Oral Health of 7- to 9-Year-Old Children Born Prematurely—A Case–Control Observational Study with Randomized Case Selection
Abstract
1. Introduction
- The prevalence of dental caries in deciduous and permanent teeth is higher in children born PT than in children born FT.
- The prevalence of DDE in deciduous and permanent teeth is higher in PT children than in FT children.
- PT children have poorer periodontal health than FT children do.
2. Materials and Methods
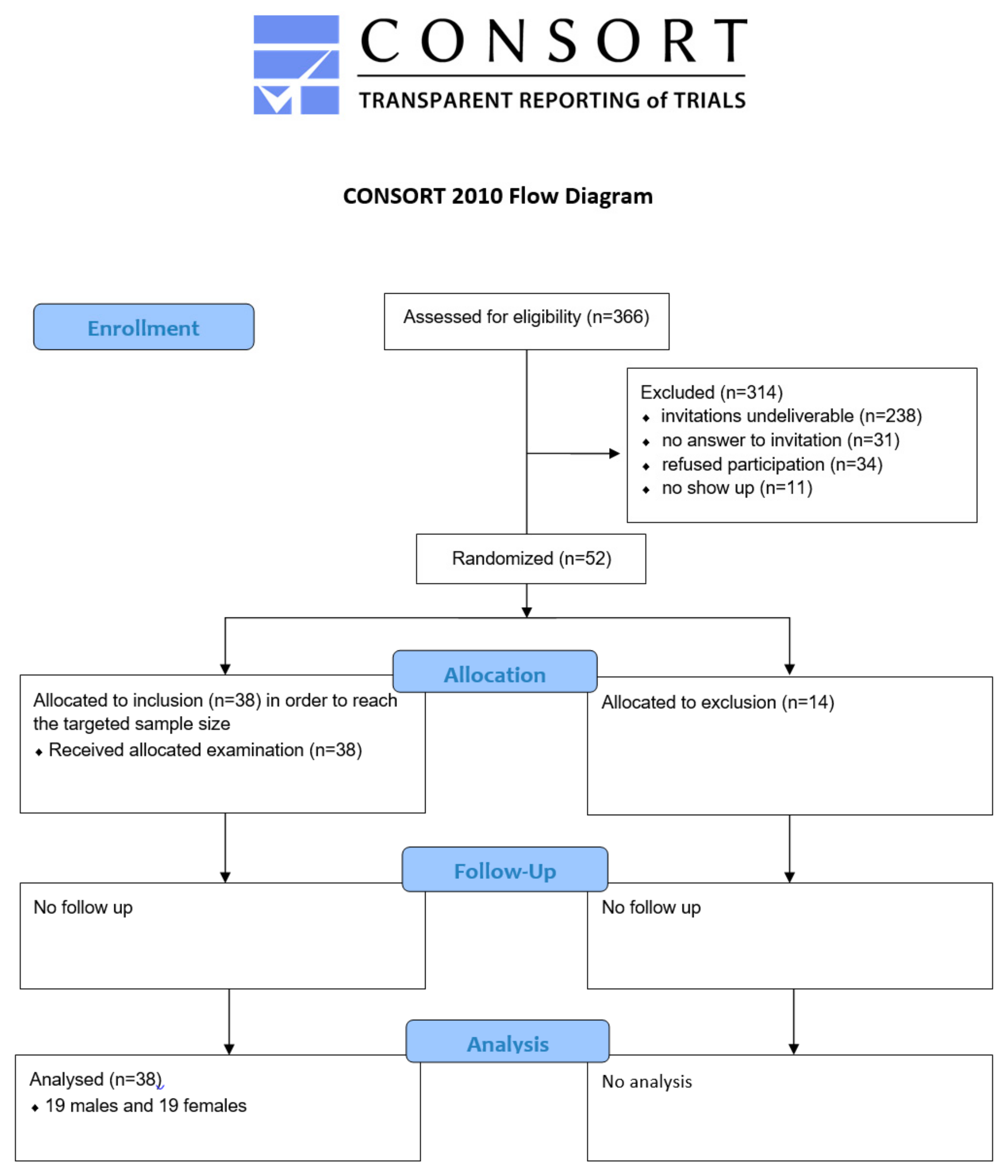
2.1. Study Sample
2.2. Dental Examination
2.3. Data Collection and Analysis
3. Results
3.1. Number of Teeth
3.2. Developmental Defects of Enamel (DDE)
3.3. Dental Caries
3.3.1. Caries Prevalence
3.3.2. Caries Experience
3.4. Periodontal Health
3.5. Socioeconomic Status
4. Discussion
4.1. Number of Teeth
4.2. Dental Caries
4.3. Develeopmental Defects of Enamel
4.4. Periodental Health
4.5. Socioeconomic Status
4.6. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Recommended definitions, terminology and format for statistical tables related to the perinatal period and use of a new certificate for cause of perinatal deaths. Acta Obstet. Gynecol. Scand. 1977, 56, 247–253. [Google Scholar] [CrossRef]
- Crump, C.; Winkleby, M.A.; Sundquist, J.; Sundquist, K. Prevalence of Survival Without Major Comorbidities Among Adults Born Prematurely. JAMA 2019, 322, 1580–1588. [Google Scholar] [CrossRef] [PubMed]
- Wolke, D.; Johnson, S.; Mendonça, M. The Life Course Consequences of Very Preterm Birth. Annu. Rev. Dev. Psychol. 2019, 1, 69–92. [Google Scholar] [CrossRef]
- Ohuma, E.O.; Moller, A.-B.; Bradley, E.; Chakwera, S.; Hussain-Alkhateeb, L.; Lewin, A.; Okwaraji, Y.B.; Mahanani, W.R.; Johansson, E.W.; Lavin, T.; et al. National, regional, and global estimates of preterm birth in 2020, with trends from 2010: A systematic analysis. Lancet 2023, 402, 1261–1271. [Google Scholar] [CrossRef]
- Schleußner, E. Drohende Frühgeburt: Prävention, Diagnostik und Therapie. Dtsch. Arztebl. 2013, 110, 227–236. (In German) [Google Scholar]
- Frick, A.P. Advanced maternal age and adverse pregnancy outcomes. Best. Pract. Res. Clin. Obstet. Gynaecol. 2021, 70, 92–100. [Google Scholar] [CrossRef]
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef]
- Manrique-Corredor, E.J.; Orozco-Beltran, D.; Lopez-Pineda, A.; Quesada, J.A.; Gil-Guillen, V.F.; Carratala-Munuera, C. Maternal periodontitis and preterm birth: Systematic review and meta-analysis. Community Dent. Oral Epidemiol. 2019, 47, 243–251. [Google Scholar] [CrossRef]
- Terzic, M.; Aimagambetova, G.; Terzic, S.; Radunovic, M.; Bapayeva, G.; Laganà, A.S. Periodontal Pathogens and Preterm Birth: Current Knowledge and Further Interventions. Pathogens 2021, 10, 730. [Google Scholar] [CrossRef]
- Gravett, M.G.; Menon, R.; Tribe, R.M.; Hezelgrave, N.L.; Kacerovsky, M.; Soma-Pillay, P.; Jacobsson, B.; McElrath, T.F. Assessment of current biomarkers and interventions to identify and treat women at risk of preterm birth. Front. Med. 2024, 11, 1414428. [Google Scholar] [CrossRef]
- Liu, M.-X.; Li, H.-F.; Wu, M.-Q.; Geng, S.-S.; Ke, L.; Lou, B.-W.; Du, W.; Hua, J. Associations of preterm and early-term birth with suspected developmental coordination disorder: A national retrospective cohort study in children aged 3–10 years. World J. Pediatr. 2023, 19, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Holsti, A.; Adamsson, M.; Hägglöf, B.; Farooqi, A.; Serenius, F. Chronic conditions and health care needs of adolescents born at 23 to 25 weeks’ gestation. Pediatrics 2017, 139, e20162215. [Google Scholar] [CrossRef] [PubMed]
- Peralta-Carcelen, M.; Schwartz, J.; Carcelen, A.C. Behavioral and Socioemotional Development in Preterm Children. Clin. Perinatol. 2018, 45, 529–546. [Google Scholar] [CrossRef] [PubMed]
- Panceri, C.; Sbruzzi, G.; Zanella, L.W.; Wiltgen, A.; Procianoy, R.S.; Silveira, R.C.; Valentini, N.C. Developmental coordination disorder in preterm children: A systematic review and meta-analysis. Eur. J. Neurosci. 2024, 60, 4128–4147. [Google Scholar] [CrossRef]
- Pineda, R.; Prince, D.; Reynolds, J.; Grabill, M.; Smith, J. Preterm infant feeding performance at term equivalent age differs from that of full-term infants. J. Perinatol. 2020, 40, 646–654. [Google Scholar] [CrossRef]
- Park, S.; Jeong, S.J.; Han, J.H.; Shin, J.E.; Lee, J.-H.; Kang, C.-M. Natal factors affecting developmental defects of enamel in preterm infants: A prospective cohort study. Sci. Rep. 2024, 14, 2089. [Google Scholar] [CrossRef]
- Paulsson, L.; Arvini, S.; Bergström, N.; Klingberg, G.; Lindh, C. The impact of premature birth on dental maturation in the permanent dentition. Clin. Oral Investig. 2019, 23, 855–861. [Google Scholar] [CrossRef]
- Buhamer, S.N.; Kaklamanos, E.; Kowash, M.; Hussein, I.; Salami, A.; Al-Halabi, M. What is the effect of preterm birth on permanent tooth crown dimensions? A systematic review and meta-analysis. PLoS ONE 2021, 16, e0259293. [Google Scholar] [CrossRef]
- Rythén, M.; Thilander, B.; Robertson, A. Dento-alveolar characteristics in adolescents born extremely preterm. Eur. J. Orthod. 2013, 35, 475–482. [Google Scholar] [CrossRef]
- Paulsson, L. Premature birth—Studies on orthodontic treatment need, craniofacial morphology and function. Swed. Dent. J. Suppl. 2009, 199, 9–66. [Google Scholar]
- Xu, S.; Zhao, C.; Jia, L.; Ma, Z.; Zhang, X.; Shi, H. Relationship between preterm, low birth weight, and development defects of enamel in the primary dentition: A meta-analysis. Front. Pediatr. 2022, 10, 975340. [Google Scholar] [CrossRef] [PubMed]
- Halperson, E.; Shafir, S.; Fux-Noy, A.; Ram, D.; Eventov-Friedman, S. Developmental defects of enamel in children born preterm. Front. Pediatr. 2022, 10, 1019586. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seow, W.K. Effects of preterm birth on oral growth and development. Aust. Dent. J. 1997, 42, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Rythén, M. Preterm Infants—Odontological Aspects. Swed. Dent. J. Suppl. 2012, 224, 2–106. [Google Scholar]
- Eastman, D.L. Dental outcomes of preterm infants. Newborn Infant Nurs. Rev. 2003, 3, 93–98. [Google Scholar] [CrossRef]
- Atar, M.; Körperich, E.J. Systemic disorders and their influence on the development of dental hard tissues: A literature review. J. Dent. 2010, 38, 296–306. [Google Scholar] [CrossRef]
- Bag, A.; Gayen, K.; Sikdar, R.; Shirolkar, S.; Sarkar, S.; Roychowdhury, S. Enlightening the Effects of Premature Birth on Dental and Orofacial Development: A Review. Int. J. Health Sci. Res. 2021, 11, 157–163. [Google Scholar] [CrossRef]
- de Carvalho, P.; Arima, L.; Abanto, J.; Bönecker, M. Maternal-Child Health Indicators Associated with Developmental Defects of Enamel in Primary Dentition. Pediatr. Dent. 2022, 44, 425–433. [Google Scholar]
- Schüler, I.M.; Haberstroh, S.; Dawczynski, K.; Lehmann, T.; Heinrich-Weltzien, R. Dental caries and developmental defects of enamel in the primary dentition of preterm infants: Case-control observational study. Caries Res. 2018, 52, 22–31. [Google Scholar] [CrossRef]
- Portella, P.D.; Dias, B.C.; Ferreira, P.; de Souza, J.F.; Wambier, L.; da Silva Assunção, L.R. The Association of Developmental Dental Defects and the Clinical Consequences in the Primary Dentition: A Systematic Review of Observational Studies. Pediatr. Dent. 2022, 44, 330–341. [Google Scholar]
- Cruvinel, V.R.N.; Gravina, D.B.L.; Azevedo, T.D.P.L.; de Rezende, C.S.; Bezerra, A.C.B.; de Toledo, O.A. Prevalence of enamel defects and associated risk factors in both dentitions in preterm and full term born children. J. Appl. Oral Sci. 2012, 20, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Rythén, M.; Sabel, N.; Dietz, W.; Robertson, A.; Norén, J.G. Chemical aspects on dental hard tissues in primary teeth from preterm infants. Eur. J. Oral Sci. 2010, 118, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Brogårdh-Roth, S.; Matsson, L.; Klingberg, G. Molar-incisor-hypomineralization and oral hygiene in 10- to-12-yr-old Swedish children born preterm. Eur. J. Oral Sci. 2011, 119, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Occhi-Alexandre, I.G.P.; Cruz, P.V.; Bendo, C.B.; Paiva, S.M.; Pordeus, I.A.; Martins, C.C. Prevalence of dental caries in preschool children born preterm and/or with low birth weight: A systematic review with meta-analysis of prevalence data. Int. J. Paediatr. Dent. 2020, 30, 265–275. [Google Scholar] [CrossRef]
- Bernabé, E.; MacRitchie, H.; Longbottom, C.; Pitts, N.B.; Sabbah, W. Birth weight, breastfeeding, maternal smoking and caries trajectories. J. Dent. Res. 2017, 96, 171–178. [Google Scholar] [CrossRef]
- Varoneckas, A.; Jasinskaite, K.; Varas, A. Relationship between Early Childhood Caries and andverse birth outcomes. Health Sci. 2021, 31, 196–200. [Google Scholar] [CrossRef]
- Caufield, P.W.; Li, Y.; Bromage, T.G. Hypoplasia-associated severe early childhood caries—A proposed definition. J. Dent. Res. 2012, 91, 544–550. [Google Scholar] [CrossRef]
- Law, V.; Seow, W.K.; Townsend, G. Factors influencing oral colonization of mutans streptococci in young children. Austr. Dent. J. 2007, 52, 93–100. [Google Scholar] [CrossRef]
- Brown, R.N.; Burnett, A.C.; Thompson, D.K.; Spittle, A.J.; Ellis, R.; Cheong, J.L.Y.; Doyle, L.W.; Pascoe, L.; Anderson, P.J. Motor performance and attention outcomes in children born very preterm. Dev. Med. Child. Neurol. 2023, 65, 1501–1510. [Google Scholar] [CrossRef]
- Fitzallen, G.C.; Sagar, Y.K.; Taylor, H.G.; Bora, S. Anxiety and Depressive Disorders in Children Born Preterm: A Meta-Analysis. J. Dev. Behav. Pediatr. 2021, 42, 154–162. [Google Scholar] [CrossRef]
- Cianetti, S.; Lombardo, G.; Lupatelli, E.; Pagano, S.; Abraha, I.; Montedori, A.; Caruso, S.; Gatto, R.; De Giorgio, S.; Salvato, R. Dental fear/anxiety among children and adolescents. A systematic review. Eur. J. Paediatr. Dent. 2017, 18, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Gao, X. Children’s dental fear and anxiety: Exploring family related factors. BMC Oral Health 2018, 18, 100. [Google Scholar] [CrossRef] [PubMed]
- Mundim, A.P.; Corrêa-Faria, P.; Costa, L.R. Do preschoolers with adverse birth outcomes have more distress during dental examination? Eur. Arch. Paediatr. Dent. 2019, 20, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Boehm, A.; Ellsäßer, G.; Lüdecke, K. Der Brandenburger Sozialindex: Ein Werkzeug für die Gesundheits- und Sozialberichterstattung auf Landes- und kommunaler Ebene bei der Analyse von Einschülerdaten. Gesundheitswesen 2007, 69, 555–559. [Google Scholar] [CrossRef]
- Löe, H. The Gingival Index, the Plaque Index and the Retention Index Systems. J. Periodontol. 1967, 38, 610–616. [Google Scholar] [CrossRef]
- Meyle, J.; Jepsen, S. Der parodontale Screening-Index (PSI). Parodontologie 2000, 11, 17–21. (In German) [Google Scholar]
- WHO. Oral Health Surveys: Basic Methods, 4th ed.; World Health Organisation: Geneva, Switzerland, 1997. [Google Scholar]
- International Caries Detection and Assessment System (ICDAS) Coordinating Committee. Rationale and Evidence for the International Caries Detection and Assessment System (ICDAS II); International Caries Detection and Assessment System (ICDAS) Coordinating Committee: Baltimore, MD, USA, 2011. [Google Scholar]
- FDI Commission on Oral Health, Research and Epidemiology. A review of the developmental defects of enamel index (DDE index). Int. Dent. J. 1992, 42, 411–426. [Google Scholar]
- Pavičin, I.S.; Dumančić, J.; Badel, T.; Vodanović, M. Timing of emergence of the first primary tooth in preterm and full-term infants. Ann. Anat. 2016, 203, 19–23. [Google Scholar] [CrossRef]
- Bozorgnia, Y.; Mafinejad, S.; Dokohaki, S.; Razavi, N.; Shabani, R. The effect of birth weight on tooth development by Demirjian’s method. Clin. Oral Investig. 2024, 28, 411. [Google Scholar] [CrossRef]
- Almonaitiene, R.; Balciuniene, I.; Tutkuviene, J. Factors influencing permanent teeth eruption. Part one—General factors. Stomatologija 2010, 12, 67–72. [Google Scholar]
- Christensen, L.B.; Twetman, S.; Sundby, A. Oral health in children and adolescents with different socio-cultural and socio-economic backgrounds. Acta Odontol. Scand. 2010, 68, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, M.; Aslam, T.; Saeed, S.; Sarfraz, A.; Sarfraz, Z.; Cherrez-Ojeda, I. Individual, Family, and Socioeconomic Contributors to Dental Caries in Children from Low- and Middle-Income Countries. Int. J. Environ. Res. Public Health 2022, 19, 7114. [Google Scholar] [CrossRef] [PubMed]
- Cruvinel, V.R.N.; Gravina, D.B.L.; Azevedo, T.D.P.L.; Bezerra, A.C.B.; de Toledo, O.A. Prevalence of dental caries and caries-related risk factors in premature and term children. Braz. Oral Res. 2010, 24, 329–335. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tanaka, K.; Miyake, Y. Low birth weight, preterm birth or small-for-gestational-age are not associated with dental caries in young Japanese children. BMC Oral Health 2014, 14, 38. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.; Lou, Y.; Tao, R.; Li, Y.; Cao, D.; Yu, M.; Ying, B.; Wang, H. The association between low birth weight and dental caries among 11-to-13-year-old school age children in Ningbo, China. BMC Pediatr. 2021, 21, 491. [Google Scholar] [CrossRef]
- Alshehhi, A.; Al Halabi, M.; Hussein, I.; Salami, A.; Hassan, A.; Kowash, M. Enamel defects and caries prevalence in preterm children aged 5–10 years in Dubai. Libyan J. Med 2020, 15, 1705633. [Google Scholar] [CrossRef]
- Castañeda-Sarmiento, S.; Koecklin, K.H.U.; Hernandez, M.B.B.; Santos, G.P.; Luyo, J.C.B.; Sotomayor, J.C.S.; Ruiz-Yasuda, C.; Apaza, Z.R.; Adasme, D.P.; Ricse, D.A.T.; et al. Association between developmental defects of enamel and early childhood caries in children under 6 years old: A systematic review and meta-analysis. Heliyon 2022, 8, e10479. [Google Scholar] [CrossRef]
- Fagrell, T.G.; Dietz, W.; Jälevik, B.; Norén, J.G. Chemical, mechanical and morphological properties of hypomineralized enamel of permanent first molars. Acta Odontol. Scand. 2010, 68, 215–222. [Google Scholar] [CrossRef]
- Massignan, C.; Ximenes, M.; da Silva Pereira, C.; Dias, L.; Bolan, M.; Cardoso, M. Prevalence of enamel defects and association with dental caries in preschool children. Eur. Arch. Paediatr. Dent. 2016, 17, 461–466. [Google Scholar] [CrossRef]
- Alkhtib, A.; Ghanim, A.; Temple-Smith, M.; Messer, L.B.; Pirotta, M.; Morgan, M. Prevalence of early childhood caries and enamel defects in four and five-year old Qatari preschool children. BMC Oral. Health 2016, 16, 73. [Google Scholar] [CrossRef]
- Corrêa-Faria, P.; Martins-Júnior, P.A.; Vieira-Andrade, R.G.; Oliveira-Ferreira, F.; Marques, L.S.; Ramos-Jorge, M.L. Developmental defects of enamel in primary teeth: Prevalence and associated factors. Int. J. Paediatr. Dent. 2013, 23, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Bensi, C.; Costacurta, M.; Belli, S.; Paradiso, D.; Docimo, R. Relationship between preterm birth and developmental defects of enamel: A systematic review and meta-analysis. Int. J. Paediatr. Dent. 2020, 30, 676–686. [Google Scholar] [CrossRef] [PubMed]
- da Silva Figueiredo Sé, M.J.; Ribeiro, A.P.D.; Santos-Pinto, L.A.M.D.; de Cassia Loiola Cordeiro, R.; Cabral, R.N.; Leal, S.C. Are Hypomineralized Primary Molars and Canines Associated with Molar-Incisor Hypomineralization? Pediatr. Dent. 2017, 39, 445–449. [Google Scholar] [PubMed]
- Mohamed, R.N.; Basha, S.; Virupaxi, S.G.; Eregowda, N.I.; Parameshwarappa, P. Hypomineralized Primary Teeth in Preterm Low Birth Weight Children and Its Association with Molar Incisor Hypomineralization-A 3-Year-Prospective Study. Children 2021, 8, 1111. [Google Scholar] [CrossRef] [PubMed]
- Masumo, R.; Bårdsen, A.; Astrøm, A.N. Developmental defects of enamel in primary teeth and association with early life course events: A study of 6–36 month old children in Manyara, Tanzania. BMC Oral Health 2013, 13, 21. [Google Scholar] [CrossRef]
| Number of Permanent Teeth | |||||||
|---|---|---|---|---|---|---|---|
| N | Group | Mean | Min-Max | SD | 95%. CI | p * | |
| All | 38 | PT | 9.6 | 0–14 | 2.9 | 8.7–10.6 | 0.007 |
| 38 | FT | 10.6 | 6–14 | 1.9 | 10.2–11.5 | ||
| Males | 19 | PT | 9.0 | 0–12 | 3.3 | 7.4–10.6 | 0.077 |
| 19 | FT | 10.7 | 6–14 | 2.1 | 9.7–11.8 | ||
| Females | 19 | PT | 10.3 | 6–14 | 2.4 | 9.1–11.4 | 0.031 |
| 19 | FT | 11.0 | 8–13 | 1.7 | 10.2–11.8 | ||
| LBW | 23 | PT | 9.4 | 4–14 | 2.7 | 8.2–10.6 | 0.010 |
| 23 | FT ** | 11.0 | 8–14 | 1.8 | 10.2–11.8 | ||
| VLBW | 7 | PT | 11.3 | 9–12 | 1.3 | 10.1–12.4 | 0.500 |
| 7 | FT ** | 10.3 | 6–12 | 2.2 | 8.2–12.3 | ||
| ELBW | 5 | PT | 7.8 | 0–12 | 4.7 | 1.9–13.7 | 0.125 |
| 5 | FT ** | 10.6 | 8–13 | 2.4 | 7.6–13.6 | ||
| DDE > 0 in Permanent Teeth | DDE > 0 in Deciduous Teeth | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | N | % | 95%-CI | p * | N | % | 95%-CI | p * | |
| All | PT | 23 | 62.2 | 44.8–77.5 | 0.346 | 21 | 55.3 | 38.3–71.4 | 0.008 |
| FT | 19 | 51.4 | 34.4–68.1 | 11 | 28.9 | 15.4–45.9 | |||
| Males | PT | 10 | 55.6 | 30.8–78.5 | 0.739 | 9 | 47.4 | 24.4–71.1 | 0.414 |
| FT | 9 | 50.0 | 26.0–74.0 | 7 | 36.8 | 16.3–61.6 | |||
| Females | PT | 13 | 68.4 | 43.4–87.4 | 0.317 | 12 | 63.2 | 38.4–83.7 | 0.005 |
| FT | 10 | 63.2 | 38.4–83.7 | 4 | 21.1 | 6.1–45.6 | |||
| LBW | PT | 14 | 60.9 | 38.5–80.3 | 0.527 | 13 | 56.5 | 34.5–76.8 | 0.102 |
| FT ** | 12 | 52.2 | 30.6–73.2 | 9 | 39.1 | 19.7–61.5 | |||
| VLBW | PT | 5 | 71.4 | 29.0–96.3 | 1.000 | 3 | 42.9 | 9.9–81.6 | 0.564 |
| FT ** | 5 | 71.4 | 29.0–96.3 | 2 | 28.6 | 3.7–71.0 | |||
| ELBW | PT | 3 | 75.0 | 19.4–99.4 | 0.564 | 2 | 40.0 | 5.3–85.3 | 0.157 |
| FT ** | 2 | 50.0 | 6.8–93.2 | 0 | 0.0 | 0.0–52.2 | |||
| Oral Health Parameters | Birthweight Groups | PT | FT | ||
|---|---|---|---|---|---|
| ELBW | VLBW | LBW | |||
| DMFT mean [95%-CI] SD p * | 1.0 [−2.2–4.2] 1.0 1.000 | 0.6 [−0.2–1.3] 0.8 1.000 | 0.3 [−0.1–0.6] 0.8 0.750 | 0.4 [0.1–0.7] 0.9 | 0.3 [−0.0–0.6] 1.0 0.688 |
| dmft mean [95%-CI] SD p * | 3.2 [−0.4–6.8] 2.9 0.438 | 1.7 [−0.2–3.6] 2.1 0.313 | 1.3 [0.4–2.3] 2.2 0.221 | 1.6 [0.9–2.3] 2.2 | 2.7 [1.7–3.7] 3.1 0.035 |
| dt mean [95%-CI] SD p * | 1.6 [−1.3–4.5] 2.3 1.000 | 1.7 [−0.2–3.6] 2.1 1.000 | 0.5 [0.1–1.0] 1.0 1.000 | 0.9 [0.4–1.4] 1.5 | 0.7 [0.3–1.2] 1.5 0.653 |
| ft mean [95%-CI] SD p * | 1.4 [−1.8–4.6] 2.6 0.500 | 0.0 0.0 0.250 | 0.8 [0.1–1.6] 1.7 0.185 | 0.7 [0.1–1.2] 1.6 | 1.8 [1.0–2.7] 2.5 0.009 |
| PSI mean [95%-CI] SD p * | 7.4 [5.0–9,8] 1.9 0.125 | 4.6 [2.4–6.7] 2.3 0.438 | 2.6 [1.5–3.7] 2.6 0.508 | 3.8 [2.8–4.7] 2.9 | 3.3 [2.1–4.6] 3.8 0.427 |
| PI mean [95%-CI] SD p * | 1.3 [0.7–1.9] 0.5 0.188 | 1.2 [1.0–1.5] 0.3 0.063 | 0.7 [0.5–1.0] 0.6 0.485 | 0.9 [0.7–1.1] 0.6 | 0.6 [0.4–0.8] 0.6 0.027 |
| Socioeconomic Status | |||||
|---|---|---|---|---|---|
| Group (n) | All Mean ± SD Range | DMFT > 0 Mean ± SD Range | DMFT = 0 Mean ± SD Range | dmft > 0 Mean ± SD Range | dmft = 0 Mean ± SD Range |
| All (76) | H 8.9 ± 1.2 5–10 | M 8.2 ± 1.8 5–10 | H 9.0 ± 1.0 6–10 | M 8.5 ± 1.4 5–10 | H 9.3 ± 0.9 7–10 |
| Males (38) | H 9.0 ± 1.3 5–10 | M 8.4 ± 1.9 5–10 | H 9.1 ± 1.1 6–10 | M 8.4 ±1.5 5–10 | H 9.6 ±0.7 8–10 |
| Females (38) | H 8.8 ± 1.2 5–10 | M 8.0 ± 1.8 6–10 | H 9.0 ± 0.9 5–10 | M 8.4 ±1.3 5–10 | H 9.0 ±1.0 7–10 |
| FT (38) | H 9.0 ± 1.3 5–10 | M 8.3 ± 2.1 5–10 | H 9.1 ±1.0 6–10 | H 8.6 ±1.5 5–10 | H 9.6 ± 0.8 7–10 |
| PT (38) | H 8.8 ± 1.1 5–10 | M 8.1 ±1.8 5–10 | H 8.9 ±0.9 7–10 | M 8.4 ±1.3 5–10 | H 9.1 ±0.9 8–10 |
| LBW (23) | H 8.8 ± 1.2 5–10 | M 7.3 ±2.1 5–9 | H 9.1 ±0.8 5–10 | M 8.5 ±1.5 5–10 | H 9.0 ±0.9 8–10 |
| VLBW (7) | H 9.0 ± 1.3 7–10 | H 9.3 ± 1.2 8–10 | H 8.8 ± 1.5 7–10 | H 9.3 ±1.5 7–10 | H 8.8 ± 1.2 8–10 |
| ELBW (5) | M 7.8 ± 0.4 7–8 | M 7.0 ± 0.0 7–7 | M 8.0 ± 0.0 8–8 | M 7.8 ± 0.5 7–8 | M 8.0 ± 0.0 8–8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schlesinger, H.L.; Heinrich-Weltzien, R.; Schüler, I.M. Oral Health of 7- to 9-Year-Old Children Born Prematurely—A Case–Control Observational Study with Randomized Case Selection. Dent. J. 2024, 12, 421. https://doi.org/10.3390/dj12120421
Schlesinger HL, Heinrich-Weltzien R, Schüler IM. Oral Health of 7- to 9-Year-Old Children Born Prematurely—A Case–Control Observational Study with Randomized Case Selection. Dentistry Journal. 2024; 12(12):421. https://doi.org/10.3390/dj12120421
Chicago/Turabian StyleSchlesinger, Heide L., Roswitha Heinrich-Weltzien, and Ina M. Schüler. 2024. "Oral Health of 7- to 9-Year-Old Children Born Prematurely—A Case–Control Observational Study with Randomized Case Selection" Dentistry Journal 12, no. 12: 421. https://doi.org/10.3390/dj12120421
APA StyleSchlesinger, H. L., Heinrich-Weltzien, R., & Schüler, I. M. (2024). Oral Health of 7- to 9-Year-Old Children Born Prematurely—A Case–Control Observational Study with Randomized Case Selection. Dentistry Journal, 12(12), 421. https://doi.org/10.3390/dj12120421






