Effect of Dentin Conditioning with EDTA and Diode Lasers on Expression of Odontoblast-like Cell Markers of Dental Pulp Stem Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Laser System
2.2. Preparation of Dentin Cylinders
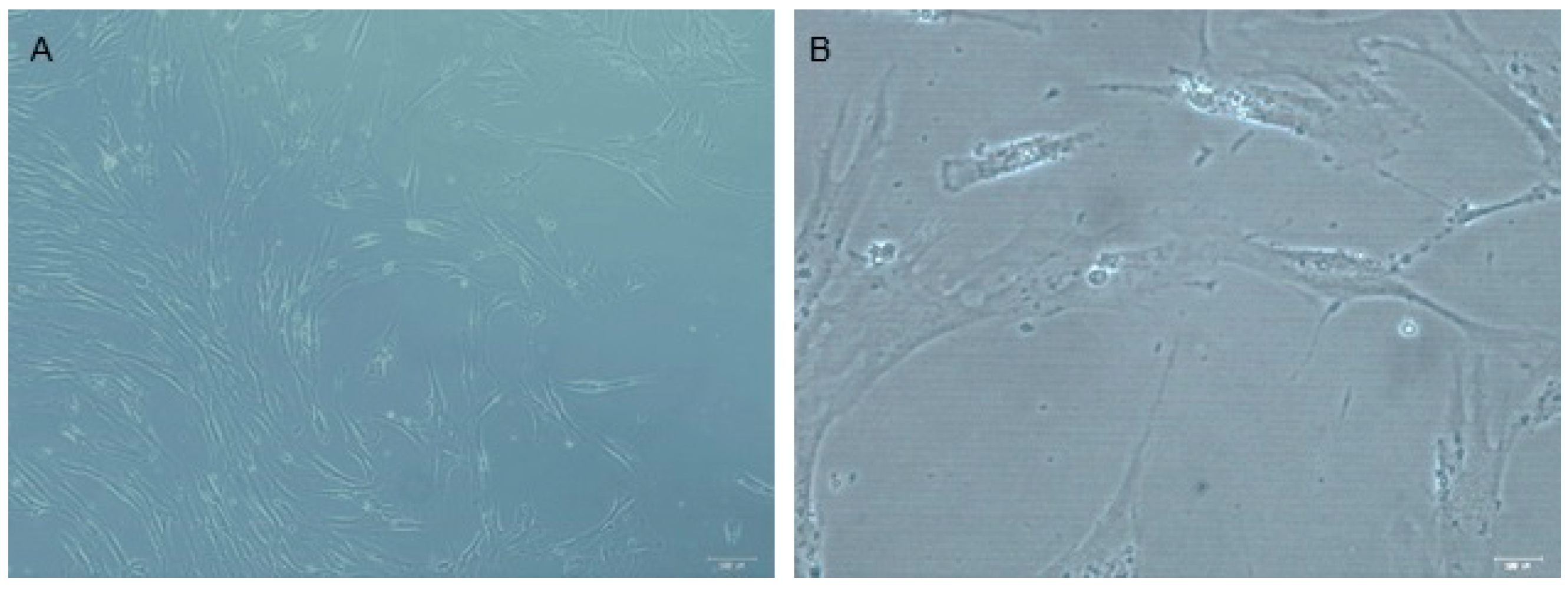
2.3. Isolation and DPSC Culture
2.4. Immunophenotypical Profile by Flow Cytometry
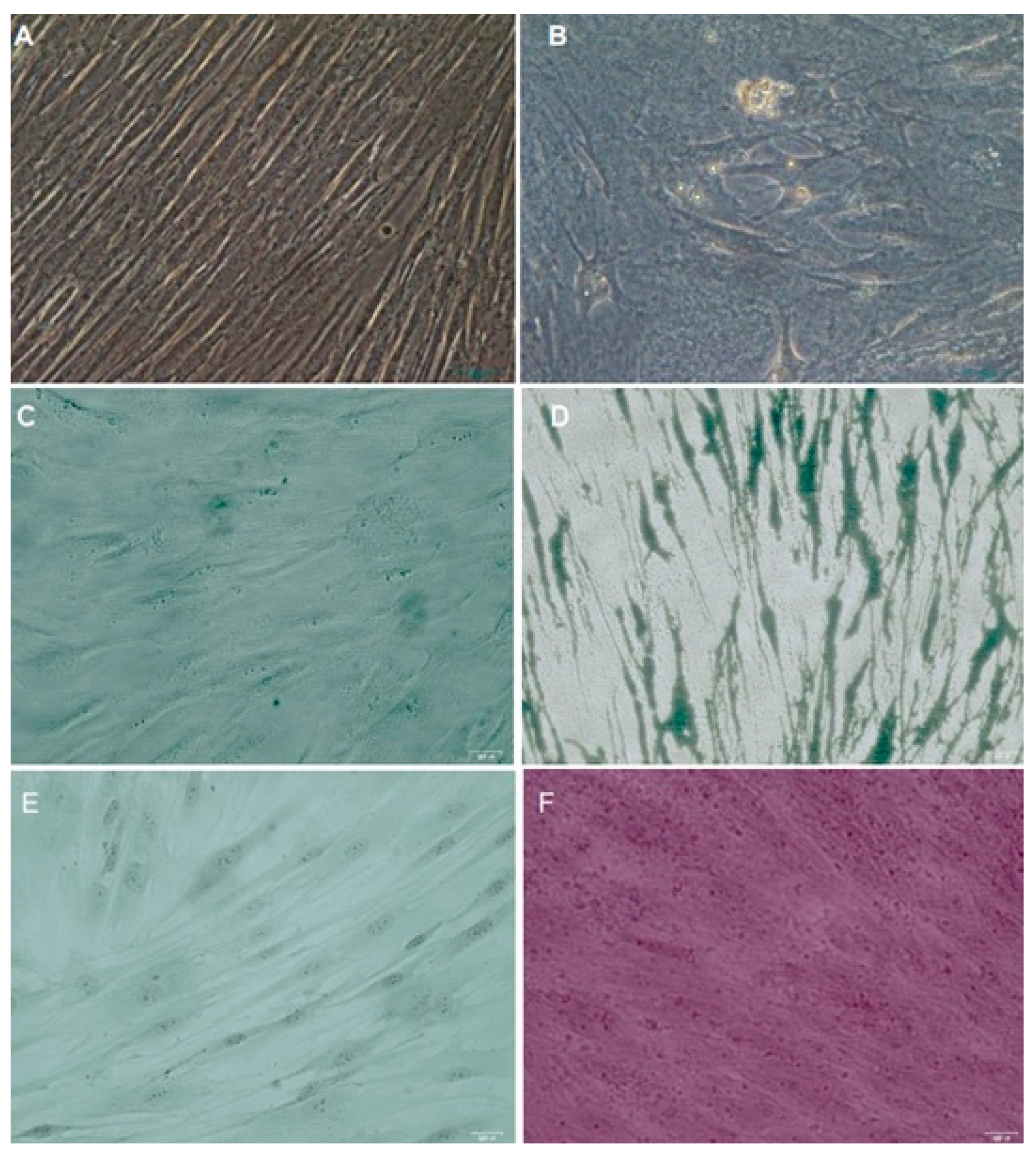
2.5. Differentiation Assays
2.5.1. Osteogenic Differentiation
2.5.2. Chondrogenic Differentiation
2.5.3. Adipogenic Differentiation
2.6. Irrigation and Laser Application
- -
- 1: phosphate-buffered saline (PBS);
- -
- 2: 17% EDTA;
- -
- 3: 17% EDTA + 808 nm GaAlAs diode laser;
- -
- 4: 17% EDTA + 980 nm GaAlAs diode laser.
2.7. Expression of Odontoblast-like Cell Markers
2.8. Statistical Analysis
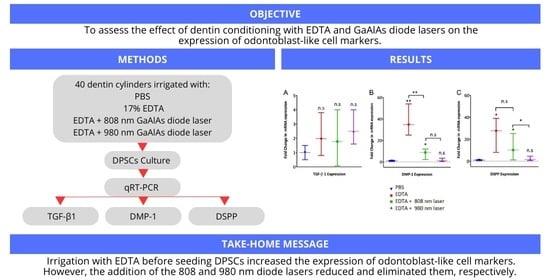
3. Results
3.1. Immunophenotypical Profile by Flow Cytometry
3.2. Differentiation Assays
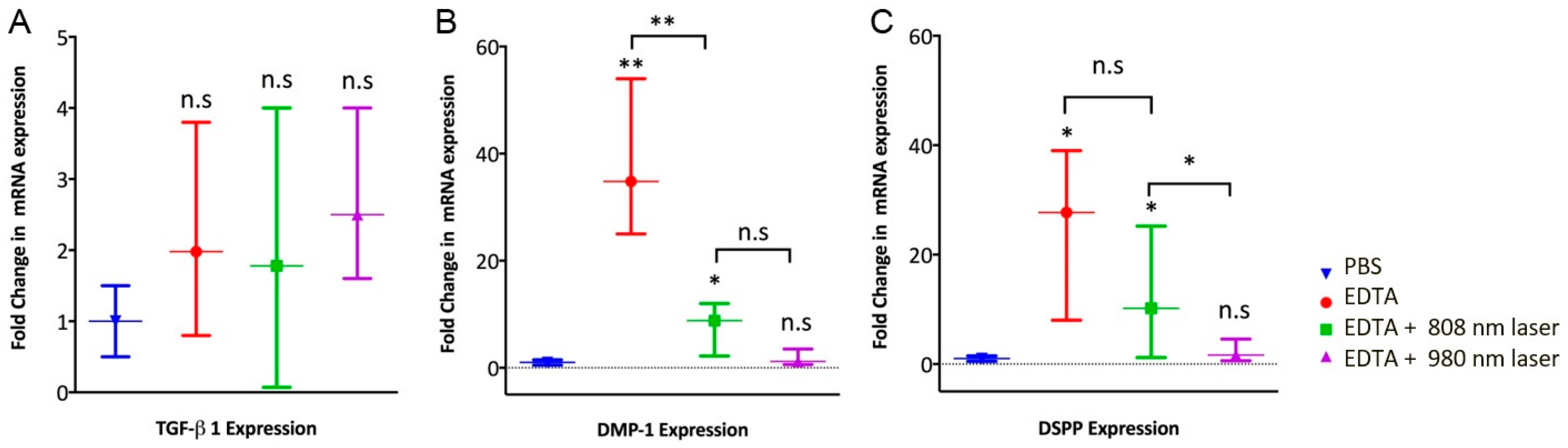
3.3. Expression of Odontoblast-like Cell Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iwaya, S.I.; Ikawa, M.; Kubota, M. Revascularization of an immature permanent tooth with apical periodontitis and sinus tract. Dent. Traumatol. 2001, 17, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Paryani, K.; Kim, S.G. Regenerative endodontic treatment of permanent teeth after completion of root development: A report of 2 cases. J. Endod. 2013, 39, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Lenzi, R.; Trope, M. Revitalization procedures in two traumatized incisors with different biological outcomes. J. Endod. 2012, 38, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Lovelace, T.W.; Henry, M.A.; Hargreaves, K.M.; Diogenes, A. Evaluation of the delivery of mesenchymal stem cells into the root canal space of necrotic immature teeth after clinical regenerative endodontic procedure. J. Endod. 2011, 37, 133–138. [Google Scholar] [CrossRef]
- Smith, A.J.; Scheven, B.A.; Takahashi, Y.; Ferracane, J.L.; Shelton, R.M.; Cooper, P.R. Dentine as a bioactive extracellular matrix. Arch. Oral Biol. 2012, 57, 109–121. [Google Scholar] [CrossRef]
- Ring, K.C.; Murray, P.E.; Namerow, K.N.; Kuttler, S.; Garcia-Godoy, F. The comparison of the effect of endodontic irrigation on cell adherence to root canal dentin. J. Endod. 2008, 34, 1474–1479. [Google Scholar] [CrossRef]
- Trevino, E.G.; Patwardhan, A.N.; Henry, M.A.; Perry, G.; Dybdal-Hargreaves, N.; Hargreaves, K.M.; Diogenes, A. Effect of irrigants on the survival of human stem cells of the apical papilla in a platelet-rich plasma scaffold in human root tips. J. Endod. 2011, 37, 1109–1115. [Google Scholar] [CrossRef]
- Galler, K.M.; D’Souza, R.N.; Federlin, M.; Cavender, A.C.; Hartgerink, J.D.; Hecker, S.; Schmalz, G. Dentin conditioning codetermines cell fate in regenerative endodontics. J. Endod. 2011, 37, 1536–1541. [Google Scholar] [CrossRef]
- Martin, D.E.; De Almeida, J.F.; Henry, M.A.; Khaing, Z.Z.; Schmidt, C.E.; Teixeira, F.B.; Diogenes, A. Concentration-dependent effect of sodium hypochlorite on stem cells of apical papilla survival and differentiation. J. Endod. 2014, 40, 51–55. [Google Scholar] [CrossRef]
- Pimentel Corrêa, A.C.; Cecchin, D.; de Almeida, J.F.; Gomes, B.P.; Zaia, A.A.; Ferraz, C.C. Sodium thiosulfate for recovery of bond strength to dentin treated with sodium hypochlorite. J. Endod. 2016, 42, 284–288. [Google Scholar] [CrossRef]
- Sahebi, S.; Sobhnamayan, F.; Moazami, F.; Naseri, M. Assessment of sodium thiosulfate neutralizing effect on micro-hardness of dentin treated with sodium hypochlorite. BMC Oral Health 2020, 20, 326. [Google Scholar] [CrossRef] [PubMed]
- Alfadda, S.; Alquria, T.; Karaismailoglu, E.; Aksel, H.; Azim, A.A. Antibacterial effect and bioactivity of innovative and currently used intracanal medicaments in regenerative endodontics. J. Endod. 2021, 47, 1294–1300. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Maruthingal, S.; Indira, R.; Divakar, D.D.; Al Kheraif, A.A.; Ramakrishnaiah, R.; Durgesh, B.H.; Basavarajappa, S.; John, J. Photoactivated disinfection (PAD) of dental root canal system—An ex-vivo study. Saudi J. Biol. Sci. 2016, 23, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Gutknecht, N.; Franzen, R.; Schippers, M.; Lampert, F. Bactericidal effect of a 980-nm diode laser in the root canal wall dentin of bovine teeth. J. Clin. Laser Med. Surg. 2004, 22, 9–13. [Google Scholar] [CrossRef]
- De Souza, E.B.; Cai, S.; Simionato, M.R.; Lage-Marques, J.L. High-power diode laser in the disinfection in depth of the root canal dentin. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008, 106, 68–72. [Google Scholar] [CrossRef]
- Kreisler, M.; Kohnen, W.; Beck, M.; Al Haj, H.; Christoffers, A.B.; Götz, H.; Duschner, H.; Jansen, B.; D’Hoedt, B. Efficacy of NaOCl/H2O2 irrigation and GaAlAs laser in decontamination of root canals in vitro. Lasers Surg. Med. 2003, 32, 189–196. [Google Scholar] [CrossRef]
- Bago, I.; Plečko, V.; Gabrić Pandurić, D.; Schauperl, Z.; Baraba, A.; Anić, I. Antimicrobial efficacy of a high-power diode laser, photo-activated disinfection, conventional and sonic activated irrigation during root canal treatment. Int. Endod. J. 2013, 46, 339–347. [Google Scholar] [CrossRef]
- Arslan, H.; Ayrancı, L.B.; Karatas, E.; Topçuoğlu, H.S.; Yavuz, M.S.; Kesim, B. Effect of agitation of EDTA with 808-nanometer diode laser on removal of smear layer. J. Endod. 2013, 39, 1589–1592. [Google Scholar] [CrossRef]
- Viapiana, R.; Sousa-Neto, M.D.; Souza-Gabriel, A.E.; Alfredo, E.; Silva-Sousa, Y.T. Microhardness of radicular dentin treated with 980-nm diode laser and different irrigant solutions. Photomed. Laser Surg. 2012, 30, 102–106. [Google Scholar] [CrossRef]
- Faria, M.I.; Sousa-Neto, M.D.; Souza-Gabriel, A.E.; Alfredo, E.; Romeo, U.; Silva-Sousa, Y.T. Effects of 980-nm diode laser on the ultrastructure and fracture resistance of dentine. Lasers Med. Sci. 2013, 28, 275–280. [Google Scholar] [CrossRef]
- Lopes, F.C.; Roperto, R.; Akkus, A.; Akkus, O.; Souza-Gabriel, A.E.; Sousa-Neto, M.D. Effects of different lasers on organic/inorganic ratio of radicular dentin. Lasers Med. Sci. 2016, 31, 415–420. [Google Scholar] [CrossRef]
- Martin, G.; Ricucci, D.; Gibbs, J.L.; Lin, L.M. Histological findings of revascularized/revitalized immature permanent molar with apical periodontitis using platelet-rich plasma. J. Endod. 2013, 39, 138–144. [Google Scholar] [CrossRef]
- Diogenes, A.R.; Ruparel, N.B.; Teixeira, F.B.; Hargreaves, K.M. Translational science in disinfection for regenerative endodontics. J. Endod. 2014, 40, S52–S57. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Adult mesenchymal stem cells and the NO pathways. Proc. Natl. Acad. Sci. USA 2013, 110, 2695–2696. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Sloa, A.J.; Murray, P.E.; Lumley, P.J.; Smith, A.J. Ultrastructural localisation of TGF-beta exposure in dentine by chemical treatment. Histochem. J. 2000, 32, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Lin, Y.; Lennon, D.P.; Correa, D.; Schluchter, M.; Caplan, A.I. Efficient lentiviral transduction of human mesenchymal stem cells that preserves proliferation and differentiation capabilities. Stem Cells Transl. Med. 2012, 1, 886–897. [Google Scholar] [CrossRef]
- Smith, A.J.; Matthews, J.B.; Hall, R.C. Transforming growth factor-beta 1(TGF-β1) in dentine matrix: Ligand activation and receptor expression. Eur. J. Oral Sci. 1998, 106, 179–184. [Google Scholar] [CrossRef]
- Sloan, A.J.; Moseley, R.; Dobie, K.; Wadington, R.J.; Smith, A.J. TGF-beta latency-associated peptides (LAPs) in human dentin matrix and pulp. Connect. Tissue Res. 2002, 43, 381–386. [Google Scholar] [CrossRef]
- Arany, P.R.; Cho, A.; Hunt, T.D.; Sidhu, G.; Shin, K.; Hahm, E.; Huang, G.X.; Weaver, J.; Chen, A.C.; Padwa, B.L.; et al. Photoactivation of endogenous latent transforming growth factor-β1 directs dental stem cell differentiation for regeneration. Sci. Transl. Med. 2014, 6, 238ra69. [Google Scholar] [CrossRef]
- Demarco, F.F.; Casagrande, L.; Zhang, Z.; Dong, Z.; Tarquinio, S.B.; Zeitlin, B.D.; Shi, S.; Smith, A.J.; Nör, J.E. Effects of morphogen and scaffold porogen on the differentiation of dental pulp stem cells. J. Endod. 2010, 36, 1805–1811. [Google Scholar] [CrossRef]
- Dostálová, T.; Jelínková, H.; Housová, D.; Sulc, J.; Nemeć, M.; Dusková, J.; Miyagi, M.; Krátky, M. Endodontic treatment with application of Er:YAG laser waveguide radiation disinfection. J. Clin. Laser Med. Surg. 2002, 20, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Ordinola-Zapata, R.; Bramante, C.M.; Aprecio, R.M.; Handysides, R.; Jaramillo, D.E. Biofilm removal by 6% sodium hypochlorite activated by different irrigation techniques. Int. Endod. J. 2014, 47, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Saghiri, M.A.; Asgar, K.; Gutmann, J.L.; Garcia-Godoy, F.; Ahmadi, K.; Karamifar, K.; Asatorian, A. Effect of laser irradiation on root canal walls after final irrigation with 17% EDTA or BioPure MTAD: X-ray diffraction and SEM analysis. Quintessence Int. 2012, 43, 127–134. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín, G.; Preve, V.; Hargreaves, K.; Diogenes, A.; Inostroza, C.; Saint-Jean, N.; Brizuela, C. Effect of Dentin Conditioning with EDTA and Diode Lasers on Expression of Odontoblast-like Cell Markers of Dental Pulp Stem Cells. Dent. J. 2023, 11, 210. https://doi.org/10.3390/dj11090210
Martín G, Preve V, Hargreaves K, Diogenes A, Inostroza C, Saint-Jean N, Brizuela C. Effect of Dentin Conditioning with EDTA and Diode Lasers on Expression of Odontoblast-like Cell Markers of Dental Pulp Stem Cells. Dentistry Journal. 2023; 11(9):210. https://doi.org/10.3390/dj11090210
Chicago/Turabian StyleMartín, Gabriela, Valentín Preve, Kenneth Hargreaves, Anibal Diogenes, Carolina Inostroza, Nicole Saint-Jean, and Claudia Brizuela. 2023. "Effect of Dentin Conditioning with EDTA and Diode Lasers on Expression of Odontoblast-like Cell Markers of Dental Pulp Stem Cells" Dentistry Journal 11, no. 9: 210. https://doi.org/10.3390/dj11090210
APA StyleMartín, G., Preve, V., Hargreaves, K., Diogenes, A., Inostroza, C., Saint-Jean, N., & Brizuela, C. (2023). Effect of Dentin Conditioning with EDTA and Diode Lasers on Expression of Odontoblast-like Cell Markers of Dental Pulp Stem Cells. Dentistry Journal, 11(9), 210. https://doi.org/10.3390/dj11090210