Hydroxyapatite from Mollusk Shells: Characteristics, Production, and Potential Applications in Dentistry
Abstract
:1. Introduction
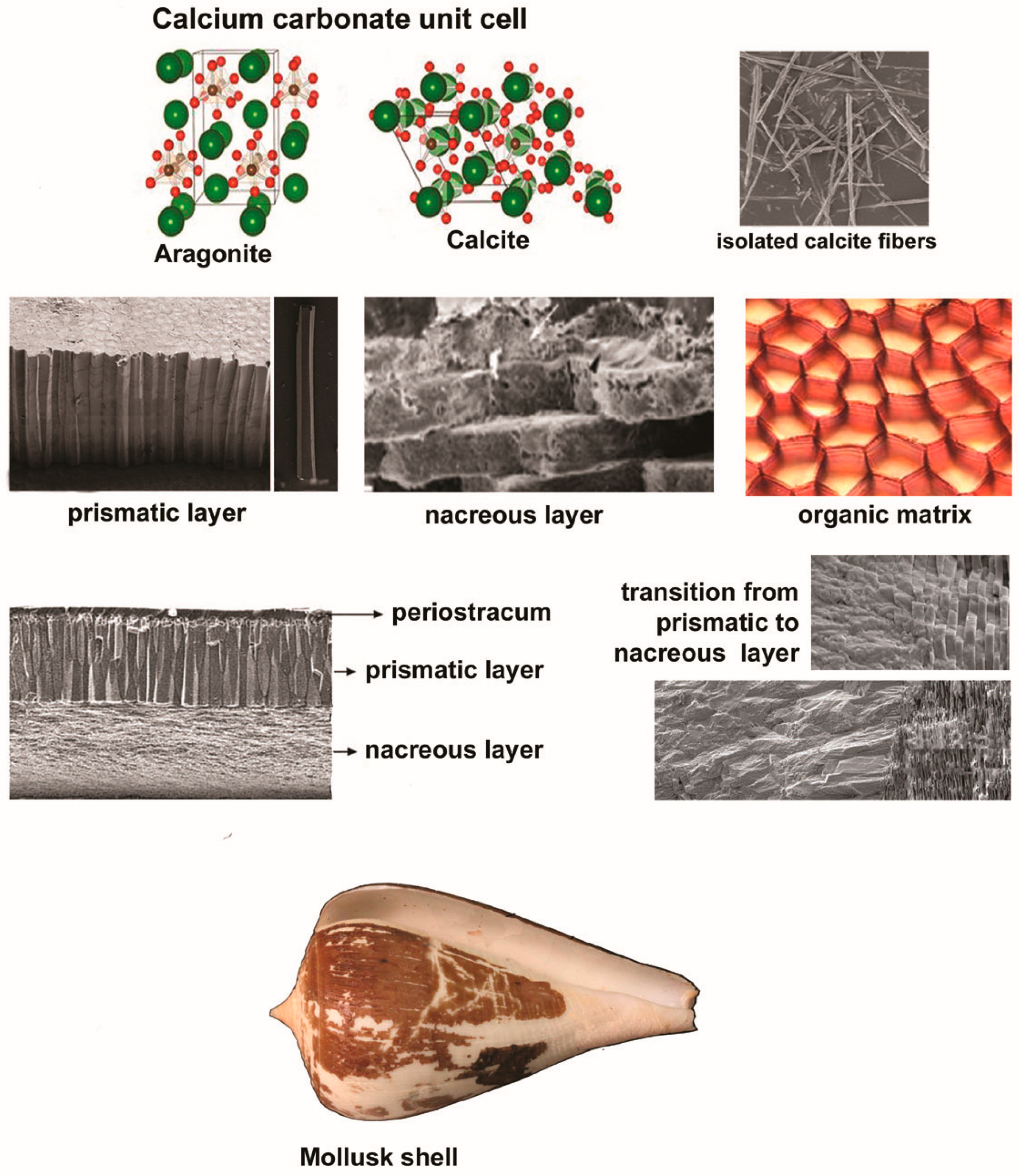
2. A Brief Overview of Mollusk Shell Architecture
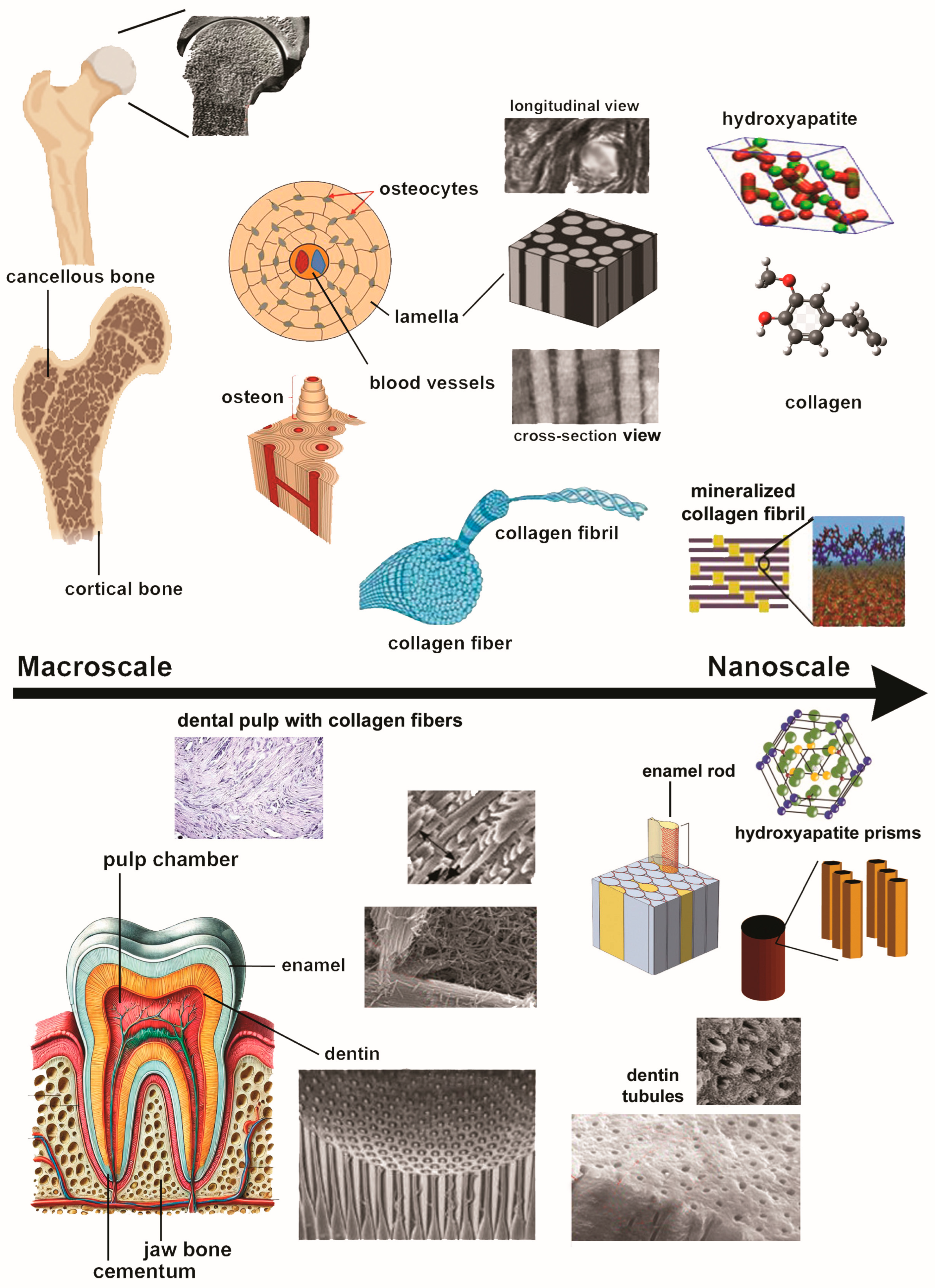
3. Shell-Derived Biomaterials in Dental Applications
3.1. Molluskan Shells as Sources of Hydroxyapatite
| Method | Advantages | Disadvantages | Reference |
|---|---|---|---|
| Thermal Treatment with Wet Precipitation |
|
| [64,65,66,67,68,69,70,71,72,73,74,75,76,77] |
| Solid-State Reaction |
|
| [78] |
| Chemical Precipitation |
|
| [79] |
| Hydrothermal Method |
|
| [80,81,82,83,84,85,86] |
| Sol–Gel Method |
|
| [87,88,89] |
3.1.1. Methods for Hydroxyapatite Synthesis from Molluskan Shells
3.1.2. Biocompatibility and Applications of Mollusk-Derived Hydroxyapatite
3.2. Mollusk-Derived Carbonated Hydroxyapatite
4. (Pre)clinical Trials
5. Conclusions
- With a hierarchical structure and compositional similarities with human bone and teeth, mollusk shells display strength, flexibility, resilience, and a strong potential for integration into the human tissues.
- Composed primarily of calcium carbonate as aragonite or calcite, these exoskeletons can be processed into different compounds of dental interest, including hydroxyapatite (HA)—a biocompatible, bioactive, osteoconductive, and osteoinductive material.
- Mollusk shell-derived HA shows great promise in oral rehabilitation, particularly as a cost-effective alternative to synthetic bone substitutes; it is low-impact, abundant, sustainable, and customizable into nanosized particles with enhanced bioactivity.
- Given their efficiency, scalability, and ability to produce highly crystalline and pure materials, thermal treatment coupled with wet chemical precipitation and hydrothermal synthesis are the most common methods used for recovering HA from mollusk shells.
- Various reaction/processing conditions (e.g., temperature, pH, phosphate sources, sintering parameters) affect the size, purity, and crystallinity of the final product.
- Carbonated hydroxyapatite (CHA) possesses higher bioactivity, biocompatibility, and solubility but lower crystallinity compared to regular HA, facilitating faster integration with natural bone.
6. Future Perspectives for Research on Mollusk Shell-Derived Hydroxyapatite in Dental Applications
- Optimization of synthesis techniques: Focus on refining methods to achieve high purity, crystallinity, and bioactivity in HA for medical and dental use.
- Sustainable production processes: Investigate green synthesis and low-energy production methods to make HA manufacturing more environmentally friendly and cost-effective.
- Nanotechnology integration: Develop nano-HA with controlled particle size, shape, and surface characteristics to improve cell attachment and bone integration.
- Bioactive ion incorporation: Explore adding ions (e.g., magnesium, zinc) to HA to replicate natural bone composition and promote tissue integration.
- Mechanical properties enhancement: Modify sintering techniques to improve HA’s mechanical properties, aiming for better structural stability in load-bearing applications.
- Reaction condition optimization: Study how conditions like temperature, pH, and sintering parameters affect HA properties to tailor it for specific clinical applications.
- Cross-disciplinary collaboration: Promote partnerships across material science, biomedicine, and environmental engineering to advance HA clinical applicability.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Yan, J.; Deng, J.; Jiao, D.; Tan, G.; Wang, Q.; Liu, Z.; Yang, P.; Wei, Y.; Yi, Z.; Deng, X.; et al. Natural mollusk shells as a potential dental material. J. Mater. Res. Technol. 2023, 25, 5196–5209. [Google Scholar] [CrossRef]
- Oktar, F.N.; Unal, S.; Gunduz, O.; Nissan, B.B.; Macha, I.J.; Akyol, S.; Duţă, L.; Ekren, N.; Altan, E.; Yetmez, M. Marine-derived bioceramics for orthopedic, reconstructive and dental surgery applications. J. Aust. Ceram. Soc. 2023, 59, 57–81. [Google Scholar] [CrossRef]
- Cheng, M.; Liu, M.; Chang, L.; Liu, Q.; Wang, C.; Hu, L.; Zhang, Z.; Ding, W.; Chen, L.; Guo, S.; et al. Overview of structure, function and integrated utilization of marine shell. Sci. Total Environ. 2023, 870, 161950. [Google Scholar] [CrossRef] [PubMed]
- Green, D.W.; Lai, W.F.; Jung, H.S. Evolving marine biomimetics for regenerative dentistry. Mar. Drugs 2014, 12, 2877–2912. [Google Scholar] [CrossRef]
- Pu’ad, N.M.; Haq, R.A.; Noh, H.M.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. Synthesis method of hydroxyapatite: A review. Mater. Today Proc. 2020, 29, 233–239. [Google Scholar] [CrossRef]
- Drăghici, G.A.; Dehelean, C.; Pinzaru, I.; Bordean, D.M.; Borozan, A.; Tsatsakis, A.M.; Kovatsi, L.; Nica, D. Soil copper uptake by land snails: A semi-field experiment with juvenile Cantareus aspersus snails. Environ. Toxicol. Pharmacol. 2019, 72, 103243. [Google Scholar] [CrossRef]
- Cochrane, N.J.; Cai, F.; Huq, N.L.; Burrow, M.F.; Reynolds, E.C. New approaches to enhanced remineralization of tooth enamel. J. Dent. Res. 2010, 89, 1187–1197. [Google Scholar] [CrossRef]
- Georgescu, M.; Drăghici, G.A.; Oancea, E.F.; Dehelean, C.A.; Şoica, C.; Vlăduţ, N.V.; Nica, D.V. Effects of cadmium sulfate on the brown garden snail Cornu aspersum: Implications for DNA Methylation. Toxics 2021, 9, 306. [Google Scholar] [CrossRef]
- Haidar, L.; Georgescu, M.; Drăghici, G.A.; Bănățean-Dunea, I.; Nica, D.V.; Șerb, A.F. DNA Methylation Machinery in Gastropod Mollusks. Life 2024, 14, 537. [Google Scholar] [CrossRef]
- Azarian, M.H.; Sutapun, W. Biogenic calcium carbonate derived from waste shells for advanced material applications: A review. Front. Mater. Sci. 2022, 9, 1024977. [Google Scholar] [CrossRef]
- Paradowska-Stolarz, A.; Mikulewicz, M.; Laskowska, J.; Karolewicz, B.; Owczarek, A. The importance of chitosan coatings in dentistry. Mar. Drugs 2023, 21, 613. [Google Scholar] [CrossRef] [PubMed]
- Yamakami, S.A.; Faraoni, J.J.; Lia, N.S.N.D.; Regula, F.B.; Ohyama, H.; Palma-Dibb, R.G. Effect of an experimental chitosan/casein gel on demineralized enamel under a cariogenic challenge. Dent. Med. Probl. 2022, 59, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Kerney, M.P.; Cameron, R.A.D. A Field Guide to the Land Snails of Britain and Northwestern Europe, 1st ed.; Collins: London, UK, 1979; pp. 23–36. [Google Scholar]
- Lowenstam, H.A.; Weiner, S. On Biomineralization, 1st ed.; Oxford University Press: Oxford, UK, 1989; pp. 89–110. [Google Scholar] [CrossRef]
- Casella, L.A.; Griesshaber, E.; Yin, X.; Ziegler, A.; Mavromatis, V.; Müller, D.; Ritter, A.C.; Hippler, D.; Harper, E.M.; Dietzel, M.; et al. Experimental diagenesis: Insights into aragonite to calcite transformation of Arctica islandica shells by hydrothermal treatment. Biogeosciences 2017, 14, 1461–1492. [Google Scholar] [CrossRef]
- Suzuki, M.; Kogure, T.; Weiner, S.; Addadi, L. Formation of aragonite crystals in the crossed lamellar microstructure of limpet shells. Cryst. Growth Des. 2011, 11, 4850–4859. [Google Scholar] [CrossRef]
- Balthasar, U.; Cusack, M. Aragonite-calcite seas—Quantifying the gray area. Geology 2015, 43, 99–102. [Google Scholar] [CrossRef]
- Chen, C.; Linse, K.; Copley, J.T.; Rogers, A.D. The ‘scaly-foot gastropod’: A new genus and species of hydrothermal vent-endemic gastropod (Neomphalina: Peltospiridae) from the Indian Ocean. J. Molluscan Stud. 2015, 81, 322–334. [Google Scholar] [CrossRef]
- Currey, J.D. The design of mineralised hard tissues for their mechanical functions. J. Exp. Biol. 1999, 202, 3285–3294. [Google Scholar] [CrossRef]
- Summa, D.; Lanzoni, M.; Castaldelli, G.; Fano, E.A.; Tamburini, E. Trends and opportunities of bivalve shells’ waste valorization in a prospect of circular blue bioeconomy. Resources 2022, 11, 48. [Google Scholar] [CrossRef]
- Marin, F.; Le Roy, N.; Marie, B. The formation and mineralization of mollusk shell. Front. Biosci 2012, 4, 1099–1125. [Google Scholar] [CrossRef]
- Suzuki, M.; Nagasawa, H. Mollusk shell structures and their formation mechanism. Can. J. Zool. 2013, 91, 349–366. [Google Scholar] [CrossRef]
- Drăghici, G.A.; Dehelean, C.A.; Moacă, A.E.; Moise, M.L.; Pînzaru, I.; Vladuț, V.N.; Banățean-Dunea, I.; Nica, D. Cadmium nitrate and DNA methylation in gastropods: Comparison between ovotestis and hepatopancreas. PeerJ 2023, 11, e15032. [Google Scholar] [CrossRef] [PubMed]
- Barthelat, F. Nacre from mollusk shells: A model for high-performance structural materials. Bioinspir. Biomim. 2010, 5, 035001. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.G.; Clark, G.R. Classification and Phylogenetic Significance of Molluscan Shell Microstructure. Notes Short Course Stud. Geol. 1985, 13, 50–71. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, J.; Wang, L.; Li, F.M. The relationship between mechanical properties and crossed-lamellar structure of mollusk shells. Mater. Sci. Eng. A 2008, 483–484, 309–312. [Google Scholar] [CrossRef]
- Moore, S.T.; Katz, J.M.; Zhukauskas, R.M.; Hernandez, R.M.; Lewis, C.S.; Supronowicz, P.R.; Cobb, R.R. Osteoconductivity and osteoinductivity of Puros® DBM putty. J. Biomater. Appl. 2011, 26, 151–171. [Google Scholar] [CrossRef]
- Zhao, R.; Yang, R.; Cooper, P.R.; Khurshid, Z.; Shavandi, A.; Ratnayake, J. Bone grafts and substitutes in dentistry: A review of current trends and developments. Molecules 2021, 26, 3007. [Google Scholar] [CrossRef]
- Cauwels, R.G.; Martens, L.C. Use of osteoconductive materials in pediatric dental medicine. Rev. Belge Med. Dent. 2004, 59, 203–214. [Google Scholar]
- Ferraz, M.P. Bone grafts in dental medicine: An overview of autografts, allografts and synthetic materials. Materials 2023, 16, 4117. [Google Scholar] [CrossRef]
- Basu, B.; Ghosh, S. Case Study: Hydroxyapatite-Based Microporous/Macroporous Scaffolds. In Biomaterials for Musculoskeletal Regeneration: Applications; Basu, B., Ghosh, S., Eds.; Springer Nature Singapore Pte Ltd.: Singapore, 2017; pp. 45–72. [Google Scholar] [CrossRef]
- Tang, G.; Liu, Z.; Liu, Y.; Yu, J.; Wang, X.; Tan, Z.; Ye, X. Recent trends in the development of bone regenerative biomaterials. Front. Cell Dev. Biol. 2021, 9, 665813. [Google Scholar] [CrossRef]
- García-Gareta, E.; Coathup, M.J.; Blunn, G.W. Osteoinduction of bone grafting materials for bone repair and regeneration. Bone 2015, 81, 112–121. [Google Scholar] [CrossRef]
- Jeyachandran, D.; Cerruti, M. Glass, ceramic, polymeric, and composite scaffolds with multiscale porosity for bone tissue engineering. Adv. Eng. Mater. 2023, 25, 2201743. [Google Scholar] [CrossRef]
- Canullo, L.; Genova, T.; Rakic, M.; Sculean, A.; Miron, R.; Muzzi, M.; Carossa, S.; Mussano, F. Effects of argon plasma treatment on the osteoconductivity of bone grafting materials. Clin. Oral Investig. 2020, 24, 2611–2623. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, M.Z.; Nadezhdin, S.V.; Kolobov, Y.R.; Ivanov, M.B.; Pavlov, N.A.; Zubareva, E.V. Relationship between osteoinductive characteristics of biocomposite material and physicochemical characteristics of coating. Bull. Exp. Biol. Med. 2009, 148, 822–824. [Google Scholar] [CrossRef] [PubMed]
- Amrollahi, P.; Shah, B.; Seifi, A.; Tayebi, L. Recent advancements in regenerative dentistry: A review. Mater. Sci. Eng. C 2016, 69, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Fanghänel, J. Biomechanics and biomaterials in oral rehabilitation and dental treatment. Biomed. Tech. 2008, 53, 197–204. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Nanodimensional and nanocrystalline apatites and other calcium orthophosphates in biomedical engineering, biology and medicine. Materials 2009, 2, 1975–2045. [Google Scholar] [CrossRef]
- Bordea, I.R.; Candrea, S.; Alexescu, G.T.; Bran, S.; Băciuț, M.; Băciuț, G.; Lucaciu, O.; Dinu, C.M.; Todea, D.A. Nano-hydroxyapatite use in dentistry: A systematic review. Drug. Metab. Rev. 2020, 52, 319–332. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; González-Calbet, J.M. Calcium phosphates as substitution of bone tissues. Prog. Solid State Chem. 2004, 32, 1–31. [Google Scholar] [CrossRef]
- Nica, D.V.; Draghici, G.A.; Andrica, F.M.; Popescu, S.; Coricovac, D.E.; Dehelean, C.A.; Gergen, I.I.; Kovatsi, L.; Coleman, M.D.; Tsatsakis, A. Short-term effects of very low dose cadmium feeding on copper, manganese and iron homeostasis: A gastropod perspective. Environ. Toxicol. Pharmacol. 2019, 65, 9–13. [Google Scholar] [CrossRef]
- Abdulkarim, A.; Isa, M.T.; Abdulsalam, S.; Muhammad, A.J.; Ameh, A.O. Extraction and characterisation of chitin and chitosan from mussel shell. Extraction 2013, 3, 108–114. [Google Scholar]
- Gerhard, E.M.; Wang, W.; Li, C.; Guo, J.; Ozbolat, I.T.; Rahn, K.M.; Armstrong, A.D.; Xia, J.; Qian, G.; Yang, J. Design strategies and applications of nacre-based biomaterials. Acta Biomater. 2017, 54, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Khrunyk, Y.; Lach, S.; Petrenko, I.; Ehrlich, H. Progress in modern marine biomaterials research. Mar. Drugs 2020, 18, 589. [Google Scholar] [CrossRef]
- González, J.A.; Vallejo, J.R. The use of shells of marine molluscs in Spanish ethnomedicine: A historical approach and present and future perspectives. Pharmaceuticals 2023, 16, 1503. [Google Scholar] [CrossRef] [PubMed]
- Bobbio, A. The first endosseous alloplastic implant in the history of man. Bull. Hist. Dent. 1972, 20, 1–6. [Google Scholar] [PubMed]
- Zampetti, P. Luci ed ombre in implantologia. In Proceedings of the 17th International Odontostomatologic Congress, Monte Carlo, Monaco, 25–30 May 2005; p. 23. [Google Scholar]
- Bobbio, A. Maya, the first authentic alloplastic, endosseous dental implant. A refinement of a priority. Rev. Assoc. Paul. Cir. Dent. 1973, 27, 27–36. [Google Scholar]
- Pasqualini, M.E. L’osteointegrazione ha origini italiane? In Proceedings of the 9th SISOS National Congress, Saronno, Italy, 5 March 2006; pp. 20–21. [Google Scholar]
- Balhuc, S.; Campian, R.; Labunet, A.; Negucioiu, M.; Buduru, S.; Kui, A. Dental applications of systems based on hydroxyapatite nanoparticles—An evidence-based update. Crystals 2021, 11, 674. [Google Scholar] [CrossRef]
- Izzetti, R.; Gennai, S.; Nisi, M.; Gulia, F.; Miceli, M.; Giuca, M.R. Clinical applications of nano-hydroxyapatite in dentistry. Appl. Sci. 2022, 12, 10762. [Google Scholar] [CrossRef]
- Teotia, A.K.; Raina, D.B.; Singh, C.; Sinha, N.; Isaksson, H.; Tägil, M.; Lidgren, L.; Kumar, A. Nano hydroxyapatite bone substitute functionalized with bone active molecules for enhanced cranial bone regeneration. ACS Appl. Mater. Interfaces 2017, 9, 6816–6828. [Google Scholar] [CrossRef]
- Mazumder, S.; Nayak, A.K.; Ara, T.J.; Hasnain, M.S. Hydroxyapatite composites for dentistry. In Applications of Nanocomposite Materials in Dentistry; Inamuddin, A.M.A., Mohammad, A., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 123–143. [Google Scholar] [CrossRef]
- Nozari, A.; Ajami, S.; Rafiei, A.; Niazi, E. Impact of nano hydroxyapatite, nano silver fluoride and sodium fluoride varnish on primary teeth enamel remineralization: An in vitro study. J. Clin. Diagn. Res. 2017, 11, ZC97–ZC100. [Google Scholar] [CrossRef]
- Antoniac, I.; Lesci, I.G.; Blajan, A.I.; Vitioanu, G.; Antoniac, A. Bioceramics and biocomposites from marine sources. Key Eng. Mater. 2016, 672, 276–292. [Google Scholar] [CrossRef]
- Nayak, A.K. Hydroxyapatite synthesis methodologies: An overview. Int. J. Chemtech Res. 2010, 2, 903–907. [Google Scholar]
- Ardiansyah, A.; Saraswaty, V.; Risdian, C. Synthesis and characterization of calcium phosphate (tricalcium phosphate/calcium pyrophosphate) from snail shells (Achatina fulica). IOP Conf. Ser. Earth Environ. Sci. 2023, 1201, 012091. [Google Scholar] [CrossRef]
- Saragih, A.S.; Pamungkas, A.; Noviyanto, A. Synthesis of hydroxyapatite from Indonesian green mussels (Perna viridis) via precipitation methods. Key Eng. Mater. 2020, 833, 199–203. [Google Scholar] [CrossRef]
- Jamilludin, M.A.; Dinatha, I.K.H.; Supii, A.I.; Partini, J.; Kusindarta, D.L.; Yusuf, Y. Chemical and morphological analysis of calcium oxide (CaO) powder from sea urchin (Diadema setosum) shell. Eng. Chem. 2023, 3, 37–43. [Google Scholar] [CrossRef]
- Crisp, D.J. Tidally deposited bands in shells of barnacles and molluscs. In Origin, Evolution, and Modern Aspects of Biomineralization in Plants and Animals; Crick, R., Ed.; Springer: Boston, MA, USA, 1989; pp. 103–124. [Google Scholar]
- Saharudin, S.H.; Shariffuddin, J.H.; Ismail, A.; Mah, J.H. Recovering value from waste: Biomaterials production from marine shell waste. Bull. Mater. Sci. 2018, 41, 162. [Google Scholar] [CrossRef]
- Manea, D.; Ienciu, A.A.; Ștef, R.; Peț, I.; Șmuleac, L.; Grozea, I.; Cărăbeț, A.; Drăghici, G.A.; Nica, D.V. The “sandwich” system: A potential solution for protecting overwintering cornu aspersum snails reared in semi-intensive heliciculture farms in colder climates. Animals 2021, 11, 1420. [Google Scholar] [CrossRef]
- Charlena, C.; Suparto, I.H.; Laia, D.P.O. Synthesis and characterization of hydroxyapatite from Polymesoda placans shell using wet precipitation method. Jurnal Bios Logos 2023, 13, 85–96. [Google Scholar] [CrossRef]
- Taqa, G.A.; Al-Hussary, B.N.; Kashmola, N.T. Preparing of nano-hydroxyapatite from seashell and mixed with gold to repair bony defect of mandibular bone in rabbits. Iraqi J. Ind. Res. 2023, 10, 120–130. [Google Scholar] [CrossRef]
- Ahmed, H.Y.; Safwat, N.; Shehata, R.; Althubaiti, E.H.; Kareem, S.; Atef, A.; Qari, S.H.; Aljahani, A.H.; Al-Meshal, A.S.; Youseef, M.; et al. Synthesis of natural nano-hydroxyapatite from snail shells and its biological activity: Antimicrobial, antibiofilm, and biocompatibility. Membranes 2022, 12, 408. [Google Scholar] [CrossRef]
- Aljaberi, K.; AlBadr, R.M.; Ziadan, K.M. A new approach to prepare nano hydroxyapatite from oyster shells used for dental applications. J. Kufa Phys. 2022, 14, 35–46. [Google Scholar] [CrossRef]
- Kristianto, N.A.; Sari, M.; Yusuf, Y. Hydroxyapatite based on abalone mussel shells coating on titanium alloy using electrophoretic deposition dip coating as a bone implant candidate. Chiang Mai Univ. J. Nat. Sci. 2022, 21, e2022021. [Google Scholar] [CrossRef]
- Puspitawati, I.N.; Tauhid, A.H.; Mnk, A.T.; Utami, L.I.; Wahyusi, K.N. Synthesis of hydroxyapatite from muscle shell waste using the precipitation method. Int. J. Eco-Innov. Sci. Eng. 2022, 3, 28–34. [Google Scholar] [CrossRef]
- Ng, C.K.; Lee, S.K.Y.; Tan, C.H.; Singh, R.; Ting, C.H.; Chuah, Y.D.; Tan, C.Y.; Sutharsini, U. Characterization and sintering properties of hydroxyapatite bioceramics synthesized from clamshell biowaste. IIUM Eng. J. 2022, 23, 228–236. [Google Scholar] [CrossRef]
- Mohd Roslan, M.R.; Mohd Nasir, N.F.; Mohammad, N.F.; Meng, C.E.; Mohd Amin, N.A.; Abdul Khalid, M.F.; Mohd Zakimi, Z.; Muhammad, M.A.; Jusoh, M. Synthesizing and optimization the hydroxyapatite based on Corbiculacea seashells. In Intelligent Manufacturing and Mechatronics, Proceedings of the SympoSIMM 2020, Perlis, Malaysia, 10 August 2020; Cavas-Martínez, F., Chaari, F., Gherardini, F., Haddar, M., Ivanov, V., Kwon, Y.W., Trojanowska, J., di Mare, F., Eds.; Springer Nature Singapore Pte Ltd.: Singapore, 2021; pp. 975–981. [Google Scholar] [CrossRef]
- Alhussary, B.N.; A Taqa, G.; Taqa, A.A.A. Preparation and characterization of natural nano hydroxyapatite from eggshell and seashell and its effect on bone healing. J. Appl. Vet. Sci. 2020, 5, 25–32. [Google Scholar] [CrossRef]
- Charlena, C.; Suparto, I.H.; Putri, D.K. Synthesis of hydroxyapatite from rice fields snail shell (Bellamya javanica) through wet method and pore modification using chitosan. Procedia Chem. 2015, 17, 27–35. [Google Scholar] [CrossRef]
- Mahmud, M.H.; Salam, K.A.; Gafur, M.A.; Rana, A.A.; Qadir, M.R.; Masum, S.M.; Sarker, M.; Karim, M.M. Chemical characteristics of hydroxyapatite from oyster shell by thermo-chemical process. Int. J. Innov. Res. Sci. Eng. Technol. 2015, 4, 5039–5047. [Google Scholar] [CrossRef]
- Santhosh, S.; Prabu, S.B. Synthesis and characterisation of nanocrystalline hydroxyapatite from sea shells. Int. J. Biomed. Nanosci. Nanotechnol. 2012, 2, 276–283. [Google Scholar] [CrossRef]
- Jones, M.I.; Barakat, H.; Patterson, D.A. Production of hydroxyapatite from waste mussel shells. IOP Conf. Ser. Mater. Sci. Eng. 2011, 18, 192002. [Google Scholar] [CrossRef]
- Singh, A.; Purohit, K.M. Chemical synthesis, characterization and bioactivity evaluation of hydroxyapatite prepared from garden snail (Helix aspersa). J. Bioproces. Biotechniq. 2011, 1, 1. [Google Scholar] [CrossRef]
- Koonawoot, R.; Thiansem, S.; Punyanitya, S.; Raksujarit, A.; Laosatirawong, S.; Pompimon, W. Synthesis of hydroxyapatite powder from mollusc shell. Adv. Mater. Res. 2011, 311, 1621–1624. [Google Scholar] [CrossRef]
- Gomez-Vazquez, O.M.; Martinez-Muñoz, P.E.; Perez-Ospina, J.L.; Rodriguez-Garcia, M.E. Comparative study between chemical precipitation and chemical precipitation by spraying for the recovery of nanometric hydroxyapatite. Mater. Today Commun. 2024, 40, 109832. [Google Scholar] [CrossRef]
- Fitriyana, D.F.; Irawan, A.P.; Bahatmaka, A.; Ismail, R.; Priharyoto, A.; Muhamadin, R.C.; Cionita, T.; Siregar, J.P.; Jehadus, E.; Baskara, G.D.; et al. The effect of temperature on the hydrothermal synthesis of carbonated apatite from calcium carbonate obtained from green mussels shells. ARPN J. Eng. Appl. Sci. 2023, 18, 1215–1224. [Google Scholar]
- Zuliantoni, Z.; Suprapto, W.; Setyarini, P.H.; Gapsari, F. Extraction and characterization of snail shell waste hydroxyapatite. Results Eng. 2022, 14, 100390. [Google Scholar] [CrossRef]
- Palaveniene, A.; Tamburaci, S.; Kimna, C.; Glambaite, K.; Baniukaitiene, O.; Tihminlioğlu, F.; Liesiene, J. Osteoconductive 3D porous composite scaffold from regenerated cellulose and cuttlebone-derived hydroxyapatite. J. Biomater. Appl. 2019, 33, 876–890. [Google Scholar] [CrossRef]
- Azis, Y.; Jamarun, N.; Arief, S.; Nur, H. Facile synthesis of hydroxyapatite particles from cockle shells (Anadara granosa) by hydrothermal method. Orient. J. Chem. 2015, 31, 1099–1105. [Google Scholar] [CrossRef]
- Sari, R.N.; Fransiska, D.; Dewi, F.R.; Sinurat, E. Karakteristik sediaan hidroksiapatit dari cangkang kerang simping (Amusium pleuronectes) dengan perlakuan suhu dan waktu sintesis. J. Pascapanen Bioteknol. Kelaut. Perikan. 2022, 17, 31–42. [Google Scholar] [CrossRef]
- Pratiwi, D.I.; Fadli, A.; Zultiniar, Z. Pengaruh suhu reaksi dan kecepatan pengadukan pada sintesa hidroksiapatit dari kulit kerang darah (Anadara granosa) dengan metode hidrotermal suhu rendah. Jom FTEKNIK 2015, 2, 1–10. [Google Scholar]
- Vecchio, K.S.; Zhang, X.; Massie, J.B.; Wang, M.; Kim, C.W. Conversion of bulk seashells to biocompatible hydroxyapatite for bone implants. Acta Biomater. 2007, 3, 910–918. [Google Scholar] [CrossRef]
- Edahwati, L.; Sutiyono, S.; Ikaputri, A.; Fuadzi, M.N. Application Of the sol-gel hydroxhapatite synthesis method from green clam shell. Int. J. Tradit. Syst. Med. 2023, 4, 866–871. [Google Scholar] [CrossRef]
- Charlena, C.; Maddu, A.; Hidayat, T. Synthesis and characterization of hydroxyapatite from green mussel shell with sol-gel method. Jurnal Kimia Valensi 2022, 8, 269–279. [Google Scholar] [CrossRef]
- Anjaneyulu, U.; Pattanayak, D.K.; Vijayalakshmi, U. Snail shell derived natural hydroxyapatite: Effects on NIH-3T3 cells for orthopedic applications. Mater. Manuf. Process. 2016, 31, 206–216. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Khorasani, M.T.; Dinpanah-Khoshdargi, E.; Jamshidi, A. Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater. 2013, 9, 7591–7621. [Google Scholar] [CrossRef]
- Malina, D.; Biernat, K.; Sobczak-Kupiec, A. Studies on sintering process of synthetic hydroxyapatite. Acta Biochim. Pol. 2013, 60, 851–855. [Google Scholar] [CrossRef]
- Scialla, S.; Carella, F.; Dapporto, M.; Sprio, S.; Piancastelli, A.; Palazzo, B.; Adamiano, A.; Esposti, L.D.; Iafisco, M.; Piccirillo, C. Mussel shell-derived macroporous 3D scaffold: Characterization and optimization study of a bioceramic from the circular economy. Mar. Drugs 2020, 18, 309. [Google Scholar] [CrossRef]
- Ge, Y.; Tang, B. Hydroxyapatite and Preparation Method Thereof. China Patent CN104211036A, 17 December 2014. Available online: https://patents.google.com/patent/CN104211036A/en (accessed on 27 August 2024).
- Ekren, N.; Gunduz, O.; Celik, S.; Ayata, B.; Sahin, Y.M.; Chou, J.; Ben-Nissan, B.; Salman, S.; Gokce, H.; Oktar, F.N. Production of apatite from snail shells for biomedical engineering applications. Key Eng. Mater. 2016, 696, 51–56. [Google Scholar] [CrossRef]
- Ramli, R.; Arawi, A.Z.O.; Talari, M.K.; Mahat, M.M.; Jais, U.S. Synthesis and structural characterization of nano-hydroxyapatite biomaterials prepared by microwave processing. AIP Conf. Proc. 2012, 1455, 45–48. [Google Scholar] [CrossRef]
- Akram, M.; Ahmed, R.; Shakir, I.; Ibrahim, W.A.W.; Hussain, R. Extracting hydroxyapatite and its precursors from natural resources. J. Mater. Sci. 2014, 49, 1461–1475. [Google Scholar] [CrossRef]
- Prihanto, A.; Muryanto, S.; Sancho Vaquer, A.; Schmahl, W.W.; Ismail, R.; Jamari, J.; Bayuseno, A.P. In-depth knowledge of the low-temperature hydrothermal synthesis of nanocrystalline hydroxyapatite from waste green mussel shell (Perna viridis). Environ. Technol. 2024, 45, 2375–2387. [Google Scholar] [CrossRef]
- Osuchukwu, O.A.; Salihi, A.; Abdullahi, I.; Abdulkareem, B.; Nwannenna, C.S. Synthesis techniques, characterization and mechanical properties of natural derived hydroxyapatite scaffolds for bone implants: A review. SN Appl. Sci. 2021, 3, 822. [Google Scholar] [CrossRef]
- Razak, R.A.; Stasya, A.; Ys, H. Effect of sintering temperature on hydroxyapatite yield of cuttlefish (Sepia sp.) using the wet deposition method. In 4th International Seminar on Science and Technology (ISST 2022), 1st ed.; Inda, N.I., Darwis, D., Sesa, E., Satrimafitrah, P., Eds.; Atlantis Press: Amsterdam, The Netherlands, 2023; pp. 286–294. [Google Scholar]
- Khiri, M.Z.A.; Matori, K.A.; Zaid, M.H.M.; Abdullah, C.A.C.; Zainuddin, N.; Alibe, I.M.; Rahman, N.A.A.; Wahab, S.A.A. The effect of the ph values and sintering temperatures on the physical, structural and mechanical properties of nano hydroxyapatite derived from ark clam shells (Anadara granosa) prepared via the wet chemical precipitate method. Ceram. Silikáty 2019, 63, 194–203. [Google Scholar] [CrossRef]
- Nurul Huda, A.; Subuki, I.; Hussain Ismail, M. Synthesized hydroxyapatite powder from clamshell via chemical precipitation method. Adv. Mater. Res. 2014, 911, 72–76. [Google Scholar] [CrossRef]
- Yanti, P.H.; Nia, N. The Effect of pH on synthesis of hydroxyapatite from Geloina coaxans shell. IPTEK J. Proc. Ser. 2017, 3, 33–38. [Google Scholar] [CrossRef]
- Muhamadin, R.C.; Ningtyas, A.H.P.; Pahlawan, I.A.; Hidayatullah, R.A.; Ismail, R.; Fitriyana, D.F.; Fadhilah, N.; Rachman, G.T. Characterization and synthesis hydroxyapatite from scallop mussel shells prepared by the microwave-assisted precipitation methods. SITEKIN J. Sains Teknol. Ind. 2023, 21, 175–182. [Google Scholar] [CrossRef]
- Ismail, R.; Laroybafih, M.B.; Fitriyana, D.F.; Nugroho, S.; Santoso, Y.I.; Hakim, A.J.; Al Mulqi, M.S.; Bayuseno, A.P. The effect of hydrothermal holding time on the characterization of hydroxyapatite synthesized from green mussel shells. J. Adv. Res. Fluid Mech. Therm. Sci. 2021, 80, 84–93. [Google Scholar] [CrossRef]
- Hussain, S.; Sabiruddin, K. Effect of heat treatment on the synthesis of hydroxyapatite from Indian clam seashell by hydrothermal method. Ceram. Int. 2021, 47, 29660–29669. [Google Scholar] [CrossRef]
- Toropkov, N.E.; Vereshchagin, V.I.; Petrovskaya, T.S.; Antonkin, N.S. Influence of synthesis conditions on the crystallinity of hydroxyapatite obtained by chemical deposition. IOP Conf. Ser. Mater. Sci. Eng. 2016, 156, 012038. [Google Scholar] [CrossRef]
- Merzougui, M.; Mezahi, F.Z.; Dakhouche, A.; Kherifi, D.; Sahnoune, F. Improvement of the reactivity of triethyl phosphate and structural behavior of hydroxyapatite versus the synthesis conditions by sol–gel route. Chem. Pap. 2022, 76, 1045–1061. [Google Scholar] [CrossRef]
- Chai, C.S.; Gross, K.A.; Ben-Nissan, B. Critical ageing of hydroxyapatite sol–gel solutions. Biomaterials 1998, 19, 2291–2296. [Google Scholar] [CrossRef]
- Dos Santos, M.L.; Riccardi, C.S.; Noronha, A.L.; Edson Filho, D.A.; Olyveira, G.M.; Guastaldi, A.C. Influence of aging time of the sol on the synthesis of hydroxyapatite powders using Ca (NO3)2·4H2O and H3PO4 as precursors. Mater. Focus 2015, 4, 189–192. [Google Scholar] [CrossRef]
- Fathi, M.H.; Hanifi, A. Sol–gel derived nanostructure hydroxyapatite powder and coating: Aging time optimisation. Adv. Appl. Ceram. 2009, 108, 363–368. [Google Scholar] [CrossRef]
- Bordean, D.M.; Nica, D.V.; Harmanescu, M.; Banatean-Dunea, I.; Gergen, I.I. Soil manganese enrichment from industrial inputs: A gastropod perspective. PLoS ONE 2014, 9, e85384. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.W.; Lee, B.W. Synthesis of calcium phosphates from abalone shells via precipitation. J. Korean Cryst. Growth Cryst. Technol. 2020, 30, 143–149. [Google Scholar] [CrossRef]
- Venkatesan, J.; Rekha, P.D.; Anil, S.; Bhatnagar, I.; Sudha, P.N.; Dechsakulwatana, C.; Kim, S.k.; Shim, M.S. Hydroxyapatite from cuttlefish bone: Isolation, characterizations, and applications. Biotechnol. Bioprocess Eng. 2018, 23, 383–393. [Google Scholar] [CrossRef]
- Xue, Q.; Xing, Y.; Zhang, Q. Preparation technology of oyster hydroxyapatite porous material for bone repair. Int. J. Biomed. Eng. 2018, 6, 291–295. [Google Scholar] [CrossRef]
- Ding, G.; Cheng, J.; Wu, X. Hydroxyapatite Toothpaste and Preparation Method Thereof. China Patent CN105640788A, 24 April 2018. Available online: https://patents.google.com/patent/CN105640788A/en (accessed on 9 September 2024).
- Cestari, F.; Agostinacchio, F.; Galotta, A.; Chemello, G.; Motta, A.M.; Sglavo, V. Nano-hydroxyapatite derived from biogenic and bioinspired calcium carbonates: Synthesis and in vitro bioactivity. Nanomaterials 2021, 11, 264. [Google Scholar] [CrossRef]
- Malau, N.D. Manufacture and characterization of hydroxyapatite from quail eggshell using precipitation methods. Int. J. Prog. Sci. Technol. 2021, 29, 484–490. [Google Scholar]
- Gomes, F.D.C.; de Amorim, J.D.P.; da Silva, G.S.; de Souza, K.C.; Pinto, A.F.; Santos, B.S.; de Santana Costa, A.F. Preparation and Characterization of Hydroxyapatite by the precipitation method and heat treatment. Res. Soc. Dev. 2020, 9, e172963549. [Google Scholar] [CrossRef]
- Dobrovol’skaya, I.P.; Tsarev, N.S.; Osmolovskaya, O.M.; Kasatkin, I.A.; Ivan’kova, E.M.; Popova, E.N.; Pankova, A.; Yudin, V.E. Effect of thermal treatment on the structure and properties of hydroxyapatite. Russ. J. Appl. Chem. 2018, 91, 368–374. [Google Scholar] [CrossRef]
- Jeong, H.J.; Gwak, S.J.; Seo, K.D.; Lee, S.; Yun, J.H.; Cho, Y.S.; Lee, S.J. Fabrication of three-dimensional composite scaffold for simultaneous alveolar bone regeneration in dental implant installation. Int. J. Mol. Sci. 2020, 21, 1863. [Google Scholar] [CrossRef]
- Permatasari, H.A.; Sari, M.; Aminatun Suciati, T.; Dahlan, K.; Yusuf, Y. Nano-carbonated hydroxyapatite precipitation from abalone shell (Haliotis asinina) waste as the bioceramics candidate for bone tissue engineering. Nanomater. Nanotechno. 2021, 11, 18479804211032851. [Google Scholar] [CrossRef]
- Ishikawa, K.; Garskaite, E.; Kareiva, A. Sol–gel synthesis of calcium phosphate-based biomaterials—A review of environmentally benign, simple, and effective synthesis routes. J. Sol-Gel Sci. Technol. 2020, 94, 551–572. [Google Scholar] [CrossRef]
- Piras, S.; Salathia, S.; Guzzini, A.; Zovi, A.; Jackson, S.; Smirnov, A.; Santulli, C. Biomimetic use of food-waste sources of calcium carbonate and phosphate for sustainable materials—A review. Materials 2024, 17, 843. [Google Scholar] [CrossRef] [PubMed]
- Alif, M.F.; Aprillia, W.; Arief, S. A hydrothermal synthesis of natural hydroxyapatite obtained from Corbicula moltkiana freshwater clams shell biowaste. Mater. Lett. 2018, 230, 40–43. [Google Scholar] [CrossRef]
- Lee, C.Y.; Hu, S.M.; Christy, J.; Chou, F.Y.; Ramli, T.C.; Chen, H.Y. Biointerface coatings with structural and biochemical properties modifications of biomaterials. Adv. Mater. Interfaces 2023, 10, 2202286. [Google Scholar] [CrossRef]
- Shavandi, A.; Bekhit, A.E.D.A.; Ali, A.; Sun, Z. Synthesis of nano-hydroxyapatite (nHA) from waste mussel shells using a rapid microwave method. Mater. Chem. Phys. 2015, 149, 607–616. [Google Scholar] [CrossRef]
- Zuo, S.; Peng, Q.; Luo, T.; Wang, Y.; Peng, Z. Microwave-assisted synthesis of composites based on titanium and hydroxyapatite for dental implantation. Biomater. Sci. 2024, 12, 92–107. [Google Scholar] [CrossRef]
- Wu, S.C.; Kao, Y.L.; Lu, Y.C.; Hsu, H.C.; Ho, W.F. Preparation and characterization of microrod hydroxyapatite bundles obtained from oyster shells through microwave irradiation. J. Aust. Ceram. Soc. 2021, 57, 1541–1551. [Google Scholar] [CrossRef]
- Dasgupta Adak, M.; Purohit, K.M. Synthesis of nano-crystalline hydroxyapatite from dead snail shells for biological implantation. Trends Biomater. Artif. Organs 2011, 25, 101–106. [Google Scholar]
- Dorcioman, G.; Grumezescu, V.; Stan, G.E.; Chifiriuc, M.C.; Gradisteanu, G.P.; Miculescu, F.; Matei, E.; Popescu-Pelin, G.; Zgura, I.; Crăciun, V.; et al. Hydroxyapatite thin films of marine origin as sustainable candidates for dental implants. Pharmaceutics 2023, 15, 1294. [Google Scholar] [CrossRef]
- Kowalski, S.; Gonciarz, W.; Belka, R.; Góral, A.; Chmiela, M.; Lechowicz, Ł.; Kaca, W.; Żórawski, W. Plasma-sprayed hydroxyapatite coatings and their biological properties. Coatings 2022, 12, 1317. [Google Scholar] [CrossRef]
- Hussain, S.; Shah, Z.A.; Sabiruddin, K.; Keshri, A.K. Characterization and tribological behaviour of Indian clam seashell-derived hydroxyapatite coating applied on titanium alloy by plasma spray technique. J. Mech. Behav. Biomed. Mater. 2023, 137, 105550. [Google Scholar] [CrossRef] [PubMed]
- Dhanaraj, K.; Suresh Kumar, C.; Socrates, S.H.; Vinoth Arulraj, J.; Suresh, G. A comparative analysis of microwave assisted natural (Murex virgineus shell) and chemical nanohydroxyapatite: Structural, morphological and biological studies. J. Aust. Ceram. Soc. 2021, 57, 173–183. [Google Scholar] [CrossRef]
- Sidauruk, S.W.; Iriani, D.; Sari, N.I.; Rusdi, R.R.; Rusadi, M.I. Evaluation of antibacterial activity of nano-hydroxyapatite (HAp) from freshwater mussel (Pilsbryoconcha sp.) shell against Escherichia coli. BIO Web Conf. 2023, 74, 02002. [Google Scholar] [CrossRef]
- Sanpo, N.; Tharajak, J. Antimicrobial property of hydroxyapatite derivative nanoparticles. Appl. Mech. Mater. 2017, 866, 81–84. [Google Scholar] [CrossRef]
- Bhavan Ram, U.; Sujatha, V.; Vidhya, S.; Jayasree, R.; Mahalaxmi, S. Oyster shell-derived nano-hydroxyapatite and proanthocyanidin pretreatment on dentinal tubule occlusion and permeability before and after acid challenge—An in vitro study. J. Mater. Sci. Mater. Med. 2020, 34, 17. [Google Scholar] [CrossRef]
- Sari, M.; Ramadhanti, D.M.; Amalina, R.; Ana, I.D.; Yusuf, Y. Development of a hydroxyapatite nanoparticle-based gel for enamel remineralization—A physicochemical properties and cell viability assay analysis. Dent. Mater. J. 2022, 41, 68–77. [Google Scholar] [CrossRef]
- Hikmah, N.; Nugroho, J.J.; Natsir, N.; Rovani, C.A.; Mooduto, L. Enamel remineralization after extracoronal bleaching using nano-hydroxyapatite (NHA) from synthesis results of blood clam (Anadara granosa) shells. J. Dentomaxillofacial Sci. 2019, 4, 28–31. [Google Scholar] [CrossRef]
- Kranz, S.; Heyder, M.; Mueller, S.; Guellmar, A.; Krafft, C.; Nietzsche, S.; Caroline Tschirpke, C.; Herold, V.; Sigusch, B.; Reise, M. Remineralization of artificially demineralized human enamel and dentin samples by zinc-carbonate hydroxyapatite nanocrystals. Materials 2022, 15, 7173. [Google Scholar] [CrossRef]
- Wang, Y.; Tsuru, K.; Ishikawa, K.; Yokoi, T.; Kawashita, M. Fibronectin adsorption on carbonate-containing hydroxyapatite. Ceram. Int. 2021, 47, 11769–11776. [Google Scholar] [CrossRef]
- Madupalli, H.; Pavan, B.; Tecklenburg, M.M. Carbonate substitution in the mineral component of bone: Discriminating the structural changes, simultaneously imposed by carbonate in A and B sites of apatite. J. Solid State Chem. 2017, 255, 27–35. [Google Scholar] [CrossRef]
- Kee, C.C.; Ismail, H.; Noor, A.F.M. Effect of synthesis technique and carbonate content on the crystallinity and morphology of carbonated hydroxyapatite. J. Mater. Sci. Technol. 2013, 29, 761–764. [Google Scholar] [CrossRef]
- Nica, D.V.; Bordean, D.M.; Borozan, A.B.; Gergen, I.; Bura, M.; Banatean-Dunea, I. Use of land snails (Pulmonata) for monitoring copper pollution in terrestrial ecosystems. Rev. Environ. Contam. Toxicol. 2013, 225, 95–137. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Darvell, B.W. Effect of carbonate on hydroxyapatite solubility. Cryst. Growth Des. 2010, 10, 845–850. [Google Scholar] [CrossRef]
- Gruselle, M.; Tonsuaadu, K.; Gredin, P.; Len, C. Apatites based catalysts: A tentative classification. Mol. Catal. 2022, 519, 112146. [Google Scholar] [CrossRef]
- Merry, J.C.; Gibson, I.R.; Best, S.M.; Bonfield, W. Synthesis and characterization of carbonate hydroxyapatite. J. Mater. Sci. Mater. Med. 1998, 9, 779–783. [Google Scholar] [CrossRef]
- Adekanmi, D.G.; Garcia, C.R.; Lopez-Badillo, C.M. Carbonate hydroxyapatite-a multifunctional bioceramics with non-medical applications. Eng. Chem. 2024, 7, 1–24. [Google Scholar] [CrossRef]
- Landi, E.; Celotti, G.; Logroscino, G.; Tampieri, A. Carbonated hydroxyapatite as bone substitute. J. Eur. Ceram. Soc. 2003, 23, 2931–2937. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, M.; Cheung, W.L.; Guo, B.C.; Jia, D.M. Synthesis of carbonated hydroxyapatite nanospheres through nanoemulsion. J. Mater. Sci. Mater. Med. 2008, 19, 103–111. [Google Scholar] [CrossRef]
- Nandhini, A.; Sudhakar, T.; Premkumar, J. Ceramics and nanoceramics in biomedical applications. In Handbook of Polymer and Ceramic Nanotechnology; Hussain, C.M., Thomas, S., Eds.; Springer Nature: Cham, Switzerland, 2021; pp. 763–779. [Google Scholar]
- Ardan, L.; Yusuf, Y. Synthesis and characterization of carbonate hydroxyapatite from pokea clam shells (Batissa violacea Var. Celebensis) by precipitation and hydrothermal methods. Key Eng. Mater. 2024, 977, 109–114. [Google Scholar] [CrossRef]
- Cahyati, N.; Sari, M.; Yusuf, Y. Properties of carbonated hydroxyapatite-based scaffold from oyster shells composited with honeycomb and polyethylene oxide for bone tissue engineering applications. Key Eng. Mater. 2024, 977, 103–108. [Google Scholar] [CrossRef]
- Januariyasa, I.K.; Yusuf, Y. Synthesis of carbonated hydroxyapatite derived from snail shells (Pilla ampulacea): Effect of carbonate precursor to the crystallographic properties. IOP Conf. Ser. Mater. Sci. Eng. 2019, 546, 042015. [Google Scholar] [CrossRef]
- Ge, Y.M.; Li, H.L.; Jiang, K.; Xue, Y.Z.B.; Tang, B. Fabrication of nano-size AB-type carbonated hydroxyapatite particles from seashells. J. Biomater. Tissue Eng. 2016, 6, 635–641. [Google Scholar] [CrossRef]
- Lemos, A.F.; Rocha, J.H.G.; Quaresma, S.S.F.; Kannan, S.; Oktar, F.N.; Agathopoulos, S.; Ferreira, J.M.F. Hydroxyapatite nano-powders produced hydrothermally from nacreous material. J. Eur. Ceram. Soc. 2006, 26, 3639–3646. [Google Scholar] [CrossRef]
- Permatasari, H.A.; Yusuf, Y. Characteristics of carbonated hydroxyapatite based on abalone mussel shells (Halioitis asinina) synthesized by precipitation method with aging time variations. IOP Conf. Ser. Mater. Sci. Eng. 2019, 546, 042031. [Google Scholar] [CrossRef]
- Nurazzi, N.M.; Norrrahim, M.N.F.; Mulla, M.H.; Kamarudin, S.H.; Rani, M.S.A.; Rushdan, A.I.; Kuzmin, A.M. Mechanical performance of seashell-reinforced polymer composites for structural applications. In Polymer Composites Derived from Animal Sources; Woodhead Publishing: Sawston, UK, 2024; pp. 243–257. [Google Scholar] [CrossRef]
- Tabrizian, P.; Sun, H.; Jargalsaikhan, U.; Sui, T.; Davis, S.; Su, B. Biomimetic nacre-like hydroxyapatite/polymer composites for bone implants. J. Funct. Biomater. 2023, 14, 393. [Google Scholar] [CrossRef]
- Wati, R.; Yusuf, Y. Effect of sintering temperature on carbonated hydroxyapatite derived from common cockle shells (Cerastoderma edule): Composition and crystal characteristics. Key Eng. Mater. 2019, 818, 37–43. [Google Scholar] [CrossRef]
- Youness, R.A.; Taha, M.A.; Ibrahim, M.A. Effect of sintering temperatures on the in vitro bioactivity, molecular structure and mechanical properties of titanium/carbonated hydroxyapatite nanobiocomposites. J. Mol. Struct. 2017, 1150, 188–195. [Google Scholar] [CrossRef]
- Almukarramah, A.; Yusuf, Y. Development of carbonated hydroxyapatite powders from oyster shells (Crassostrea gigas) by carbonate content variations. Mater. Sci. Forum 2020, 975, 76–81. [Google Scholar] [CrossRef]
- Anggraini, R.M.; Yusuf, Y. The effect of stirring time on the characteristics of carbonated hydroxyapatite from pearl shells (Pinctada maxima). IOP Conf. Ser. Mater. Sci. Eng. 2019, 546, 042002. [Google Scholar] [CrossRef]
- Megawati, M.; Patty, D.J.; Yusuf, Y. Synthesis and characterization of carbonate hydroxyapatite from pinctada maxima shell with short aging time for bone biomaterial candidate. Eng. Chem. 2023, 3, 13–18. [Google Scholar] [CrossRef]
- Wang, X.; Wan, C.; Feng, X.; Zhao, F.; Wang, H. In vivo and in vitro analyses of titanium-hydroxyapatite functionally graded material for dental implants. Biomed. Res. Int. 2021, 2021, 8859945. [Google Scholar] [CrossRef] [PubMed]
- Anggraini, R.M.; Supii, A.I.; Suparta, G.B.; Yusuf, Y. The effect of pH on the characteristics of carbonate hydroxyapatite based on pearl shell (Pinctada maxima). Key Eng. Mater. 2019, 818, 44–49. [Google Scholar] [CrossRef]
- Setyoko, B.; Verisandri, A.L.A.; Damayanti, A.T.; Fitriana, F.A.; Julieta, B.S.; Noviasari, P.; Alhasyimi, A.A. Effect of carbonated hydroxyapatite synthesis from cuttlefish shells on orthodontic relapse prevention: In silico study. Odonto Dent. J. 2023, 10, 19–27. [Google Scholar] [CrossRef]
- Ryu, S.C.; Kim, H.S. A study on the properties of hydroxyapatite powders prepared from oyster shells. Korean J. Mater. Res. 2003, 13, 703–707. [Google Scholar] [CrossRef]
- Canullo, L.; Wiel Marin, G.; Tallarico, M.; Canciani, E.; Musto, F.; Dellavia, C. Histological and histomorphometrical evaluation of postextractive sites grafted with Mg-enriched nano-hydroxyapatite: A randomized controlled trial comparing 4 versus 12 months of healing. Clin. Implant Dent. Relat. Res. 2016, 18, 973–983. [Google Scholar] [CrossRef]
- Canullo, L.; Patacchia, O.; Sisti, A.; Heinemann, F. Implant restoration 3 months after one-stage sinus lift surgery in severely resorbed maxillae: 2-year results of a multicenter prospective clinical study. Clin. Implant Dent. Relat. Res. 2012, 14, 412–420. [Google Scholar] [CrossRef]
- Kattimani, V.; Lingamaneni, K.P.; Yalamanchili, S.; Mupparapu, M. Use of eggshell-derived nano-hydroxyapatite as novel bone graft substitute-A randomized controlled clinical study. J. Biomater. Appl. 2019, 34, 597–614. [Google Scholar] [CrossRef]
- Vickers, P.; Slater, G.; Mathen, L. Case series: Use of coralline hydroxyapatite graft in faciomaxillary surgery. J. Regen. Biol. Med. 2021, 3, 1–16. [Google Scholar] [CrossRef]
- Almeida, A.C.D.; Silva, A.R.P.D.; Nakamura Filho, A.; Carvalho, M.D.D.; Cardoso, A.V. Nacre compared to aragonite as a bone substitute: Evaluation of bioactivity and biocompatibility. Mater. Res. 2015, 18, 395–403. [Google Scholar] [CrossRef]
- Coringa, R.; de Sousa, E.M.; Botelho, J.N.; Diniz, R.S.; de Sá, J.C.; da Cruz, M.C.F.N.; Paschoal, M.A.B.; Gonçalves, L.M. Bone substitute made from a Brazilian oyster shell functions as a fast stimulator for bone-forming cells in an animal model. PLoS ONE 2018, 13, e0198697. [Google Scholar] [CrossRef]
| Method Type | Specific Method | Brief Description | Reference |
|---|---|---|---|
| In Vitro Cell Culture | Cell Viability and Proliferation Assays | Assays like MTT, Alamar Blue, and live/dead staining determine the viability and proliferation of osteoblasts on hydroxyapatite | [59] |
| Osteoblast Differentiation Assays | Determines alkaline phosphatase activity, mineralization (e.g., Alizarin Red staining), and expression of osteogenic markers (e.g., Runx2, OCN, OPN) | [78] | |
| Cell Adhesion and Morphology | Employs SEM and immunofluorescence to observe cell attachment, spreading, and morphology on hydroxyapatite surface | [81] | |
| In Vivo Animal Models | Implantation Studies | HA implants are inserted into bone defects or subcutaneous sites in animals to assess new bone formation and material integration | [128] |
| Histological Analysis | Bone tissues around the implant are sectioned and stained (e.g., H&E, Masson’s Trichrome) to examine bone formation and bone-material interface | [98] | |
| Micro-Computed Tomography (Micro-CT) | Offers high-resolution 3D images on bone features (volume, density, architecture) around the hydroxyapatite implant | [59] | |
| Mechanical Testing | Push-Out or Pull-Out Tests | Measures the force needed to dislodge the HA implant from the surrounding bone, reflecting the strength of bone-material integration | [78] |
| Compression and Bending Tests | Evaluates the mechanical properties of the bone-HA composite | [81] | |
| Biochemical Assays | Calcium and Phosphate Content | Measures mineral deposition on HA using techniques like inductively coupled plasma mass spectrometry (ICP-MS) | [98] |
| Osteocalcin and Osteopontin Assays | Quantifies these bone-specific proteins in the tissue or culture medium to indicate osteogenic activity | [128] | |
| Surface Characterization | X-Ray Diffraction (XRD) | Analyzes the crystalline structure of HA and its similarity to natural bone | [59] |
| Fourier Transform Infrared Spectroscopy (FTIR) | Identifies chemical bonds and functional groups, revealing the presence of bone mineralization | [78] | |
| Energy-Dispersive X-Ray Spectroscopy (EDS) | Offers elemental composition data of HA and the newly formed bone | [81] | |
| Surface and Structural Analysis | Atomic Force Microscopy (AFM) | Measures surface roughness and topography (with effect on cell adhesion and proliferation) | [59] |
| Scanning Electron Microscopy (SEM) | Offers detailed images of the surface morphology and microstructure of HA, illustrating its interaction with bone cells | [98] | |
| Transmission Electron Microscopy (TEM) | Provides high-resolution images to analyze the fine structural details of HA and its integration with bone tissue | [128] | |
| Biodegradability and Bioactivity Tests | In Vitro Degradation Studies | Assesses the rate at which HA degrades in simulated body fluid (SBF) or other physiological conditions | [78] |
| Bioactivity Tests | Evaluates the formation of apatite on the HA surface when immersed in SBF, indicating the material’s ability to bond with natural bone | [81] |
| Characteristic | Hydroxyapatite | Carbonated Hydroxyapatite | |
|---|---|---|---|
| Chemical composition |
|
| [147] |
| Structure |
|
| [146] |
| Properties |
|
| [147,148] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muntean, F.L.; Olariu, I.; Marian, D.; Olariu, T.; Petrescu, E.L.; Olariu, T.; Drăghici, G.A. Hydroxyapatite from Mollusk Shells: Characteristics, Production, and Potential Applications in Dentistry. Dent. J. 2024, 12, 409. https://doi.org/10.3390/dj12120409
Muntean FL, Olariu I, Marian D, Olariu T, Petrescu EL, Olariu T, Drăghici GA. Hydroxyapatite from Mollusk Shells: Characteristics, Production, and Potential Applications in Dentistry. Dentistry Journal. 2024; 12(12):409. https://doi.org/10.3390/dj12120409
Chicago/Turabian StyleMuntean, Florin Lucian, Iustin Olariu, Diana Marian, Teodora Olariu, Emanuela Lidia Petrescu, Tudor Olariu, and George Andrei Drăghici. 2024. "Hydroxyapatite from Mollusk Shells: Characteristics, Production, and Potential Applications in Dentistry" Dentistry Journal 12, no. 12: 409. https://doi.org/10.3390/dj12120409
APA StyleMuntean, F. L., Olariu, I., Marian, D., Olariu, T., Petrescu, E. L., Olariu, T., & Drăghici, G. A. (2024). Hydroxyapatite from Mollusk Shells: Characteristics, Production, and Potential Applications in Dentistry. Dentistry Journal, 12(12), 409. https://doi.org/10.3390/dj12120409







