Primary Stability of Zirconia Dental Implants with Cylindrical and Tapered Designs Across Varying Bone Densities: An In Vitro Evaluation
Abstract
1. Introduction
2. Materials and Methods
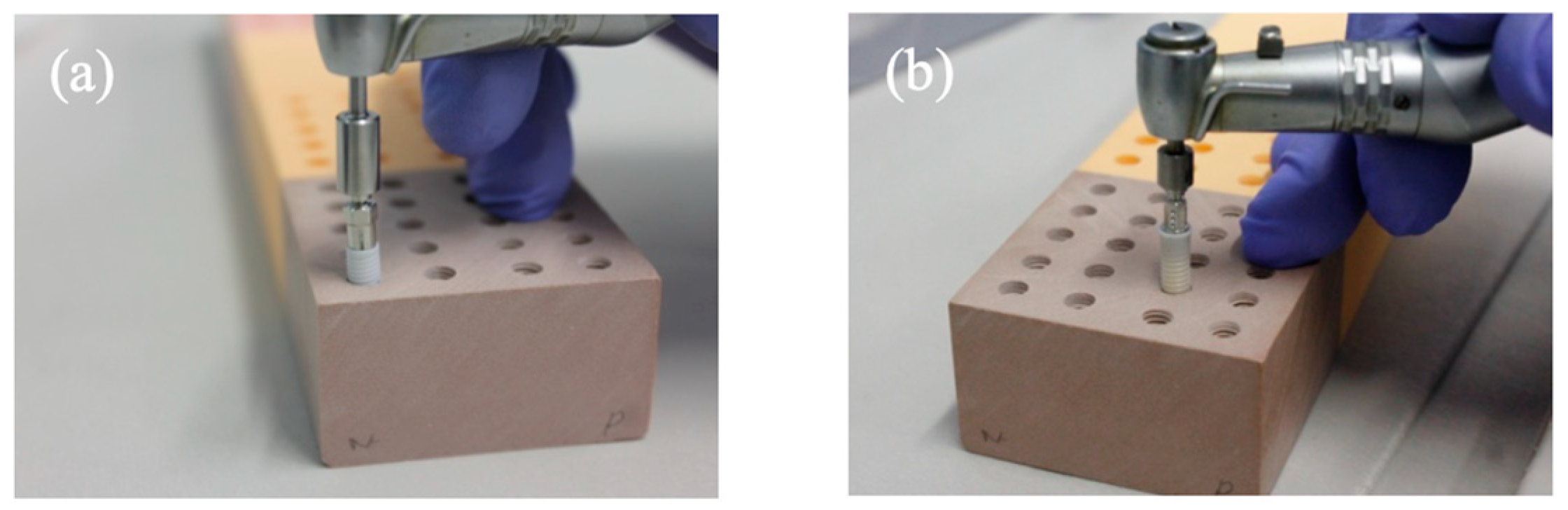
2.1. Implants and Polyurethane Blocks
- ▪
- Block 1: Density of 10 PCF (0.16 g/cm3) and light-green color.
- ▪
- Block 2: Density of 15 PCF (0.24 g/cm3) and pink color.
- ▪
- Block 3: Density of 20 PCF (0.32 g/cm3) and light-orange color.
- ▪
- Block 4: Density of 30 PCF (0.48 g/cm3) and dark-orange color.
- ▪
- Block 5: Density of 40 PCF (0.96 g/cm3) and brown color.
2.2. Sample Description
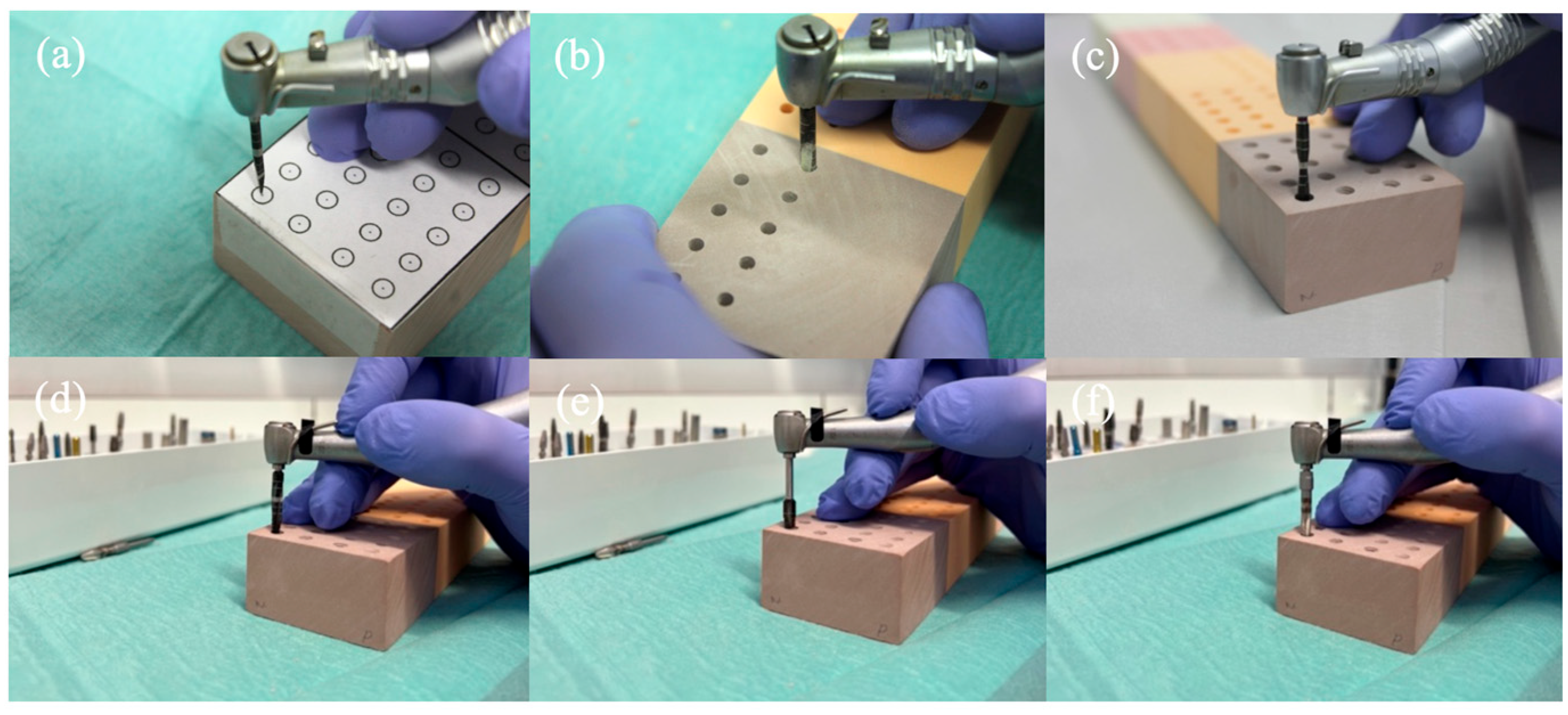
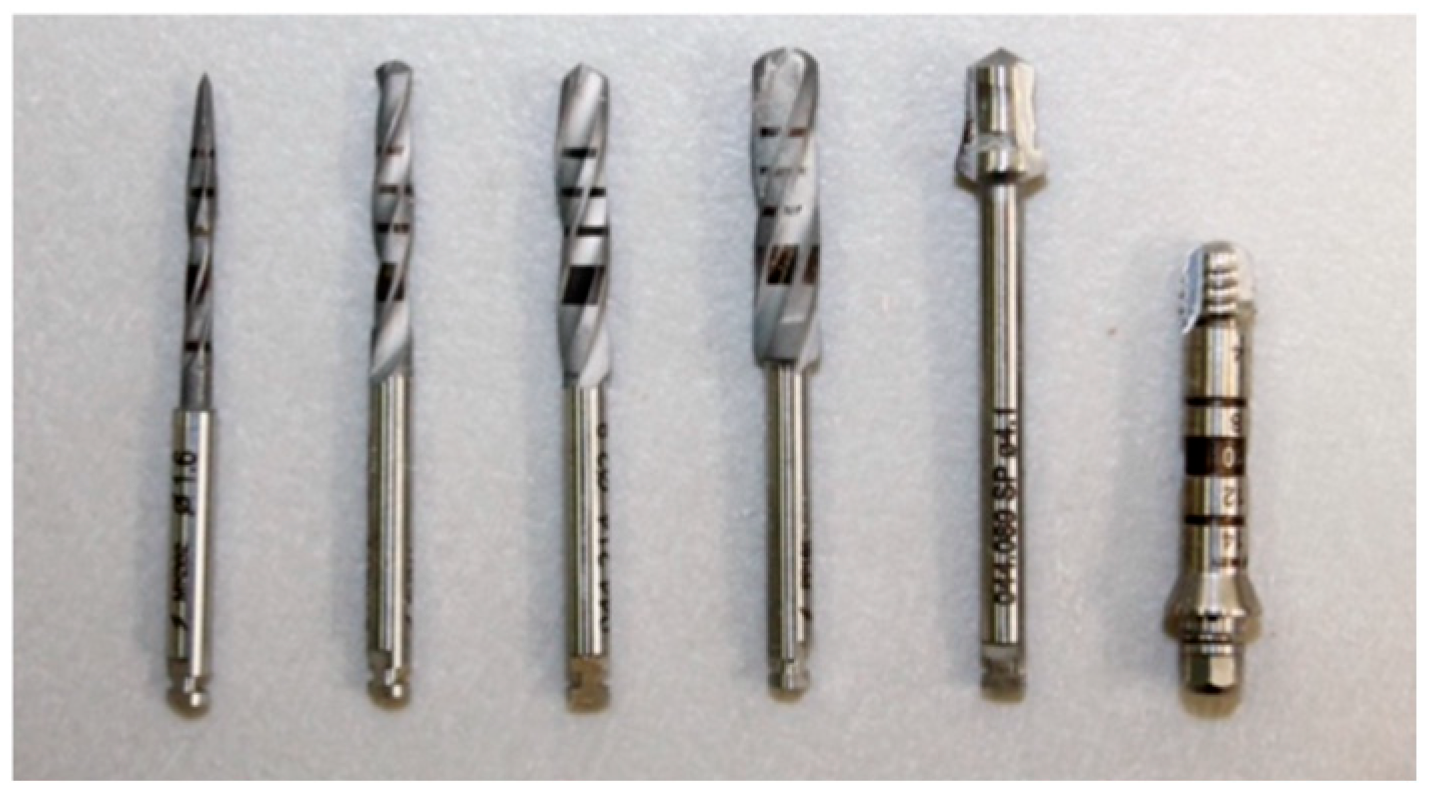
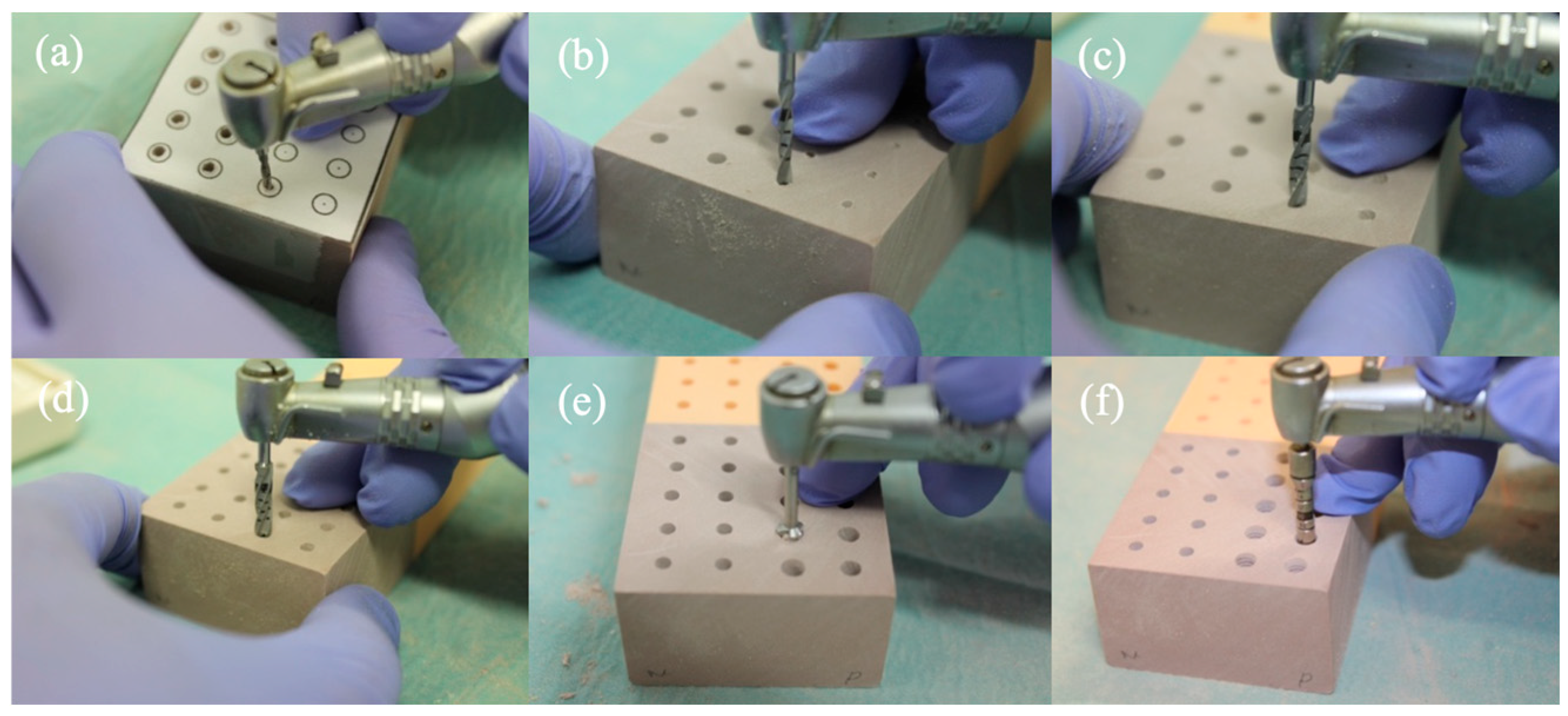
2.3. Drilling and Insertion Protocols
- Initial Needle Drill (1200 rpm);
- Tapered Drill Ø 2.0 mm (1200 rpm);
- Tapered Drill Ø 3.5 mm (1200 rpm);
- Tapered Drill Ø 4.3 mm (1200 rpm);
- Countersink Drill Ø 4.3 mm in type III bone (300 rpm);
- Bone tap Ø 4.3 mm in type I and II bone (30 rpm);
- Needle Drill Ø 1.6 mm (800 rpm);
- Pilot Drill 1 Ø 2.2 mm (800 rpm);
- Pilot Drill 2 Ø 2.8 mm (600 rpm);
- Twist Drill PRO Ø 3.5 mm (500 rpm);
- BL Profile Drill Ø 4.1 mm (300 rpm);
- BL Tap for Adapter Ø 4.1 mm in type I bone (300 rpm);
2.4. Test for Implant Stability
2.5. Statistical Analysis
3. Results
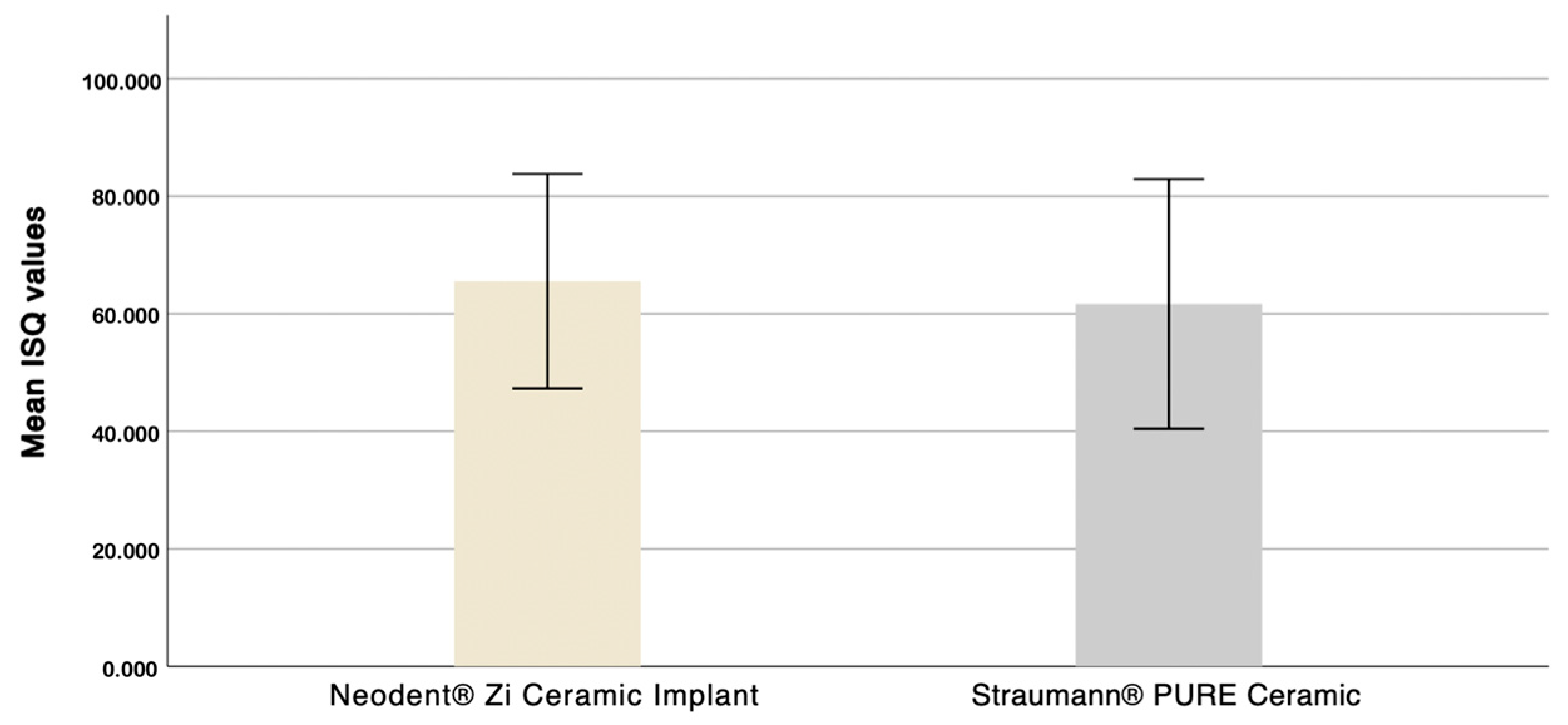
3.1. Evaluation of the Influence of Macrogeometry on Primary Stability
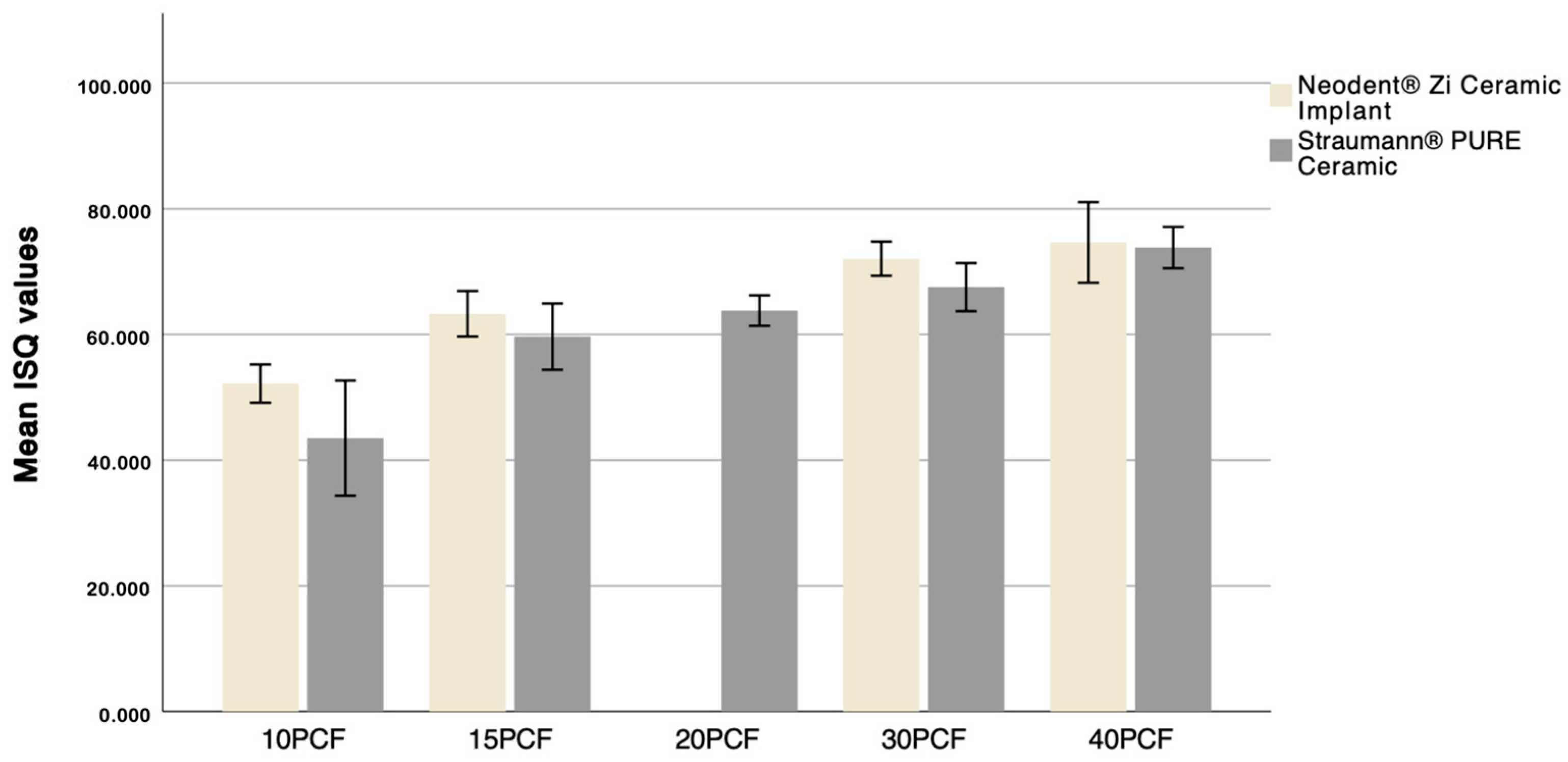
3.2. Evaluation of Straumann® Implant Primary Stability in Different Bone Densities
3.3. Evaluation of Neodent® Implant Stability in Different Bone Densities
4. Discussion
5. Conclusions
- ▪
- The macrogeometry of zirconia dental implants influences primary stability values.
- ▪
- The tapered implant demonstrated superior ISQ results compared to the cylindrical design in every tested bone density.
- ▪
- Higher primary stability values are associated with increased bone density.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fernandes, P.; Otero, A.; Fernandes, J.; Nassani, L.; Castilho, R.; De Oliveira Fernandes, G. Clinical Performance Comparing Titanium and Titanium–Zirconium or Zirconia Dental Implants: A Systematic Review of Randomized Controlled Trials. Dent. J. 2022, 10, 83. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Sennerby, L.; De Bruyn, H. Modern Implant Dentistry Based on Osseointegration: 50 Years of Progress, Current Trends and Open Questions. Periodontology 2017, 73, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Howe, M.-S.; Keys, W.; Richards, D. Long-Term (10-Year) Dental Implant Survival: A Systematic Review and Sensitivity Meta-Analysis. J. Dent. 2019, 84, 9–21. [Google Scholar] [CrossRef]
- Manzano, G.; Herrero, L.R.; Montero, J. Comparison of Clinical Performance of Zirconia Implants and Titanium Implants in Animal Models: A Systematic Review. Int. J. Oral Maxillofac. Implant. 2014, 29, 311–320. [Google Scholar] [CrossRef]
- Elnayef, B.; Lázaro, A.; Suárez-López Del Amo, F.; Galindo-Moreno, P.; Wang, H.-L.; Gargallo-Albiol, J.; Hernández-Alfaro, F. Zirconia Implants as an Alternative to Titanium: A Systematic Review and Meta-Analysis. Int. J. Oral Maxillofac. Implant. 2017, 32, e125–e134. [Google Scholar] [CrossRef]
- Gargallo-Albiol, J.; Böhm, K.; Wang, H.-L. Clinical and Radiographic Outcomes of Zirconia Dental Implants—A Clinical Case Series Study. Materials 2022, 15, 2437. [Google Scholar] [CrossRef]
- Nicholson, J.W. Titanium Alloys for Dental Implants: A Review. Prosthesis 2020, 2, 100–116. [Google Scholar] [CrossRef]
- Re, D.; Borgonovo, A.E.; Luca, A.E.; Ferrario, S.; Noumbissi, S.; Vavassori, V. The Evolution in Ceramic Implantology: A Review of the Literature and Report of Two Cases with Two-Piece Zirconia Implants. Ital. J. Dent. Med. 2019, 4, 3–16. [Google Scholar]
- Cionca, N.; Hashim, D.; Mombelli, A. Zirconia Dental Implants: Where Are We Now, and Where Are We Heading? Periodontology 2017, 73, 241–258. [Google Scholar] [CrossRef]
- Safioti, L.M.; Kotsakis, G.A.; Pozhitkov, A.E.; Chung, W.O.; Daubert, D.M. Increased Levels of Dissolved Titanium Are Associated With Peri-Implantitis—A Cross-Sectional Study. J. Periodontol. 2017, 88, 436–442. [Google Scholar] [CrossRef]
- Kunrath, M.F.; Gupta, S.; Lorusso, F.; Scarano, A.; Noumbissi, S. Oral Tissue Interactions and Cellular Response to Zirconia Implant-Prosthetic Components: A Critical Review. Materials 2021, 14, 2825. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, A.; Khan, A.S.; Zafar, S. Thirty Years of Translational Research in Zirconia Dental Implants: A Systematic Review of the Literature. J. Oral Implantol. 2017, 43, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, F.; Noumbissi, S.; Francesco, I.; Rapone, B.; Khater, A.G.A.; Scarano, A. Scientific Trends in Clinical Research on Zirconia Dental Implants: A Bibliometric Review. Materials 2020, 13, 5534. [Google Scholar] [CrossRef] [PubMed]
- Kniha, K.; Kniha, H.; Grunert, I.; Edelhoff, D.; Hölzle, F.; Modabber, A. Esthetic Evaluation of Maxillary Single-Tooth Zirconia Implants in the Esthetic Zone. Int. J. Periodont. Restor. Dent. 2019, 39, e195–e201. [Google Scholar] [CrossRef]
- Prakash, M.; Audi, K.; Vaderhobli, R.M. Long-Term Success of All-Ceramic Dental Implants Compared with Titanium Implants. J. Long Term Eff. Med. Implant. 2021, 31, 73–89. [Google Scholar] [CrossRef]
- Hanawa, T. Zirconia versus Titanium in Dentistry: A Review. Dent. Mater. J. 2020, 39, 24–36. [Google Scholar] [CrossRef]
- Arlucea, N.; Brizuela-Velasco, A.; Dieguez-Pereira, M.; Punset, M.; Molmeneu, M.; Sánchez Lasheras, F.; deLlanos-Lanchares, H.; Álvarez-Arenal, Á. Zirconia vs. Titanium Dental Implants: Primary Stability In-Vitro Analysis. Materials 2021, 14, 7886. [Google Scholar] [CrossRef]
- Roehling, S.; Schlegel, K.A.; Woelfler, H.; Gahlert, M. Performance and Outcome of Zirconia Dental Implants in Clinical Studies: A Meta-analysis. Clin. Oral Implant. Res. 2018, 29, 135–153. [Google Scholar] [CrossRef]
- Bormann, K.-H.; Gellrich, N.-C.; Kniha, H.; Schild, S.; Weingart, D.; Gahlert, M. A Prospective Clinical Study to Evaluate the Performance of Zirconium Dioxide Dental Implants in Single-Tooth Edentulous Area: 3-Year Follow-Up. BMC Oral Health 2018, 18, 181. [Google Scholar] [CrossRef]
- Balmer, M.; Spies, B.C.; Vach, K.; Kohal, R.; Hämmerle, C.H.F.; Jung, R.E. Three-year Analysis of Zirconia Implants Used for Single-tooth Replacement and Three-unit Fixed Dental Prostheses: A Prospective Multicenter Study. Clin. Oral Implant. Res. 2018, 29, 290–299. [Google Scholar] [CrossRef]
- Balmer, M.; Spies, B.C.; Kohal, R.; Hämmerle, C.H.; Vach, K.; Jung, R.E. Zirconia Implants Restored with Single Crowns or Fixed Dental Prostheses: 5-year Results of a Prospective Cohort Investigation. Clin. Oral Implant. Res. 2020, 31, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Bosshardt, D.D.; Chappuis, V.; Buser, D. Osseointegration of Titanium, Titanium Alloy and Zirconia Dental Implants: Current Knowledge and Open Questions. Periodontology 2017, 73, 22–40. [Google Scholar] [CrossRef] [PubMed]
- Heimes, D.; Becker, P.; Pabst, A.; Smeets, R.; Kraus, A.; Hartmann, A.; Sagheb, K.; Kämmerer, P.W. How Does Dental Implant Macrogeometry Affect Primary Implant Stability? A Narrative Review. Int. J. Implant. Dent. 2023, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Kreve, S.; Ferreira, I.; Da Costa Valente, M.L.; Dos Reis, A.C. Relationship between Dental Implant Macro-Design and Osseointegration: A Systematic Review. Oral Maxillofac. Surg. 2022, 28, 1–14. [Google Scholar] [CrossRef]
- Misch, C.E. (Ed.) Contemporary Implant Dentistry, 3rd ed.; Elsevier Mosby: St. Louis, MO, USA, 2008. [Google Scholar]
- Kittur, N.; Oak, R.; Dekate, D.; Jadhav, S.; Dhatrak, P. Dental Implant Stability and Its Measurements to Improve Osseointegration at the Bone-Implant Interface: A Review. Mater. Today Proc. 2021, 43, 1064–1070. [Google Scholar] [CrossRef]
- Herrero-Climent, M.; Falcão, A.; López-Jarana, P.; Díaz-Castro, C.M.; Ríos-Carrasco, B.; Ríos-Santos, J.V. In Vitro Comparative Analysis of Two Resonance Frequency Measurement Devices: Osstell Implant Stability Coefficient and Penguin Resonance Frequency Analysis. Clin. Implant. Dent. Relat. Res. 2019, 21, 1124–1131. [Google Scholar] [CrossRef]
- Swami, V.; Vijayaraghavan, V.; Swami, V. Current Trends to Measure Implant Stability. J. Indian Prosthodont. Soc. 2016, 16, 124. [Google Scholar] [CrossRef]
- Osstell® Beacon-Instruções de Utilização. Available online: https://www.osstell.com/app/uploads/25087-05-PT-Osstell-Beacon-IFU-FINAL.pdf (accessed on 11 April 2024).
- The Osstell ISQ Scale: Evidence-Based. Available online: https://www.osstell.com/clinical-guidelines/the-osstell-isq-scale/ (accessed on 27 November 2023).
- ASTM F-1839-08(2021); Standard Specification for Rigid Polyurethane Foam for Use as a Standard Material for Testing Orthopedic Devices and Instruments. ASTM International: West Conshohocken, PA, USA, 2021. [CrossRef]
- Calvert, K.L.; Trumble, K.P.; Webster, T.J.; Kirkpatrick, L.A. Characterization of Commercial Rigid Polyurethane Foams Used as Bone Analogs for Implant Testing. J. Mater. Sci Mater. Med. 2010, 21, 1453–1461. [Google Scholar] [CrossRef]
- Kästel, I.; de Quincey, G.; Neugebauer, J.; Sader, R.; Gehrke, P. Does the Manual Insertion Torque of Smartpegs Affect the Outcome of Implant Stability Quotients (ISQ) during Resonance Frequency Analysis (RFA)? Int. J. Implant. Dent. 2019, 5, 42. [Google Scholar] [CrossRef]
- Barikani, H.; Rashtak, S.; Akbari, S.; Fard, M.K.; Rokn, A. The Effect of Shape, Length and Diameter of Implants on Primary Stability Based on Resonance Frequency Analysis. Dent. Res. J. 2014, 11, 87–91. [Google Scholar]
- Comuzzi, L.; Tumedei, M.; D’Arcangelo, C.; Piattelli, A.; Iezzi, G. An In Vitro Analysis on Polyurethane Foam Blocks of the Insertion Torque (IT) Values, Removal Torque Values (RTVs), and Resonance Frequency Analysis (RFA) Values in Tapered and Cylindrical Implants. Int. J. Environ. Res. Public Health 2021, 18, 9238. [Google Scholar] [CrossRef] [PubMed]
- Comuzzi, L.; Tumedei, M.; Di Pietro, N.; Romasco, T.; Heydari Sheikh Hossein, H.; Montesani, L.; Inchingolo, F.; Piattelli, A.; Covani, U. A Comparison of Conical and Cylindrical Implants Inserted in an In Vitro Post-Extraction Model Using Low-Density Polyurethane Foam Blocks. Materials 2023, 16, 5064. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.; Chen, S.; Davies, H.; Fitzgerald, W.; Xu, J.; Darby, I. Primary Stability and Healing Outcomes of Apically Tapered and Straight Implants Placed into Fresh Extraction Sockets. A Pre-clinical in Vivo Study. Clin. Oral Implant. Res. 2020, 31, 705–714. [Google Scholar] [CrossRef] [PubMed]
- García-Vives, N.; Andrés-García, R.; Rios-Santos, V.; Fernández-Palacín, A.; Bullón-Fernández, P.; Herrero-Climent, M.; Herrero-Climent, F. In Vitro Evaluation of the Type of Implant Bed Preparation with Osteotomes in Bone Type IV and Its Influence on the Stability of Two Implant Systems. Med. Oral Patol. Oral Cir. Bucal 2009, 14, e455–e460. [Google Scholar]
- Lozano-Carrascal, N.; Salomó-Coll, O.; Gilabert-Cerdà, M.; Farré-Pagés, N.; Gargallo-Albiol, J.; Hernández-Alfaro, F. Effect of Implant Macro-Design on Primary Stability: A Prospective Clinical Study. Med. Oral Patol. Oral Cir. Bucal 2016, 21, e214–e221. [Google Scholar] [CrossRef]
- Rokn, A.; Ghahroudi, A.R.; Mesgarzadeh, A.; Miremadi, A.; Yaghoobi, S. Evaluation of Stability Changes in Tapered and Parallel Wall Implants: A Human Clinical Trial. J. Dent. 2011, 8, 186–200. [Google Scholar]
- Heitzer, M.; Kniha, K.; Katz, M.S.; Winnand, P.; Peters, F.; Möhlhenrich, S.C.; Hölzle, F.; Modabber, A. The Primary Stability of Two Dental Implant Systems in Low-Density Bone. Int. J. Oral Maxillofac. Surg. 2022, 51, 1093–1100. [Google Scholar] [CrossRef]
- Bilhan, H.; Geckili, O.; Mumcu, E.; Bozdag, E.; Sünbüloğlu, E.; Kutay, O. Influence of Surgical Technique, Implant Shape and Diameter on the Primary Stability in Cancellous Bone. J. Oral Rehabil. 2010, 37, 900–907. [Google Scholar] [CrossRef]
- Atieh, M.A.; Alsabeeha, N.; Duncan, W.J. Stability of Tapered and Parallel-walled Dental Implants: A Systematic Review and Meta-analysis. Clin. Implant. Dent. Relat. Res. 2018, 20, 634–645. [Google Scholar] [CrossRef]
- Ivanova, V.; Chenchev, I.; Zlatev, S.; Mijiritsky, E. Correlation between Primary, Secondary Stability, Bone Density, Percentage of Vital Bone Formation and Implant Size. Int. J. Environ. Res. Public Health 2021, 18, 6994. [Google Scholar] [CrossRef]
- Duddeck, D. Effects of Multiple Reuse, Remounting and Consecutive Autoclave Sterilization on Osstell SmartPegs. Available online: https://www.physicsforceps.com/pdf/duddeck-2015-clinical_oral_implants_research.pdf (accessed on 11 April 2024).
- Di Stefano, D.A.; Arosio, P.; Gastaldi, G.; Gherlone, E. The Insertion Torque-Depth Curve Integral as a Measure of Implant Primary Stability: An in Vitro Study on Polyurethane Foam Blocks. J. Prosthet. Dent. 2018, 120, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Chávarri-Prado, D.; Brizuela-Velasco, A.; Diéguez-Pereira, M.; Pérez-Pevida, E.; Jiménez-Garrudo, A.; Viteri-Agustín, I.; Estrada-Martínez, A.; Montalbán-Vadillo, O. Influence of Cortical Bone and Implant Design in the Primary Stability of Dental Implants Measured by Two Different Devices of Resonance Frequency Analysis: An in Vitro Study. J. Clin. Exp. Dent. 2020, 12, e242–e248. [Google Scholar] [CrossRef] [PubMed]
- Geckili, O.; Bilhan, H.; Geckili, E.; Barca-Dayan, E.; Dayan, C.; Bural, C. Is Clinical Experience Important for Obtaining the Primary Stability of Dental Implants with Aggressive Threads? An Ex Vivo Study. Med. Oral Patol. Oral Cir. Bucal 2019, 24, e254–e259. [Google Scholar] [CrossRef] [PubMed]
- Ostman, P.-O.; Hellman, M.; Wendelhag, I.; Sennerby, L. Resonance Frequency Analysis Measurements of Implants at Placement Surgery. Int. J. Prosthodont. 2006, 19, 77–83; discussion 84. [Google Scholar] [PubMed]
- Gottlow, J.; Sennerby, L. Influence of Diameter and Length on Primary Stability in Various Implant Site Densities-An in Vitro Study in Polyurethane Blocks. Clin. Implant. Dent. Relat. Res. 2024, 26, 327–332. [Google Scholar] [CrossRef]
- Moreira, A.; Rosa, J.; Freitas, F.; Francisco, H.; Luís, H.; Caramês, J. Influence of Implant Design, Length, Diameter, and Anatomic Region on Implant Stability: A Randomized Clinical Trial. Rev. Port. Estomatol. Med. Dent. Cir. Maxilofac. 2021, 62, 9–15. [Google Scholar] [CrossRef]





| Densities | Mean ± SD | p-Value | |
|---|---|---|---|
| Neodent® | Straumann® | ||
| 10 PCF | 52.18 ± 1.52 | 43.5 ± 4.58 | <0.001 |
| 15 PCF | 63.28 ± 1.81 | 59.65 ± 2.64 | 0.001 |
| 20 PCF | 63.80 ± 1.21 | ||
| 30 PCF | 72.05 ± 1.36 | 67.53 ± 1.91 | <0.001 |
| 40 PCF | 74.63 ± 3.21 | 73.82 ± 1.64 | 0.048 |
| Comparisons | Sig. | Adj. Sig. * |
|---|---|---|
| 10 PCF–15 PCF | 0.104 | 1.000 |
| 10 PCF–20 PCF | 0.002 | 0.024 |
| 10 PCF–30 PCF | <0.001 | 0.000 |
| 10 PCF–40 PCF | <0.001 | 0.000 |
| 15 PCF–20 PCF | 0.158 | 1.000 |
| 15 PCF–30 PCF | 0.004 | 0.036 |
| 15 PCF–40 PCF | <0.001 | 0.000 |
| 20 PCF–30 PCF | 0.133 | 1.000 |
| 20 PCF–40 PCF | 0.002 | 0.019 |
| 30 PCF–40 PCF | 0.111 | 1.000 |
| Comparisons | Sig. | Adj. Sig. * |
|---|---|---|
| 10 PCF–15 PCF | 0.056 | 0.334 |
| 10 PCF–30 PCF | <0.001 | 0.000 |
| 10 PCF–40 PCF | <0.001 | 0.000 |
| 15 PCF–30 PCF | 0.020 | 0.118 |
| 15 PCF–40 PCF | <0.001 | 0.004 |
| 30 PCF–40 PCF | 0.284 | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, D.; Cavaco, F.; Freitas, F.; Marques, D.; Caramês, J.; Moreira, A. Primary Stability of Zirconia Dental Implants with Cylindrical and Tapered Designs Across Varying Bone Densities: An In Vitro Evaluation. Dent. J. 2024, 12, 356. https://doi.org/10.3390/dj12110356
Fernandes D, Cavaco F, Freitas F, Marques D, Caramês J, Moreira A. Primary Stability of Zirconia Dental Implants with Cylindrical and Tapered Designs Across Varying Bone Densities: An In Vitro Evaluation. Dentistry Journal. 2024; 12(11):356. https://doi.org/10.3390/dj12110356
Chicago/Turabian StyleFernandes, Diogo, Francisco Cavaco, Filipe Freitas, Duarte Marques, João Caramês, and André Moreira. 2024. "Primary Stability of Zirconia Dental Implants with Cylindrical and Tapered Designs Across Varying Bone Densities: An In Vitro Evaluation" Dentistry Journal 12, no. 11: 356. https://doi.org/10.3390/dj12110356
APA StyleFernandes, D., Cavaco, F., Freitas, F., Marques, D., Caramês, J., & Moreira, A. (2024). Primary Stability of Zirconia Dental Implants with Cylindrical and Tapered Designs Across Varying Bone Densities: An In Vitro Evaluation. Dentistry Journal, 12(11), 356. https://doi.org/10.3390/dj12110356








