Multicentre Prospective Study Analysing Relevant Factors Related to Marginal Bone Loss: A Two-Year Evolution
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Statistical Analysis
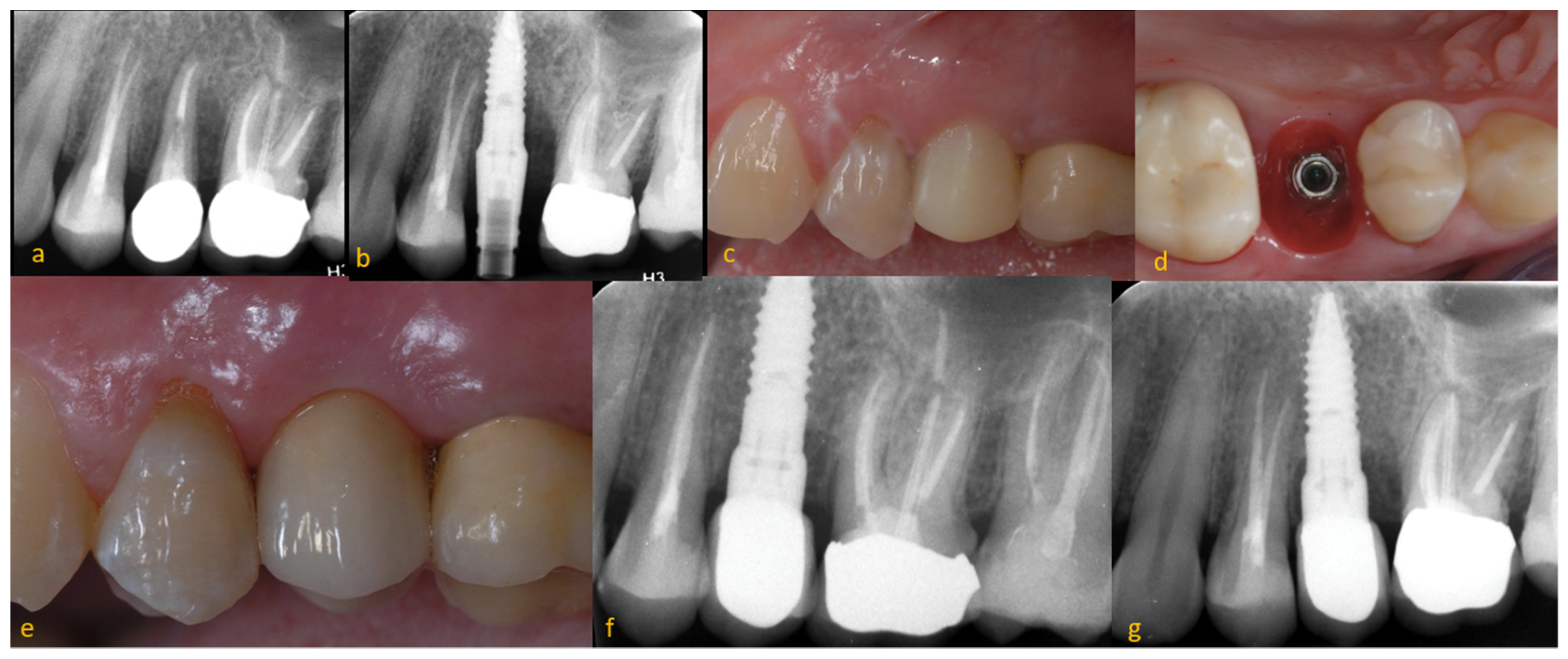
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buser, D.; Belser, U.C.; Schroeder, A. Progress and current trends in oral implantology. Switz. Monatsschr Zahnmed. 1998, 108, 326–350. [Google Scholar]
- Peñarrocha-Diago, M.; Maestre-Ferrin, L.; Cervera-Ballester, J.; Penarrocha-Oltra, D. Implant periapical lesion: Diagnosis and treatment. Med. Oral Patol. Oral Cir. Bucal. 2012, 17, e1023–e1027. [Google Scholar] [CrossRef] [PubMed]
- Atieh, M.A.; Alsabeeha, N.H.; Faggion, C.M., Jr.; Duncan, W.J. The frequency of peri-implant diseases: A systematic review and meta-analysis. J. Periodontol. 2013, 84, 1586–1598. [Google Scholar] [CrossRef] [PubMed]
- Hämmerle, C.H.; Cordaro, L.; Alccayhuaman, K.A.A.; Botticelli, D.; Esposito, M.; Colomina, L.E.; Gil, A.; Gulje, F.L.; Ioannidis, A.; Meijer, H.; et al. Biomechanical aspects: Summary and consensus statements of group 4. The 5th EAO Consensus Conference 2018. Clin. Oral Implant. Res. 2018, 29 (Suppl. S18), 326–331. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Klinge, B.; Alcoforado, G.; Bienz, S.P.; Cosyn, J.; De Bruyn, H.; Derks, J.; Figuero, E.; Gurzawska, K.; Heitz-Mayfield, L.; et al. Biological aspects: Summary and consensus statements of group 2. The 5th EAO Consensus Conference 2018. Clin. Oral Implant. Res. 2018, 29 (Suppl. S18), 152–156. [Google Scholar] [CrossRef]
- Papaspyridakos, P.; Chen, C.J.; Singh, M.; Weber, H.P.; Gallucci, G.O. Success criteria in implant dentistry: A systematic review. J. Dent. Res. 2012, 91, 242–248. [Google Scholar] [CrossRef]
- Albrektsson, T.; Zarb, G.; Worthington, P.; Eriksson, A.R. The long-term efficacy of currently used dental implants: A review and proposed criteria of success. Int. J. Oral Maxillofac. Implants 1986, 1, 11–25. [Google Scholar]
- Sargolzaie, N.; Zarch, H.H.; Arab, H.; Koohestani, T.; Ramandi, M.F. Marginal bone loss around crestal or subcrestal dental implants: Prospective clinical study. J. Korean Assoc. Oral Maxillofac. Surg. 2022, 48, 159–166, Erratum in J. Korean Assoc. Oral Maxillofac. Surg. 2022, 48, 245. [Google Scholar] [CrossRef]
- Palacios-Garzón, N.; Mauri-Obradors, E.; Ayuso-Montero, R.; Velasco-Ortega, E.; Anglada-Cantarell, J.M.; López-López, J. Marginal Bone Loss in Internal Conical Connection Implants Placed at the Crestal and Subcrestal Levels before Prosthetic Loading: A Randomized Clinical Study. Materials 2022, 15, 3729. [Google Scholar] [CrossRef]
- Stacchi, C.; Lamazza, L.; Rapani, A.; Troiano, G.; Messina, M.; Antonelli, A.; Giudice, A.; Lombardi, T. Marginal bone changes around platform-switched conical connection implants placed 1 or 2 mm subcrestally: A multicenter crossover randomized controlled trial. Clin. Implant Dent. Relat. Res. 2023, 25, 398–408. [Google Scholar] [CrossRef]
- Fernández-Olavarria, A.; Gutiérrez-Corrales, A.; González-Martín, M.; Torres-Lagares, D.; Torres-Carranza, E.; Serrera-Figallo, M. Influence of different drilling protocols and bone density on the insertion torque of dental Implants. Med. Oral Patol. Oral Cir. Bucal. 2023, 28, e385–e394. [Google Scholar] [CrossRef]
- Al Amri, M.D.; Al-Johany, S.S.; Al Baker, A.M.; Al Rifaiy, M.Q.; Abduljabbar, T.S.; Al-Kheraif, A.A. Soft tissue changes and crestal bone loss around platform-switched implants placed at crestal and subcrestal levels: 36-month results from a prospective split-mouth clinical trial. Clin. Oral Implants Res. 2017, 28, 1342–1347. [Google Scholar] [CrossRef] [PubMed]
- Fickl, S.; Zuhr, O.; Stein, J.M.; Hurzeler, M.B. Peri-implant bone level around implants with platform-switched abutments. Int. J. Oral Maxillofac. Implants 2010, 25, 577–581. [Google Scholar] [PubMed]
- Misch, K.A.; Yi, E.S.; Sarment, D.P. Accuracy of cone beam computed tomography for periodontal defect measurements. J. Periodontol. 2006, 77, 1261–1266. [Google Scholar] [CrossRef]
- Raes, F.; Renckens, L.; Aps, J.; Cosyn, J.; De Bruyn, H. Reliability of circumferential bone level assessment around single implants in healed ridges and extraction sockets using cone beam CT. Clin. Implant Dent. Relat. Res. 2013, 15, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Azpur, G.; Lau, M.; Valdivia, E.; Rojas, J.; Munoz, H.; Nevins, M. Assessment of Marginal Peri-implant Bone-Level Short-Length Implants Compared with Standard Implants Supporting Single Crowns in a Controlled Clinical Trial: 12-Month Follow-up. Int. J. Periodontics Restor. Dent. 2016, 36, 791–795. [Google Scholar] [CrossRef]
- Corcuera-Flores, J.R.; Alonso-Dominguez, A.M.; Serrera-Figallo, M.A.; Torres-Lagares, D.; Castellanos-Cosano, L.; Machuca-Portillo, G. Relationship Between Osteoporosis and Marginal Bone Loss in Osseointegrated Implants: A 2-Year Retrospective Study. J. Periodontol. 2016, 87, 14–20. [Google Scholar] [CrossRef]
- Penarrocha-Oltra, D.; Palau, I.; Cabanes, G.; Tarazona, B.; Penarrocha-Diago, M. Comparison of digital protocols for the measurement of peri-implant marginal bone loss. J. Clin. Exp. Dent. 2018, 10, e1216–e1222. [Google Scholar] [CrossRef]
- Negri, M.; Galli, C.; Smerieri, A.; Macaluso, G.M.; Manfredi, E.; Ghiacci, G.; Toffoli, A.; Bonanini, M.; Lumetti, S. The effect of age, gender, and insertion site on marginal bone loss around endosseous implants: Results from a 3-year trial with premium implant system. Biomed. Res. Int. 2014, 2014, 369051. [Google Scholar] [CrossRef]
- Koutouzis, T.; Fetner, M.; Fetner, A.; Lundgren, T. Retrospective evaluation of crestal bone changes around implants with reduced abutment diameter placed non-submerged and at subcrestal positions: The effect of bone grafting at implant placement. J. Periodontol. 2011, 82, 234–242. [Google Scholar] [CrossRef]
- Bressan, E.; Grusovin, M.G.; D’Avenia, F.; Neumann, K.; Sbricoli, L.; Luongo, G.; Esposito, M. The influence of repeated abutment changes on peri-implant tissue stability: 3-year post-loading results from a multicenter randomised controlled trial. Eur. J. Oral Implantol. 2017, 10, 373–390. [Google Scholar]
- Khorsand, A.; Rasouli-Ghahroudi, A.A.; Naddafpour, N.; Shayesteh, Y.S.; Khojasteh, A. Effect of Microthread Design on Marginal Bone Level Around Dental Implants Placed in Fresh Extraction Sockets. Implant. Dent. 2016, 25, 90–96. [Google Scholar] [CrossRef]
- Kadkhodazadeh, M.; Heidari, B.; Abdi, Z.; Mollaverdi, F.; Amid, R. Radiographic evaluation of marginal bone levels around dental implants with different designs after 1 year. Acta Odontol. Scand. 2013, 71, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Maghaireh, H.; Pistilli, R.; Grusovin, M.G.; Lee, S.T.; Gualini, F.; Yoo, J.; Buti, J. Dental implants with internal versus external connections: 1-year post-loading results from a pragmatic multicenter randomised controlled trial. Eur. J. Oral Implantol. 2015, 8, 331–344. [Google Scholar] [CrossRef]
- Esposito, M.; Maghaireh, H.; Pistilli, R.; Grusovin, M.G.; Lee, S.T.; Trullenque-Eriksson, A.; Gualini, F. Dental implants with internal versus external connections: 5-year post-loading results from a pragmatic multicenter randomised controlled trial. Eur. J. Oral Implantol. 2016, 9 (Suppl. S1), 129–141. [Google Scholar] [CrossRef]
- Cooper, L.F.; Tarnow, D.; Froum, S.; Moriarty, J.; De Kok, I.J. Comparison of Marginal Bone Changes with Internal Conus and External Hexagon Design Implant Systems: A Prospective, Randomized Study. Int. J. Periodontics Restor. Dent. 2016, 36, 631–642. [Google Scholar] [CrossRef]
- Pessoa, R.S.; Sousa, R.M.; Pereira, L.M.; Neves, F.D.; Bezerra, F.J.; Jaecques, S.V.; Sloten, J.V.; Quirynen, M.; Teughels, W.; Spin-Neto, R. Bone Remodeling Around Implants with External Hexagon and Morse-Taper Connections: A Randomized, Controlled, Split-Mouth, Clinical Trial. Clin. Implant Dent. Relat. Res. 2017, 19, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Becker, W.; Becker, B.E.; Hujoel, P.; Abu Ras, Z.; Goldstein, M.; Smidt, A. Prospective clinical trial evaluating a new implant system for implant survival, implant stability and radiographic bone changes. Clin. Implant Dent. Relat. Res. 2013, 15, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Felice, P.; Barausse, C.; Blasone, R.; Favaretto, G.; Stacchi, C.; Calvo, M.; Marin, C.; Buti, J.; Esposito, M. A comparison of two dental implant systems in partially edentulous patients: 1-year post-loading results from a pragmatic multicentre randomised controlled trial. Eur. J. Oral Implantol. 2014, 7, 397–409. [Google Scholar]
- Vercruyssen, M.; van de Wiele, G.; Teughels, W.; Naert, I.; Jacobs, R.; Quirynen, M. Implant- and patient-centred outcomes of guided surgery, a 1-year follow-up: An RCT comparing guided surgery with conventional implant placement. J. Clin. Periodontol. 2014, 41, 1154–1160. [Google Scholar] [CrossRef]
- Jung, R.E.; Grohmann, P.; Sailer, I.; Steinhart, Y.N.; Fehér, A.; Hämmerle, C.; Strub, J.R.; Kohal, R. Evaluation of a one-piece ceramic implant used for single-tooth replacement and three-unit fixed partial dentures: A prospective cohort clinical trial. Clin. Oral Implants Res. 2016, 27, 751–761. [Google Scholar] [CrossRef]
- Den Hartog, L.; Meijer, H.J.A.; Vissink, A.; Raghoebar, G.M. Anterior single implants with different neck designs: 5 Year results of a randomized clinical trial. Clin. Implant Dent. Relat. Res. 2017, 19, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Sahrmann, P.; Naenni, N.; Jung, R.E.; Held, U.; Truninger, T.; Hämmerle, C.H.F.; Attin, T.; Schmidlin, P.R. Success of 6-mm Implants with Single-Tooth Restorations: A 3-year Randomized Controlled Clinical Trial. J. Dent. Res. 2016, 95, 623–628. [Google Scholar] [CrossRef]
- Gulje, F.L.; Raghoebar, G.M.; Vissink, A.; Meijer, H.J. Single crowns in the resorbed posterior maxilla supported by either 6-mm implants or by 11-mm implants combined with sinus floor elevation surgery: A 1-year randomised controlled trial. Eur. J. Oral Implantol. 2014, 7, 247–255. [Google Scholar] [PubMed]
- Schincaglia, G.P.; Rubin, S.; Thacker, S.; Dhingra, A.; Trombelli, L.; Ioannidou, E. Marginal Bone Response Around Immediate- and Delayed-Loading Implants Supporting a Locator-Retained Mandibular Overdenture: A Randomized Controlled Study. Int. J. Oral Maxillofac. Implants 2016, 31, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Felice, P.; Checchi, L.; Barausse, C.; Pistilli, R.; Sammartino, G.; Masi, I.; Ippolito, D.R.; Esposito, M. Posterior jaws rehabilitated with partial prostheses supported by 4.0 x 4.0 mm or by longer implants: One-year post-loading results from a multicenter randomised controlled trial. Eur. J. Oral Implantol. 2016, 9, 35–45. [Google Scholar]
- Hadzik, J.; Krawiec, M.; Slawecki, K.; Kunert-Keil, C.; Dominiak, M.; Gedrange, T. The Influence of the Crown-Implant Ratio on the Crestal Bone Level and Implant Secondary Stability: 36-Month Clinical Study. Biomed. Res. Int. 2018, 2018, 4246874. [Google Scholar] [CrossRef]
- Ryu, H.S.; Namgung, C.; Heo, Y.K.; Lee, J.H.; Lim, Y.J. Early loading of splinted implants supporting a two-unit fixed partial denture in the posterior maxilla: 13-month results from a randomized controlled clinical trial of two different implant systems. Clin. Oral Implants Res. 2016, 27, 1017–1025. [Google Scholar] [CrossRef]
- Patil, R.C.; den Hartog, L.; van Heereveld, C.; Jagdale, A.; Dilbaghi, A.; Cune, M.S. Comparison of two different abutment designs on marginal bone loss and soft tissue development. Int. J. Oral Maxillofac. Implants 2014, 29, 675–681. [Google Scholar] [CrossRef]
- Koutouzis, T.; Koutouzis, G.; Gadalla, H.; Neiva, R. The effect of healing abutment reconnection and disconnection on soft and hard peri-implant tissues: A short-term randomized controlled clinical trial. Int. J. Oral Maxillofac. Implants 2013, 28, 807–814. [Google Scholar] [CrossRef]
- Esposito, M.; Bressan, E.; Grusovin, M.G.; D’Avenia, F.; Neumann, K.; Sbricoli, L.; Luongo, G. Do repeated changes of abutments have any influence on the stability of peri-implant tissues? One-year post-loading results from a multicenter randomised controlled trial. Eur. J. Oral Implantol. 2017, 10, 57–72. [Google Scholar]
- Pisoni, L.; Ordesi, P.; Siervo, P.; Bianchi, A.E.; Persia, M.; Siervo, S. Flapless Versus Traditional Dental Implant Surgery: Long-Term Evaluation of Crestal Bone Resorption. J. Oral Maxillofac. Surg. 2016, 74, 1354–1359. [Google Scholar] [CrossRef]
- Froum, S.J.; Khouly, I. Survival Rates and Bone and Soft Tissue Level Changes Around One-Piece Dental Implants Placed with a Flapless or Flap Protocol: 8.5-Year Results. Int. J. Periodontics Restor. Dent. 2017, 37, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Simunek, A.; Strnad, J.; Kopecka, D.; Brazda, T.; Pilathadka, S.; Chauhan, R.; Slezak, R.; Capek, L. Changes in stability after healing of immediately loaded dental Implants. Int. J. Oral Maxillofac. Implants 2010, 25, 1085–1092. [Google Scholar] [PubMed]
- Monje, A.; Ravidà, A.; Wang, H.L.; Helms, J.A.; Brunski, J.B. Relationship Between Primary/Mechanical and Secondary/Biological Implant Stability. Int. J. Oral Maxillofac. Implants 2019, 34, s7–s23. [Google Scholar] [CrossRef]
- Barone, A.; Alfonsi, F.; Derchi, G.; Tonelli, P.; Toti, P.; Marchionni, S.; Covani, U. The Effect of Insertion Torque on the Clinical Outcome of Single Implants: A Randomized Clinical Trial. Clin. Implant Dent. Relat. Res. 2016, 18, 588–600. [Google Scholar] [CrossRef] [PubMed]
- Stacchi, C.; Troiano, G.; Montaruli, G.; Mozzati, M.; Lamazza, L.; Antonelli, A.; Giudice, A.; Lombardi, T. Changes in implant stability using different site preparation techniques: Osseodensification drills versus piezoelectric surgery. A multi-center prospective randomized controlled clinical trial. Clin. Implant Dent. Relat. Res. 2023, 25, 133–140. [Google Scholar] [CrossRef] [PubMed]

| n | % | ||
|---|---|---|---|
| Maxillary—mandibular position | Maxillary | 119 | 54.6 |
| Mandibular | 99 | 45.4 | |
| Anteroposterior position | Anterior | 100 | 45.9 |
| Posterior | 118 | 54.1 | |
| “Crossed” position | Maxillary—anterior | 57 | 26.1 |
| Maxillary—posterior | 62 | 28.4 | |
| Mandibular—anterior | 43 | 19.7 | |
| Mandibular—posterior | 56 | 25.7 | |
| Implant model | Internal conical | 39 | 17.9 |
| External conical | 133 | 61.0 | |
| Internal cylindrical | 0 | 0.0 | |
| External cylindrical | 46 | 21.1 | |
| Diameter | Up to 4 mm | 197 | 90.4 |
| More than 4 mm | 21 | 9.6 | |
| Mean | S.D. | ||
| 3.94 | 0.25 | ||
| Length | Up to 10 mm | 24 | 11.0 |
| More than 10 mm | 194 | 89.0 | |
| Mean | S.D. | ||
| 12.61 | 1.50 | ||
| Bone type | D1 | 41 | 18.8 |
| D2 | 108 | 49.5 | |
| D3 | 67 | 30.7 | |
| D4 | 2 | 0.9 | |
| Type of prosthetic connection | Direct to implant | 39 | 17.9 |
| Transepithelial straight | 170 | 78.0 | |
| Transepithelial angled | 9 | 4.1 | |
| Multi-unit | 0 | 0.0 | |
| Restoration type | Full | 193 | 88.5 |
| Partial | 13 | 6.0 | |
| Unitary | 12 | 5.5 | |
| Torque > 35 N/cm | Yes | 203 | 93.1 |
| No | 15 | 6.9 | |
| Crestal expansion | Yes | 0 | 0.0 |
| No | 218 | 100.0 | |
| Failure (2 months) | Yes | 4 | 1.8 |
| No | 214 | 98.2 | |
| Cause of failure (2 months) | Mobility | 3 | 75.0 |
| Peri-implant infection | 1 | 25.0 | |
| Failure (6 months) | Yes | 4 | 1.8 |
| No | 214 | 98.2 | |
| Cause of failure (6 months) | Mobility | 3 | 75.0 |
| Peri-implant infection | 1 | 25.0 | |
| Failure (12 months) | Yes | 4 | 1.8 |
| No | 214 | 98.2 | |
| Cause of failure (12 months) | Mobility | 3 | 75.0 |
| Peri-implant infection | 1 | 25.0 | |
| Failure (24 months) | Yes | 4 | 1.8 |
| No | 214 | 98.2 | |
| Cause of failure (24 months) | Mobility | 3 | 75.0 |
| Peri-implant infection | 1 | 25.0 |
| Variable | No. | Mean | S.D. | Normality |
|---|---|---|---|---|
| ISQ initial value | 97 | 74.55 | 7.96 | Yes |
| ISQ osseointegration value | 90 | 80.81 | 5.77 | No |
| ISQ difference (osseointegration—initial) | 90 | 6.54 | 7.74 | Yes |
| Bone loss at two months (mm) | 214 | 0.09 | 0.20 | No |
| Bone loss at six months (mm) | 214 | 0.20 | 0.26 | No |
| Bone loss at 12 months (mm) | 214 | 0.45 | 0.41 | No |
| Bone loss at 24 months (mm) | 214 | 0.65 | 0.59 | No |
| Bone loss difference (2–6 months) (mm) | 214 | 0.11 | 0.25 | No |
| Bone loss difference (2–12 months) (mm) | 214 | 0.36 | 0.42 | No |
| Bone loss difference (2–24 months) (mm) | 214 | 0.56 | 0.60 | No |
| Bone loss difference (6–12 months) (mm) | 214 | 0.25 | 0.38 | No |
| Bone loss difference (6–24 months) (mm) | 214 | 0.45 | 0.55 | No |
| Bone loss difference (12–24 months) (mm) | 214 | 0.20 | 0.30 | No |
| Bone Loss m | 2–6 Months | 2–12 Months | 2–24 Months | ||||
|---|---|---|---|---|---|---|---|
| Variable | Mean | Standard Deviation | Mean | Standard Deviation | Mean | Standard Deviation | |
| Sample | 0.11 | 0.25 | 0.36 | 0.42 | 0.56 | 0.60 | |
| Gender | |||||||
| Female | 0.14 | 0.24 | 0.47 | 0.43 | 0.74 | 0.61 | |
| Male | 0.07 | 0.25 | 0.22 | 0.37 | 0.34 | 0.51 | |
| p | <0.05 | <0.0001 | <0.0001 | ||||
| Age | |||||||
| Under 55 | 0.06 | 0.24 | 0.14 | 0.41 | 0.25 | 0.56 | |
| From 55 to 64 years | 0.15 | 0.27 | 0.47 | 0.42 | 0.74 | 0.57 | |
| 65 or over | 0.09 | 0.19 | 0.43 | 0.35 | 0.63 | 0.55 | |
| p | - | <0.0001 | <0.0001 | ||||
| Location | |||||||
| Maxillary—anterior | 0.12 | 0.23 | 0.38 | 0.41 | 0.57 | 0.58 | |
| Maxillary—posterior | 0.12 | 0.31 | 0.33 | 0.42 | 0.49 | 0.58 | |
| Mand—anterior | 0.12 | 0.22 | 0.42 | 0.41 | 0.65 | 0.57 | |
| Mand—posterior | 0.08 | 0.19 | 0.32 | 0.45 | 0.55 | 0.67 | |
| p | - | - | - | ||||
| Implant Model | |||||||
| Internal conical | 0.07 | 0.17 | 0.14 | 0.23 | 0.17 | 0.24 | |
| External conical | 0.10 | 0.25 | 0.29 | 0.42 | 0.48 | 0.61 | |
| External cylindrical | 0.17 | 0.26 | 0.73 | 0.33 | 1.12 | 0.32 | |
| p | - | <0.0001 | <0.0001 | ||||
| Implant Diameter | |||||||
| Up to 4 mm | 0.10 | 0.24 | 0.32 | 0.42 | 0.49 | 0.58 | |
| More than 4 mm | 0.19 | 0.25 | 0.69 | 0.33 | 1.21 | 0.30 | |
| p | - | <0.001 | <0.0001 | ||||
| Implant Length | |||||||
| Up to 10 mm | 0.21 | 0.33 | 0.44 | 0.54 | 0.71 | 0.64 | |
| More than 10 mm | 0.10 | 0.23 | 0.35 | 0.41 | 0.54 | 0.59 | |
| p | <0.05 | - | - | ||||
| Bone Type | |||||||
| D1 | 0.08 | 0.18 | 0.46 | 0.43 | 0.75 | 0.62 | |
| D2 | 0.11 | 0.23 | 0.28 | 0.39 | 0.43 | 0.57 | |
| D3 | 0.11 | 0.29 | 0.42 | 0.45 | 0.65 | 0.60 | |
| p | - | <0.05 | <0.01 | ||||
| Type Of Prosthetic Connection | |||||||
| Direct to implant | 0.00 | 0.26 | 0.03 | 0.41 | 0.11 | 0.58 | |
| Transepithelial straight | 0.13 | 0.23 | 0.43 | 0.40 | 0.67 | 0.57 | |
| Transepithelial angled | 0.22 | 0.26 | 0.33 | 0.25 | 0.33 | 0.25 | |
| p | <0.01 | <0.0001 | <0.0001 | ||||
| Type of Rehabilitation | |||||||
| Full | 0.12 | 0.23 | 0.38 | 0.41 | 0.59 | 0.59 | |
| Partial | −0.04 | 0.40 | 0.25 | 0.69 | 0.50 | 0.85 | |
| Unitary | 0.04 | 0.14 | 0.08 | 0.19 | 0.08 | 0.19 | |
| p | - | <0.05 | <0.05 | ||||
| Insertion Torque > 35 N/cm | |||||||
| Yes | 0.10 | 0.23 | 0.34 | 0.42 | 0.53 | 0.58 | |
| No | 0.31 | 0.33 | 0.62 | 0.46 | 1.04 | 0.63 | |
| p | <0.01 | <0.05 | <0.01 | ||||
| Bone Loss m | 2–6 Months | 2–12 Months | 2–24 Months | ||||
|---|---|---|---|---|---|---|---|
| Variable | Median | IQ | Median | IQ | Median | IQ | |
| Sample | 0.00 | [0.00–0.00] | 0.50 | [0.00–0.50] | 0.50 | [0.00–1.00] | |
| Gender | |||||||
| Female | 0.00 | [0.00–0.50] | 0.50 | [0.00–1.00] | 1.00 | [0.00–1.25] | |
| Male | 0.00 | [0.00–0.00] | 0.00 | [0.00–0.50] | 0.00 | [0.00–1.00] | |
| p | <0.05 | <0.0001 | <0.0001 | ||||
| Age | |||||||
| Under 55 | 0.00 | [0.00–0.00] | 0.00 | [0.00–0.50] | 0.00 | [0.00–0.50] | |
| From 55 to 64 years | 0.00 | [0.00–0.50] | 0.50 | [0.00–1.00] | 1.00 | [0.00–1.00] | |
| 65 or over | 0.00 | [0.00–0.00] | 0.50 | [0.00–0.50] | 0.50 | [0.00–1.00] | |
| p | - | <0.0001 | <0.0001 | ||||
| Location | |||||||
| Maxillary—anterior | 0.00 | [0.00–0.38] | 0.50 | [0.00–0.50] | 0.50 | [0.00–1.00] | |
| Maxillary—posterior | 0.00 | [0.00–0.50] | 0.50 | [0.00–0.50] | 0.50 | [0.00–1.00] | |
| Mand—anterior | 0.00 | [0.00–0.13] | 0.50 | [0.00–1.00] | 0.75 | [0.00–1.00] | |
| Mand—posterior | 0.00 | [0.00–0.00] | 0.00 | [0.00–0.50] | 0.50 | [0.00–1.00] | |
| p | - | - | - | ||||
| Implant Model | |||||||
| Internal conical | 0.00 | [0.00–0.00] | 0.00 | [0.00–0.50] | 0.00 | [0.00–0.50] | |
| External conical | 0.00 | [0.00–0.00] | 0.00 | [0.00–0.50] | 0.50 | [0.00–1.00] | |
| External cylindrical | 0.00 | [0.00–0.50] | 1.00 | [0.50–1.00] | 1.00 | [1.00–1.50] | |
| p | - | <0.0001 | <0.0001 | ||||
| Implant Diameter | |||||||
| Up to 4 mm | 0.00 | [0.00–0.00] | 0.00 | [0.00–0.50] | 0.50 | [0.00–1.00] | |
| More than 4 mm | 0.00 | [0.00–0.50] | 0.50 | [0.50–1.00] | 1.00 | [1.00–1.50] | |
| p | - | <0.001 | <0.0001 | ||||
| Implant Length | |||||||
| Up to 10 mm | 0.00 | [0.00–0.50] | 0.50 | [0.00–1.00] | 1.00 | [0.13–1.00] | |
| More than 10 mm | 0.00 | [0.00–0.00] | 0.50 | [0.00–0.50] | 0.50 | [0.00–1.00] | |
| p | <0.05 | - | - | ||||
| Bone Type | |||||||
| D1 | 0.00 | [0.00–0.00] | 0.50 | [0.00–1.00] | 1.00 | [0.00–1.38] | |
| D2 | 0.00 | [0.00–0.00] | 0.00 | [0.00–0.50] | 0.00 | [0.00–1.00] | |
| D3 | 0.00 | [0.00–0.50] | 0.50 | [0.00–1.00] | 0.75 | [0.00–1.00] | |
| p | - | <0.05 | <0.01 | ||||
| Type Of Prosthethic Connection | |||||||
| Direct to implant | 0.00 | [0.00–0.00] | 0.00 | [0.00–0.00] | 0.00 | [0.00–0.00] | |
| Transepithelial straight | 0.00 | [0.00–0.50] | 0.50 | [0.00–0.95] | 0.50 | [0.00–1.00] | |
| Transepithelial angled | 0.00 | [0.00–0.50] | 0.50 | [0.00–0.50] | 0.50 | [0.00–0.50] | |
| p | <0.01 | <0.0001 | <0.0001 | ||||
| Type of Rehabilitation | |||||||
| Full | 0.00 | [0.00–0.50] | 0.50 | [0.00–0.50] | 0.50 | [0.00–1.00] | |
| Partial | 0.00 | [0.00–0.00] | 0.25 | [−0.38–1.00] | 1.00 | [−0.38–1.00] | |
| Unitary | 0.00 | [0.00–0.00] | 0.00 | [0.00–0.00] | 0.00 | [0.00–0.00] | |
| p | - | <0.05 | <0.05 | ||||
| Insertion Torque > 35 N/cm | |||||||
| Yes | 0.00 | [0.00–0.00] | 0.50 | [0.00–0.50] | 0.50 | [0.00–1.00] | |
| No | 0.50 | [0.00–0.50] | 1.00 | [0.00–1.00] | 1.50 | [0.50–1.50] | |
| p | <0.01 | <0.05 | <0.01 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Figares-Conde, I.; Castellanos-Cosano, L.; Fernandez-Ruiz, J.-A.; Soriano-Santamaria, I.; Hueto-Madrid, J.-A.; Gómez-Lagunas, J.; Romano-Laureato, R.; Torres-Lagares, D. Multicentre Prospective Study Analysing Relevant Factors Related to Marginal Bone Loss: A Two-Year Evolution. Dent. J. 2023, 11, 185. https://doi.org/10.3390/dj11080185
Fernández-Figares-Conde I, Castellanos-Cosano L, Fernandez-Ruiz J-A, Soriano-Santamaria I, Hueto-Madrid J-A, Gómez-Lagunas J, Romano-Laureato R, Torres-Lagares D. Multicentre Prospective Study Analysing Relevant Factors Related to Marginal Bone Loss: A Two-Year Evolution. Dentistry Journal. 2023; 11(8):185. https://doi.org/10.3390/dj11080185
Chicago/Turabian StyleFernández-Figares-Conde, Iñigo, Lizett Castellanos-Cosano, Juan-Alberto Fernandez-Ruiz, Ismael Soriano-Santamaria, Juan-Antonio Hueto-Madrid, Javier Gómez-Lagunas, Roberto Romano-Laureato, and Daniel Torres-Lagares. 2023. "Multicentre Prospective Study Analysing Relevant Factors Related to Marginal Bone Loss: A Two-Year Evolution" Dentistry Journal 11, no. 8: 185. https://doi.org/10.3390/dj11080185
APA StyleFernández-Figares-Conde, I., Castellanos-Cosano, L., Fernandez-Ruiz, J.-A., Soriano-Santamaria, I., Hueto-Madrid, J.-A., Gómez-Lagunas, J., Romano-Laureato, R., & Torres-Lagares, D. (2023). Multicentre Prospective Study Analysing Relevant Factors Related to Marginal Bone Loss: A Two-Year Evolution. Dentistry Journal, 11(8), 185. https://doi.org/10.3390/dj11080185







