL and PD at 12 and 24 months was seen in the primary teeth. Merchant et al. (2014) [23] reported the results for 22 out of the 27 subjects presented in the article by Miller et al. (2017) [4]. They followed similar treatment protocols (SRP + amoxicillin and metronidazole) and reported significant reductions in PD and CAL gain at 3, 6, and 12 months following the treatment. There was a significantly higher gain in CAL at 3, 6, and 12 months for the primary teeth when compared to the permanent teeth. Additionally, 13/22 subjects showed the normal eruption of permanent teeth, while the remaining patients had primary teeth with LAP at the end of the follow-up period.
The third interventional study included in this review was conducted by Mros et al. (2010) [33] and involved 11 children between the ages of 7 and 13 with LAP. All the affected primary molars were extracted. The subjects were recalled at 14 to 19 years after treatment for a clinical examination with PD and BOP measurements being recorded and a radiographic examination including bitewings of the permanent teeth. At the follow-up examination, four of the recorded cases (36.3%) exhibited the development of periodontitis in permanent teeth, and the rest were free of periodontitis (Table 2). These findings, therefore, suggest that treatment of the primary teeth has the potential to prevent the spread of periodontitis to the permanent teeth.
3.3. Description of the Included Case Reports/Case Series
A total of 19 cases have been reported in the 12 included case reports/case series (Table 3). Of the ten cases reported in the paper by Bimstein et al. (2003) [24], one case did not include information regarding periodontitis affecting the primary teeth and was thus excluded; another case did not report the primary or secondary outcomes and was thus excluded, whereas the remaining 8 cases were included.
The included cases described children who were between 3 and 9 years of age. The common symptoms were bleeding from the gums, the mobility of one or more teeth, and the loss of teeth. In a case reported by Seremidi et al. (2012) [30], the mean PD of 7.5 mm was reduced to 3.8 mm following treatment over 18 months. Hazan-Molina et al. (2012) [5] reported a case where the initial PD was greater than 7 mm, which was reduced to 3 mm following treatment. Spoerri et al. (2014) [7] reported a PD of less than 4 mm at follow-up, while Ngan et al. (1985) [32] reported a PD within the normal range and Suzuki et al. (2003) reported a PD of within 2.5 mm after treatment.
Of the included case reports/case series, no cases reported periodontitis affecting the permanent teeth after treatment of the primary teeth. For case reports that pursued extraction as the treatment of choice, space maintenance options were reported in four case reports; these included pedi-partials [29], vacuum-formed removable retainers [5], space maintainers [24], and complete dentures (allowing space for the erupting permanent teeth) [6].
3.4. Quality Assessment of the Included Studies
The quality of the three interventional studies [4,23,33] was assessed using the NIH risk of bias tool and the provided guidance. Mros et al. (2010) [33] achieved a score of 6/12, Merchant et al. (2014) [23] achieved a score of 10/12, and Miller et al. (2017) [4] achieved a score of 10/12. Overall, the three studies showed a fair to low risk of bias (Table 4).

Table 4.
Quality assessment of the included interventional studies, using the NIH risk of bias tool for those intervention studies with no control group. The criteria were derived from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed 5 January 2023).
The quality of the included case reports/case series was evaluated using the CARE guidelines. All case reports except one were considered to be of high quality (>20) and the exception [32] was found to be of medium quality (10–20), while no articles were found to be of low quality (<10) (Table 5).

Table 5.
Quality assessment of the case reports/case series using the CARE guidelines.
4. Discussion
Treatment outcomes for permanent teeth that have been affected by Grade C molar-incisor pattern periodontitis (formerly known as AP) have a better prognosis with early diagnosis and treatment [11]. In mild cases of Grade C, molar-incisor pattern periodontitis can be managed with non-surgical therapy using systemic antibiotics, followed by periodontal maintenance at regular intervals [34,35,36]. In more advanced cases, surgical treatment for the residual deep pockets and bone defects, followed by periodontal maintenance at regular intervals, is often needed [11,37,38,39].
Studies have reported that periodontitis can occur in healthy children [24,40], which can be an early sign of the potential involvement of permanent dentition [3,41]. If the affected primary teeth are not treated at an early stage, this can lead to spontaneous exfoliation or the need to extract the teeth at an early age, due to rapid attachment loss and disease progression [29,42]. The early diagnosis and treatment of periodontitis (affecting both the primary and mixed dentition) can provide an opportunity to limit the damage and prevent disease progression to permanent dentition [23], thereby improving the quality of life [43].
The included studies in this systematic review predominantly utilized SRP and extraction as the most common treatment modality for primary teeth that have been affected by periodontitis. Although the two treatment modalities (extraction and SRP) widely vary in their outcomes, the principal goal for primary teeth affected by periodontitis is to prevent the involvement of permanent dentition. In the studies included in this systematic review, both treatment modalities showed similar outcomes in terms of the potential to limit the involvement of the developing permeant teeth [4,23,33]. When SRP was used as a treatment modality, the included studies reported significant improvement in the clinical parameters (PD and CAL), especially when combined with adjunct systemic antibiotics [4,23]. The data available from the included case reports/series [5,7,30] in the systematic reviews reported similar findings to the included interventional studies. However, the limited sample size outlines the need for studies that are longitudinal in nature to confirm the findings and make evidence-based clinical recommendations.
The results of the included interventional studies in this systematic review suggest that the primary teeth responded better to SRP and adjunct antibiotics than the permanent teeth [4]. Although a direct comparison cannot be made, a reduction in PD after the SRP of permanent teeth is by about 1.3–2.1 mm, and the improvement in CAL is by about 0.6–1.2 mm [44]. When adjunct systemic antibiotics are prescribed along with SRP for permanent teeth, there is an additional 0.3–0.4 mm in terms of PD reduction and 0.15–0.2 mm in terms of CAL gain [45]. In contrast, the two included studies reported a 2.5-millimeter reduction in PD and a 3-millimeter improvement in CAL after SRP and systemic antibiotics were employed for the treatment of primary teeth with periodontitis [4,23], suggesting that early intervention in cases involving the primary teeth has better outcomes than in the case of permanent teeth, but future trials are required to confirm this observation.
This systematic review is not without its limitations. In the protocol for this systematic review, we included both interventional studies and case reports. Although case reports provide a low level of evidence, in the absence of randomized trials and limited observational studies, the data provided by case reports can be beneficial [46,47]. In the interventional studies included in the review, there was the absence of a control group and the relative sample sizes were low. Additionally, due to a lack of specific diagnostic terminology (according to the new 2018 classification of periodontal diseases) for periodontitis affecting the primary teeth, we have used the generic term “periodontitis affecting primary teeth” [9]. The search screening process aimed to identify only those articles written in the English language. The CARE guidelines were used to evaluate the risk of bias for the included case reports/series. Despite this being a reporting checklist, the CARE guidelines have been used previously for assessing the risk of bias in systematic reviews that have included case reports/series [46,47].
The systematic review identified the issue that there is a lack of standardized treatment protocols for periodontitis affecting the primary teeth, unlike the permanent teeth. SRP and extraction were most commonly employed in the included studies. The majority of the studies that employed extraction of the primary teeth as the treatment of choice did not report any information regarding space management. The management of concerns regarding space loss, the loss of esthetics, and the interruption of speech due to tooth loss need to be considered before choosing extraction as a treatment option [5,24,29]. Following treatment of the primary teeth, the results need to be evaluated by measuring PD and CAL and longitudinally reporting the effect on permanent dentition, as well as the effect on oral health-related quality of life. Future interventional trials are needed to determine the optimal treatment options for periodontitis affecting the primary teeth and to confirm if early intervention can prevent the involvement of permanent teeth.
5. Conclusions
Within the limitations of this systematic review, which is based on a limited number of studies, periodontitis affecting the primary teeth is currently managed by means of extraction or SRP (with or without antibiotic therapy). The included studies showed that treatment using SRP with antibiotic therapy results in a favorable reduction in PD and CAL. Additionally, the extraction and SRP of the affected primary teeth have the potential to prevent periodontitis from developing in the permanent teeth. Further interventional studies are required to standardize the treatment protocols.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/dj11070171/s1, Supplementary Material S1. References [48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, E.K. and A.M.; methodology, E.K., A.M. and P.G.B.; formal analysis, E.K., A.M. and P.G.B.; investigation, P.G.B. and E.K.; data curation, P.G.B., E.K. and A.M.; writing—original draft preparation, A.M.; writing—review and editing, E.K. and P.G.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used in this study is taken from previously published literature.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus Report of Workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. 1), S173–S182. [Google Scholar] [CrossRef] [PubMed]
- Fine, D.H.; Patil, A.G.; Loos, B.G. Classification and Diagnosis of Aggressive Periodontitis. J. Clin. Periodontol. 2018, 45 (Suppl. 20), S95–S111. [Google Scholar] [CrossRef] [PubMed]
- Bimstein, E. Radiographic Description of the Distribution of Aggressive Periodontitis in Primary Teeth. J. Clin. Pediatr. Dent. 2018, 42, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.A.F.S.; Branco-de-Almeida, L.S.; Wolf, S.; Hovencamp, N.; Treloar, T.; Harrison, P.; Aukhil, I.; Gong, Y.; Shaddox, L.M. Long-Term Clinical Response to Treatment and Maintenance of Localized Aggressive Periodontitis: A Cohort Study. J. Clin. Periodontol. 2017, 44, 158–168. [Google Scholar] [CrossRef]
- Hazan-Molina, H.; Zigdon, H.; Einy, S.; Aizenbud, D. Periodontal and Space Maintenance Considerations for Primary Teeth Presenting with Aggressive Periodontitis: A Case Report. Pediatr. Dent. 2012, 34, 254–258. [Google Scholar]
- Cunha, R.F.; Machado, A.C.; Watanabe, S.; Freire, I.R.; Goiato, M.C.; Júnior, E.G.-J. A Combination of Clinical and Microbiological Management of Generalized Aggressive Periodontitis in Primary Teeth. A Case Report. Int. J. Paediatr. Dent. 2012, 22, 310–316. [Google Scholar] [CrossRef]
- Spoerri, A.; Signorelli, C.; Erb, J.; van Waes, H.; Schmidlin, P.R. Rare Case of Generalised Aggressive Periodontitis in the Primary Dentition. Eur. Arch. Paediatr. Dent. 2014, 15, 443–447. [Google Scholar] [CrossRef]
- Miller, K.; Treloar, T.; Guelmann, M.; Rody, W.J.; Shaddox, L.M. Clinical Characteristics of Localized Aggressive Periodontitis in Primary Dentition. J. Clin. Pediatr. Dent. 2018, 42, 95–102. [Google Scholar] [CrossRef]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.C.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A New Classification Scheme for Periodontal and Peri-Implant Diseases and Conditions—Introduction and Key Changes from the 1999 Classification. J. Periodontol. 2018, 89 (Suppl. 1), S1–S8. [Google Scholar] [CrossRef]
- Kinane, D. Periodontal Disease in Children and Adolescents: Introduction and Classification. Periodontol. 2000 2001, 26, 7–15. [Google Scholar] [CrossRef]
- Teughels, W.; Dhondt, R.; Dekeyser, C.; Quirynen, M. Treatment of Aggressive Periodontitis. Periodontol. 2000 2014, 65, 107–133. [Google Scholar] [CrossRef] [PubMed]
- Bimstein, E.; McIlwain, M.; Katz, J.; Jerrell, G.; Primosch, R. Aggressive Periodontitis of the Primary Dentition Associated with Idiopathic Immune Deficiency: Case Report and Treatment Considerations. J. Clin. Pediatr. Dent. 2004, 29, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, W.M.; Papapanou, P.N. Epidemiology of Periodontal Disease in Children and Adolescents. Periodontol. 2000 2001, 26, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Mass, E.; Hershkovitz, F.; Zilberman, U. Localised Aggressive Periodontitis in a 3-Year-Old-Boy. Eur. Arch. Paediatr. Dent. 2018, 19, 61–63. [Google Scholar] [CrossRef] [PubMed]
- Keestra, J.A.J.; Grosjean, I.; Coucke, W.; Quirynen, M.; Teughels, W. Non-Surgical Periodontal Therapy with Systemic Antibiotics in Patients with Untreated Aggressive Periodontitis: A Systematic Review and Meta-Analysis. J. Periodont. Res. 2015, 50, 689–706. [Google Scholar] [CrossRef]
- Rabelo, C.C.; Feres, M.; Gonçalves, C.; Figueiredo, L.C.; Faveri, M.; Tu, Y.-K.; Chambrone, L. Systemic Antibiotics in the Treatment of Aggressive Periodontitis. A Systematic Review and a Bayesian Network Meta-Analysis. J. Clin. Periodontol. 2015, 42, 647–657. [Google Scholar] [CrossRef]
- Souza, E.; Medeiros, A.C.; Gurgel, B.C.; Sarmento, C. Antimicrobial Photodynamic Therapy in the Treatment of Aggressive Periodontitis: A Systematic Review and Meta-Analysis. Lasers Med. Sci. 2016, 31, 187–196. [Google Scholar] [CrossRef]
- Díaz-Faes, L.; Fernández-Somoano, A.; Magán-Fernández, A.; Mesa, F. Efficacy of Regenerative Therapy in Aggressive Periodontitis: A Systematic Review and Meta-Analysis of Randomised Controlled Clinical Trials. Clin. Oral Investig. 2020, 24, 1369–1378. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Mohan, A.; Kandaswamy, E. Anusha Mohan; Eswar Kandaswamy Treatment of Aggressive Periodontitis Affecting the Primary Dentition—A Systematic Review. Available online: Crd.york.ac.uk/prospero/display_record.php?RecordID=127469 (accessed on 10 April 2023).
- Study Quality Assessment Tools|NHLBI, NIH. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 21 September 2022).
- Riley, D.S.; Barber, M.S.; Kienle, G.S.; Aronson, J.K.; von Schoen-Angerer, T.; Tugwell, P.; Kiene, H.; Helfand, M.; Altman, D.G.; Sox, H.; et al. CARE Guidelines for Case Reports: Explanation and Elaboration Document. J. Clin. Epidemiol. 2017, 89, 218–235. [Google Scholar] [CrossRef]
- Merchant, S.N.; Vovk, A.; Kalash, D.; Hovencamp, N.; Aukhil, I.; Harrison, P.; Zapert, E.; Bidwell, J.; Varnado, P.; Shaddox, L.M. Localized Aggressive Periodontitis Treatment Response in Primary and Permanent Dentitions. J. Periodontol. 2014, 85, 1722–1729. [Google Scholar] [CrossRef] [PubMed]
- Bimstein, E. Seven-Year Follow-up of 10 Children with Periodontitis. Pediatr. Dent. 2003, 25, 389–396. [Google Scholar] [PubMed]
- Bimstein, E.; Sela, M.N.; Shapira, L. Clinical and Microbial Considerations for the Treatment of an Extended Kindred with Seven Cases of Prepubertal Periodontitis: A 2-Year Follow-Up. Pediatr. Dent. 1997, 19, 396–403. [Google Scholar]
- Yoshida-Minami, I.; Kishimoto, K.; Suzuki, A.; Fujiwara, T.; Shintani, S.; Morisaki, I.; Sobue, S.; Miyamoto, M.; Nagai, A.; Kurihara, H. Clinical, Microbiological and Host Defense Parameters Associated with a Case of Localized Prepubertal Periodontitis. J. Clin. Periodontol. 1995, 22, 56–62. [Google Scholar] [CrossRef]
- Sixou, J.L.; Robert, J.C.; Bonnaure-Mallet, M. Loss of Deciduous Teeth and Germs of Permanent Incisors in a 4-Year-Old Child. An Atypic Prepubertal Periodontitis? A Clinical, Microbiological, Immunological and Ultrastructural Study. J. Clin. Periodontol. 1997, 24, 836–843. [Google Scholar] [CrossRef]
- Suzuki, J.; Okada, M.; Wang, Y.; Nii, N.; Miura, K.; Kozai, K. Localized Aggressive Periodontitis in Primary Dentition: A Case Report. J. Periodontol. 2003, 74, 1060–1066. [Google Scholar] [CrossRef] [PubMed]
- Portaro, C.P.; Chópite, Y.G.; Cárdenas, A.C. Generalized Aggressive Periodontitis in Preschoolers: Report of a Case in a 3-1/2 Year Old. J. Clin. Pediatr. Dent. 2008, 33, 155–159. [Google Scholar] [CrossRef]
- Seremidi, K.; Gizani, S.; Madianos, P. Therapeutic Management of a Case of Generalised Aggressive Periodontitis in an 8-Year Old Child: 18-Month Results. Eur. Arch. Paediatr. Dent. 2012, 13, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Hilgers, K.K.; Dean, J.W.; Mathieu, G.P. Localized Aggressive Periodontitis in a Six-Year-Old: A Case Report. Pediatr. Dent. 2004, 26, 345–351. [Google Scholar] [PubMed]
- Ngan, P.W.; Tsai, C.C.; Sweeney, E. Advanced Periodontitis in the Primary Dentition: Case Report. Pediatr. Dent. 1985, 7, 255–258. [Google Scholar] [PubMed]
- Mros, S.T.; Berglundh, T. Aggressive Periodontitis in Children: A 14-19-Year Follow-Up. J. Clin. Periodontol. 2010, 37, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Branco-de-Almeida, L.S.; Cruz-Almeida, Y.; Gonzalez-Marrero, Y.; Kudsi, R.; de Oliveira, I.C.V.; Dolia, B.; Huang, H.; Aukhil, I.; Harrison, P.; Shaddox, L.M. Treatment of Localized Aggressive Periodontitis Alters Local Host Immunoinflammatory Profiles: A Long-Term Evaluation. J. Clin. Periodontol. 2021, 48, 237–248. [Google Scholar] [CrossRef]
- Branco-de-Almeida, L.S.; Velsko, I.M.; de Oliveira, I.C.V.; de Oliveira, R.C.G.; Shaddox, L.M. Impact of Treatment on Host Responses in Young Individuals with Periodontitis. J. Dent. Res. 2023, 102, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Velsko, I.M.; Harrison, P.; Chalmers, N.; Barb, J.; Huang, H.; Aukhil, I.; Shaddox, L. Grade C Molar-Incisor Pattern Periodontitis Subgingival Microbial Profile before and after Treatment. J. Oral Microbiol. 2020, 12, 1814674. [Google Scholar] [CrossRef] [PubMed]
- da Silva Cirino, C.C.; do Vale, H.F.; Casati, M.Z.; Sallum, E.A.; Casarin, R.C.V.; Sallum, A.W. Clinical and Microbiological Evaluation of Surgical and Nonsurgical Treatment of Aggressive Periodontitis. Braz. Dent. J. 2019, 30, 577–586. [Google Scholar] [CrossRef]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Beglundh, T.; Sculean, A.; Tonetti, M.S.; EFP Workshop Participants and Methodological Consultants. Treatment of Stage I-III Periodontitis-The EFP S3 Level Clinical Practice Guideline. J. Clin. Periodontol. 2020, 47 (Suppl. 22), 4–60. [Google Scholar] [CrossRef]
- Delatola, C.; Loos, B.G.; Laine, M.L. Three Periodontitis Phenotypes: Bone Loss Patterns, Antibiotic-Surgical Treatment and the New Classification. J. Clin. Periodontol. 2020, 47, 1371–1378. [Google Scholar] [CrossRef]
- Periodontal Diseases of Children and Adolescents. Pediatr. Dent. 2016, 38, 388–396.
- Petit, C.; Huck, O.; Amar, S.; Tenenbaum, H. Management of Localized Aggressive Periodontitis: A 30-Year Follow-Up. Quintessence Int. 2018, 49, 615–624. [Google Scholar] [CrossRef]
- Sharma, G.; Whatling, R. Case Report: Premature Exfoliation of Primary Teeth in a 4-Year-Old Child, a Diagnostic Dilemma. Eur. Arch. Paediatr. Dent. 2011, 12, 312–317. [Google Scholar] [CrossRef]
- Ferreira, M.C.; Dias-Pereira, A.C.; Branco-de-Almeida, L.S.; Martins, C.C.; Paiva, S.M. Impact of Periodontal Disease on Quality of Life: A Systematic Review. J. Periodontal. Res. 2017, 52, 651–665. [Google Scholar] [CrossRef] [PubMed]
- Cobb, C.M. Non-Surgical Pocket Therapy: Mechanical. Ann. Periodontol. 1996, 1, 443–490. [Google Scholar] [CrossRef] [PubMed]
- Walters, J.; Lai, P.-C. Should Antibiotics Be Prescribed to Treat Chronic Periodontitis? Dent. Clin. N. Am. 2015, 59, 919–933. [Google Scholar] [CrossRef]
- Halai, H.; Somani, C.; Donos, N.; Nibali, L. Periodontal Status of Children with Primary Immunodeficiencies: A Systematic Review. Clin. Oral Investig. 2020, 24, 1939–1951. [Google Scholar] [CrossRef]
- Nambiema, A.; Sembajwe, G.; Lam, J.; Woodruff, T.; Mandrioli, D.; Chartres, N.; Fadel, M.; Le Guillou, A.; Valter, R.; Deguigne, M.; et al. A Protocol for the Use of Case Reports/Studies and Case Series in Systematic Reviews for Clinical Toxicology. Front. Med. 2021, 8, 708380. [Google Scholar] [CrossRef]
- Abbott, B. Periodontal disease in children and adolescents. J. Macomb. Dent. Soc. 1989, 26, 29. [Google Scholar]
- Allin, N.; Cruz-Almeida, Y.; Velsko, I.; Vovk, A.; Hovemcamp, N.; Harrison, P.; Huang, H.; Aukhil, I.; Wallet, S.; Shaddox, L. Inflammatory Response Influences Treatment of Localized Aggressive Periodontitis. J. Dent. Res. 2016, 95, 635–641. [Google Scholar] [CrossRef]
- Barber, S.; Day, P.; Judge, M.; Toole, E.O.; Fayle, S. Variant Carvajal syndrome with additional dental anomalies. Int. J. Paediatr. Dent. 2012, 22, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Beliveau, D.; Magnusson, I.; Bidwell, J.A.; Zapert, E.F.; Aukhil, I.; Wallet, S.M.; Shaddox, L.M. Benefits of early systemic antibiotics in localized aggressive periodontitis: A retrospective study. J. Clin. Periodontol. 2012, 39, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Bimstein, E. Periodontal Health and Disease in Children and Adolescents. Pediatr. Clin. North Am. 1991, 38, 1183–1207. [Google Scholar] [CrossRef] [PubMed]
- Bodur, A.; Bodur, H.; Bal, B.; Balos, K. Generalized aggressive periodontitis in a prepubertal patient: A case report. Quin-Tessence Int. 2001, 32, 303–308. [Google Scholar]
- Budtz Jorgensen, E.; Ellegaard, J.; Ellegaard, B. CMI in juvenile periodontitis and the effect of levamisole treatment. Allergol. Immunopathol. 1977, 5, 314–315. [Google Scholar]
- Buduneli, N.; Baylas, H.; Aksu, G.; Kütükçüler, N. Prepubertal periodontitis associated with chronic granulomatous disease. J. Clin. Periodontol. 2001, 28, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Burgess, D.K.; Huang, H.; Harrison, P.; Kompotiati, T.; Aukhil, I.; Shaddox, L.M. Non-Surgical Therapy Reduces Presence of JP2 Clone in Localized Aggressive Periodontitis. J. Periodontol. 2017, 88, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.H. A Familial Pattern of Juvenile Periodontitis (Periodontosis). J. Periodontol. 1969, 40, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Celenligil, H.; Kansu, E.; Eratalay, K.; Yavuzyilmaz, E. Prepubertal periodontitis. A case report with an analysis of lymphocyte populations. J. Clin. Periodontol. 1987, 14, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Christersson, L.A.; Slots, J.; Rosling, B.G.; Genco, R.J. Microbiological and clinical effects of surgical treatment of localized juvenile periodontitis. J. Clin. Periodontol. 1985, 12, 465–476. [Google Scholar] [CrossRef]
- Christersson, L.A. Actinobacillus actinomycetemcomitans and localized juvenile periodontitis. Clinical, microbiologic and histologic studies. Swed. Dent. J. Suppl. 1993, 90, 1–46. [Google Scholar]
- Caufield, P.W.; Stanley, D.H.; Donaldson, D.K. Periodontal disease in healthy children: Two clinical reports. Pediatr. Dent. 1984, 6, 41–45. [Google Scholar]
- Compton, D.W.; Claiborne, W.J.; Hutchens, L.H. Combined periodontal, orthodontic and fixed prosthetic treatment of juvenile periodontitis: A case report. Int. J. Periodontics Restor. Dent. 1983, 3, 20–33. [Google Scholar]
- Debevc, T.M.; Silver, J.G. Periodontal diseases affecting children and young adults. J. Can. Dent. Assoc. 1996, 62, 650–652. [Google Scholar]
- Dibart, S.; Chapple, I.L.; Skobe, Z.; Shusterman, S.; Nedleman, H.L. Microbiological findings in prepubertal periodontitis. A case report. J. Periodontol. 1998, 69, 1172–1175. [Google Scholar] [CrossRef]
- Dopico, J.; Nibali, L.; Donos, N. Disease progression in aggressive periodontitis patients. A Retrospective Study. J. Clin. Periodontol. 2016, 43, 531–537. [Google Scholar] [CrossRef]
- Dosumu, E.B.; Bankole, O.O.; Dosumu, O.O. Simultaneous occurrence of periodontal and skin abscesses in a Nigerian girl: Case report. Afr. J. Biomed. Res. 2015, 18, 249–255. [Google Scholar]
- Emingil, G.; Darcan, S.; Keskinoǧlu, A.; Kütükçüler, N.; Atilla, G. Localized aggressive periodontitis in a patient with type 1 diabetes mellitus: A case report. J. Periodontol. 2001, 72, 1265–1270. [Google Scholar] [CrossRef]
- Epstein, S.R. Localized prepubertal periodontitis--nonsurgical treatment of an adolescent patient: A case report. Pract. Periodontics Aesthet. Dent. 1995, 7, 55–59. [Google Scholar]
- Fretwell, L.D.; Leinbach, T.E.; Wiley, D.O. Juvenile periodontitis: Report of cases. J. Am. Dent. Assoc. 1982, 105, 1022–1025. [Google Scholar] [CrossRef] [PubMed]
- Goepferd, S.J. Advanced alveolar bone loss in the primary dentition: A case report. J. Periodontol. 1981, 52, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, P.F.; Huang, H.; McAninley, S.; Alfant, B.; Harrison, P.; Aukhil, I.; Walker, C.; Shaddox, L.M. Periodontal treatment reduces matrix metalloproteinase levels in localized aggressive periodontitis. J. Periodontol. 2013, 84, 1801–1808. [Google Scholar] [CrossRef] [PubMed]
- Haring, J.I. Case study. Early-onset periodontitis. RDH 1999, 19, 11–102. [Google Scholar] [PubMed]
- Hempton, T.J.; Wilkins, E.; Lancaster, D. Localized juvenile periodontitis. RDH 1997, 17, 30–32. [Google Scholar] [PubMed]
- Hoffman, I.D. Familial occurrence of juvenile periodontitis with varied treatment of one of the siblings with five-year follow-up: Case reports. J. Periodontol. 1983, 54, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Kalash, D.; Vovk, A.; Huang, H.; Aukhil, I.; Wallet, S.M.; Shaddox, L.M. Influence of Periodontal Therapy on Systemic Lipopolysaccharides in Children with Localized Aggressive Periodontitis. Pediatr. Dent. 2015, 37, 35–40. [Google Scholar]
- Katz, J.; Ben-Yehuda, A.; Machtei, E.E.; Danon, Y.L.; Metzker, A. Tumoral calcinosis associated with early onset periodontitis. J. Clin. Periodontol. 1989, 16, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Kornman, K.S.; Robertson, P.B. Clinical and microbiological evaluation of therapy for juvenile periodontitis. J. Periodontol. 1985, 56, 443–446. [Google Scholar] [CrossRef]
- Kunihira, D.M.; Caine, F.A.; Palcanis, K.G.; Best, A.M.; Ranney, R.R. A clinical trial of phenoxymethyl penicillin for adjunctive treatment of juvenile periodontitis. J. Periodontol. 1985, 56, 352–358. [Google Scholar] [CrossRef]
- Linden, G.; Fleming, P.; Coulter, W.; Lynn, G. Localized prepubertal periodontitis in a 5-year-old child: Investigations and clinical observations over a 3-year period. Int. J. Paediatr. Dent. 2009, 4, 47–53. [Google Scholar] [CrossRef]
- Liyange, S.; Edgar, D.; Shields, M.D.; Linden, G.J. Gingival Inflammation and Aggressive Periodontitis in a Child with a Specific Antibody Deficiency. Dent. Updat. 2016, 43, 130–136. [Google Scholar] [CrossRef]
- López, N.J. Clinical, Laboratory, and Immunological studies of a family with a high prevalence of generalized prepubertal and juvenile periodontitis. J. Periodontol. 1992, 63, 457–468. [Google Scholar] [CrossRef]
- Mandell, R.L.; Siegal, M.D.; Umland, E. Localized juvenile periodontitis of the primary dentition. ASDC J. Dent. Child 1986, 53, 193–196. [Google Scholar]
- Mandell, R.L.; Tripodi, L.S.; Savitt, E.; Goodson, J.M.; Socransky, S.S. The effect of treatment on Actinobacillus actinomycetemcomitans in localized juvenile periodontitis. J. Periodontol. 1986, 57, 94–99. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, T.R. Treatment of juvenile periodontitis. Ont. Dent. 1989, 66, 16–17. [Google Scholar]
- McLain, J.B.; Proffit, W.R.; Davenport, R.H. Adjunctive orthodontic therapy in the treatment of juvenile periodontitis: Report of a case and review of the literature. Am. J. Orthod. 1983, 83, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Mengel, R.; Lehmann, K.M.; Metke, W.; Wolf, J.; Flores-de-Jacoby, L. A telescopic crown concept for the restoration of partially edentulous patients with aggressive generalized periodontitis: Two case reports. Int. J. Periodontics Restor. Dent. 2002, 22, 129–137. [Google Scholar]
- Michel, J.F.; Michel, M.G.; Nadan, J.; Nowzari, H. The street children of Manila are affected by early-in-life periodontal infection: Description of a treatment modality: Sea salt. Refuat Hapeh Vehashinayim (1993) 2013, 30, 6–13. [Google Scholar] [PubMed]
- Mishkin, D.J.; Grant, N.C.; Bergeron, R.A.; Young, W.L. Prepubertal periodontitis: A recently defined clinical entity. Pediatr. Dent. 1986, 8, 235–238. [Google Scholar]
- Modin, C.; Abadji, D.; Adler, L.; Jansson, L. Treatment compliance in patients with aggressive periodontitis—A retrospective case-control study. Acta Odontol. Scand. 2016, 75, 94–99. [Google Scholar] [CrossRef]
- Mombelli, A.; Cionca, N.; Almaghlouth, A.; Décaillet, F.; Courvoisier, D.S.; Giannopoulou, C. Are There Specific Benefits of Amoxicillin Plus Metronidazole in Aggregatibacter actinomycetemcomitans-Associated Periodontitis? Double-Masked, Randomized Clinical Trial of Efficacy and Safety. J. Periodontol. 2013, 84, 715–724. [Google Scholar] [CrossRef]
- Mombelli, A.; Almaghlouth, A.; Cionca, N.; Courvoisier, D.S.; Giannopoulou, C. Differential Benefits of Amoxicillin–Metronidazole in Different Phases of Periodontal Therapy in a Randomized Controlled Crossover Clinical Trial. J. Periodontol. 2015, 86, 367–375. [Google Scholar] [CrossRef]
- Monteiro, M.F.; Tonelli, H.; Reis, A.A.; Casati, M.Z.; Silvério, K.G.; Junior, F.H.N.; Sallum, E.A.; Casarin, R.C.V. Triclosan toothpaste as an adjunct therapy to plaque control in children from periodontitis families: A crossover clinical trial. Clin. Oral Investig. 2020, 24, 1421–1430. [Google Scholar] [CrossRef]
- Morimoto, S.; Hirano, K.; Tabata, K.; Asaumi, H.; Morikawa, Y.; Matsumi, Y.; Naka, S.; Matsumoto-Nakano, M. Case of autoimmune neutropenia with severe marginal periodontitis. Pediatr. Dent. J. 2019, 29, 138–145. [Google Scholar] [CrossRef]
- Muppa, R.; Nallanchakrava, S.; Chinta, M.; Manthena, R.T. Nonsyndromic localized aggressive periodontitis of primary dentition: A rare case report. Contemp. Clin. Dent. 2016, 7, 262–264. [Google Scholar] [CrossRef] [PubMed]
- Myers, D.R.; O’Dell, N.L.; Clark, J.W.; Cross, R.L. Localized prepubertal periodontitis: Literature review and report of case. ASDC J. Dent. Child 1989, 56, 107–111. [Google Scholar] [PubMed]
- Nowzari, H.; Jorgensen, M.G.; Ta, T.T.; Contreras, A.; Slots, J. Aggressive periodontitis associated with Fanconi’s anemia. A case report. J. Periodontol. 2001, 72, 1601–1606. [Google Scholar] [CrossRef] [PubMed]
- Ogunsalu, C.; Daisley, H.; Akpaka, P.E. Prevalence and antimicrobial susceptibility pattern of pathogens isolated from patients with juvenile periodontitis in Jamaica: A prospective multi-centre study of 15 cases over a 15-year period. West. Indian Med. J. 2011, 60, 235–239. [Google Scholar]
- Olgun-Erdemir, E.; Yildirim, M.S.; Karşiyaka, M. Generalized aggressive periodontitis in a child with 92, XXYY / 46,XY mosaicism: Report of a second case. Turk. J. Pediatr. 2010, 52, 94–96. [Google Scholar]
- Page, R.C.; Vandesteen, G.E.; Ebersole, J.L.; Williams, B.L.; Dixon, I.L.; Altman, L.C. Clinical and laboratory studies of a family with a high prevalence of juvenile periodontitis. J. Periodontol. 1985, 56, 602–610. [Google Scholar] [CrossRef]
- Palmer, R.; Watts, T.L.P.; Wilson, R. A double-blind trial of tetracycline in the management of early onset periodontitis. J. Clin. Periodontol. 1996, 23, 670–674. [Google Scholar] [CrossRef]
- Piergallini, G.; Malerba, A.; Mazzucchelli, L.; Strohmenger, L. Generalized aggressive periodontitis in prepubertal age: Description and comparison of two cases. Pediatr. Medica Chir. 2014, 36, 95. [Google Scholar] [CrossRef]
- Roy, E.; Soueidan, A.; Fouassier, M. Multidisciplinary Clinical Management of a Localized Aggressive Periodontitis diagnosed in a Child with Glanzmann\’s Thrombasthenia. Int. J. Clin. Pediatr. Dent. 2018, 11, 344–348. [Google Scholar] [CrossRef]
- Qi, G.; Yu, K.; Feng, Y.; Zhang, Y.; Shao, Q.; Yu, M.; Wang, Y.; Ren, L.; Zhu, D.; Yang, G.; et al. 1α,25-dihydroxyvitamin D3 promotes early osteogenic differentiation of PDLSCs and a 12-year follow-up case of early-onset vitamin D deficiency periodontitis. J. Steroid Biochem. Mol. Biol. 2021, 208, 105805. [Google Scholar] [CrossRef]
- Ram, D.; Bimstein, E. Subgingival bacteria in a case of prepubertal periodontitis, before and one year after extractions of the affected primary teeth. J. Clin. Pediatr. Dent. 1994, 19, 45–47. [Google Scholar]
- Ramich, T.; Asendorf, A.; Nickles, K.; Oremek, G.M.; Schubert, R.; Nibali, L.; Wohlfeil, M.; Eickholz, P. Inflammatory serum markers up to 5 years after comprehensive periodontal therapy of aggressive and chronic periodontitis. Clin. Oral Investig. 2018, 22, 3079–3089. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.A.; Paz, H.E.D.S.; Monteiro, M.D.F.; Casati, M.Z.; Steiner-Oliveira, C.; Pascon, F.M.; Casarin, R.C.V. Early Manifestation of Periodontal Disease in Children and Its Association with Familial Aggregation. J. Dent. Child. 2021, 88, 140–143. [Google Scholar]
- Rosenthal, S.L. Case Report: Periodontosis in a Child Resulting in Exfoliation of the Teeth. J. Periodontol. 1951, 22, 101–104. [Google Scholar] [CrossRef]
- Shaddox, L.; Gonçalves, P.; Vovk, A.; Allin, N.; Huang, H.; Hou, W.; Aukhil, I.; Wallet, S. LPS-induced inflammatory response after therapy of aggressive periodontitis. J. Dent. Res. 2013, 92, 702–708. [Google Scholar] [CrossRef]
- Shapira, L.; Smidt, A.; Van Dyke, T.E.; Barak, V.; Soskolne, A.W.; Brautbar, C.; Sela, M.N.; Bimstein, E. Sequential manifestation of different forms of early-onset periodontitis. A case report. J. Periodontol. 1994, 65, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Sood, P.B.; Sood, M.; Mescarenhas, A.K. Prepubertal periodontitis--a case report. J. Indian Soc. Pedod. Prev. Dent. 1988, 6, 45–47. [Google Scholar] [PubMed]
- Spektor, M.D.; Vandesteen, G.E.; Page, R.C. Clinical studies of one family manifesting rapidly progressive, juvenile and prepubertal periodontitis. J. Periodontol. 1985, 56, 93–101. [Google Scholar] [CrossRef]
- Steiger, J.; Zeevi, A.; Bhola, M. Aggressive periodontitis: A case report. J. Mich. Dent. Assoc. 2008, 90, 40–46. [Google Scholar]
- Suzuki, J.B.; Risom, L.; Falkler, W.A.; Collison, C.; Bowers, G. Effect of periodontal therapy on spontaneous lymphocyte response and neutrophil chemotaxis in localized and generalized juvenile periodontitis patients*. J. Clin. Periodontol. 1985, 12, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, T.T.; Gholami, F.; Huang, H.; Gonçalves, P.F.; Villasante-Tezanos, A.; Aukhil, I.; Oliveira, R.C.G.; Hovencamp, N.; Wallet, S.; Ioannidou, E.; et al. Gender differences in immunological response of African-American juveniles with Grade C molar incisor pattern periodontitis. J. Periodontol. 2021, 93, 392–402. [Google Scholar] [CrossRef]
- Tinoco, E.M.; Beldi, M.I.; Campedelli, F.; Lana, M.; Loureiro, C.A.; Bellini, H.T.; Rams, T.E.; Tinoco, N.M.; Gjermo, P.; Preus, H.R. Clinical and microbiological effects of adjunctive antibiotics in treatment of localized juvenile periodontitis. A controlled clinical trial. J. Periodontol. 1998, 69, 1355–1363. [Google Scholar] [CrossRef]
- Vandana, K.L.; Redy, B.V.R. Prepubertal periodontitis: A report of 2 cases. J. Dent. Child 2003, 70, 82–85. [Google Scholar]
- Watanabe, K. Generalized juvenile periodontitis in a thirteen-year-old child. ASDC J. Dent. Child 1991, 58, 390–395. [Google Scholar] [PubMed]
- Yalçın, S.; Yalçın, F.; Günay, Y.; Bellaz, B.; Önal, Ş.; Firatli, E. Treatment of aggressive periodontitis by osseointegrated dental implants. A case report. J. Periodontol. 2001, 72, 411–416. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).