Intraoral Applications of Lasers in the Prosthetic Rehabilitation with Fixed Partial Dentures—A Narrative Review
Abstract
1. Introduction
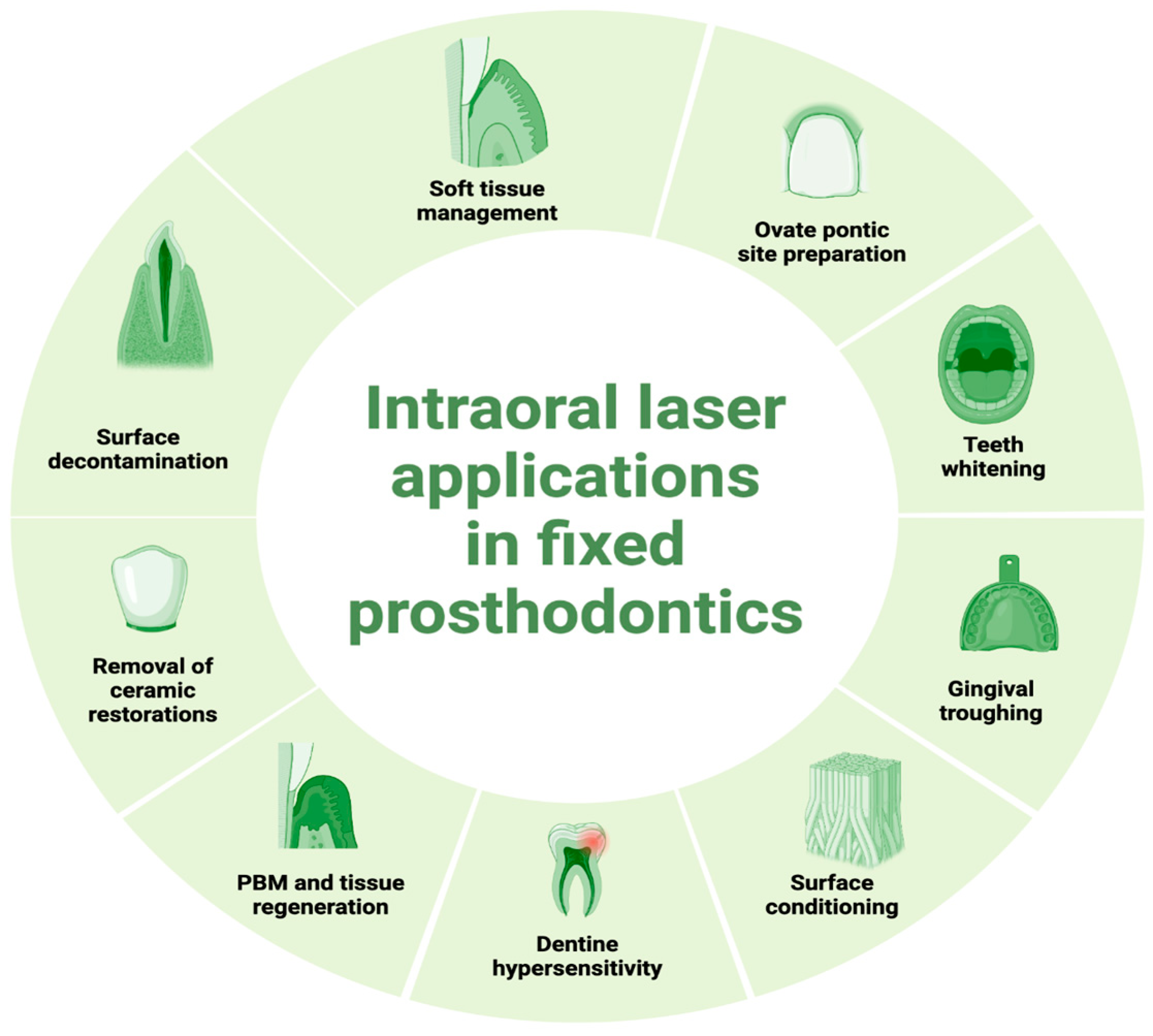
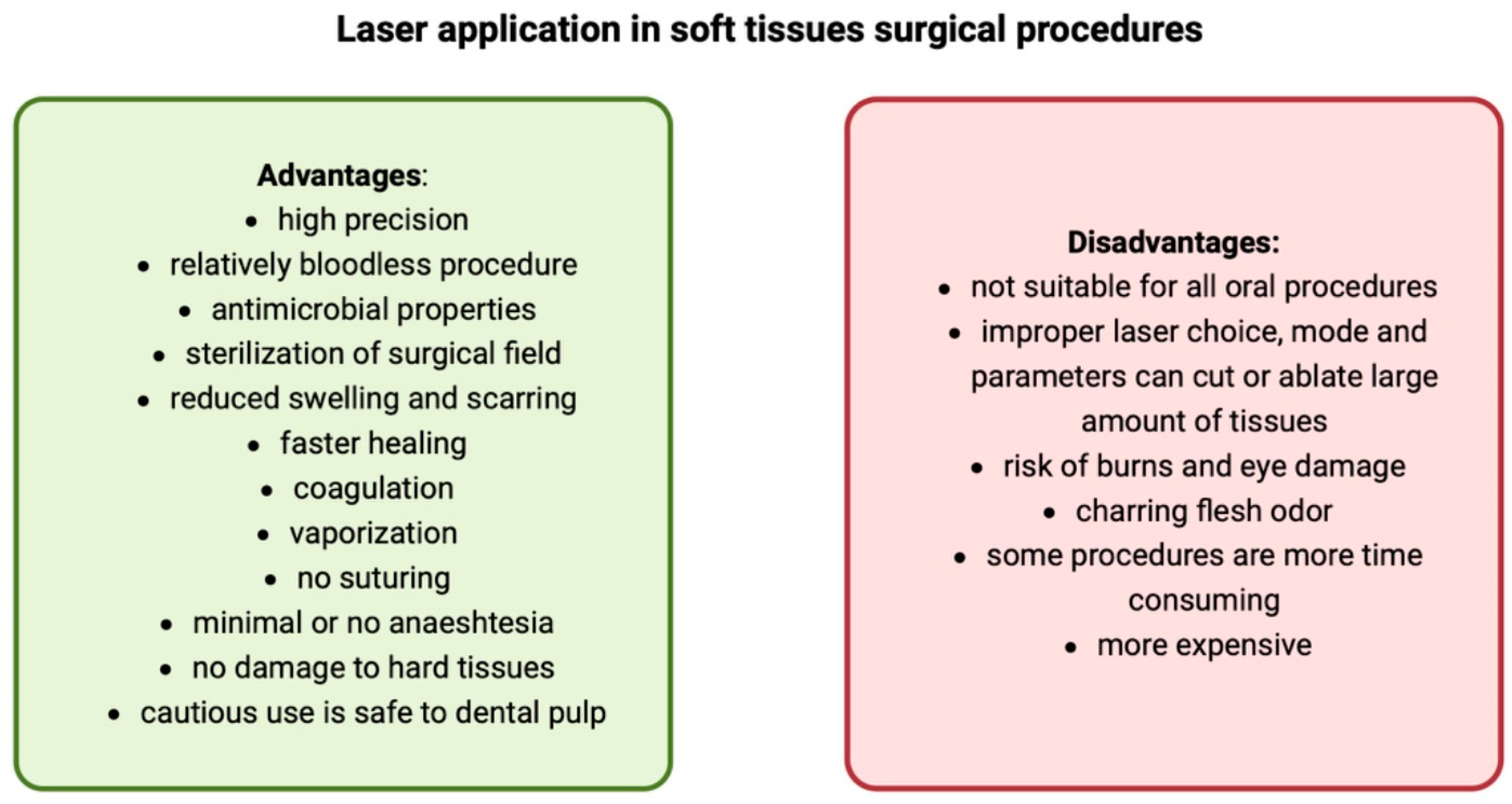
2. Intraoral Applications of Lasers
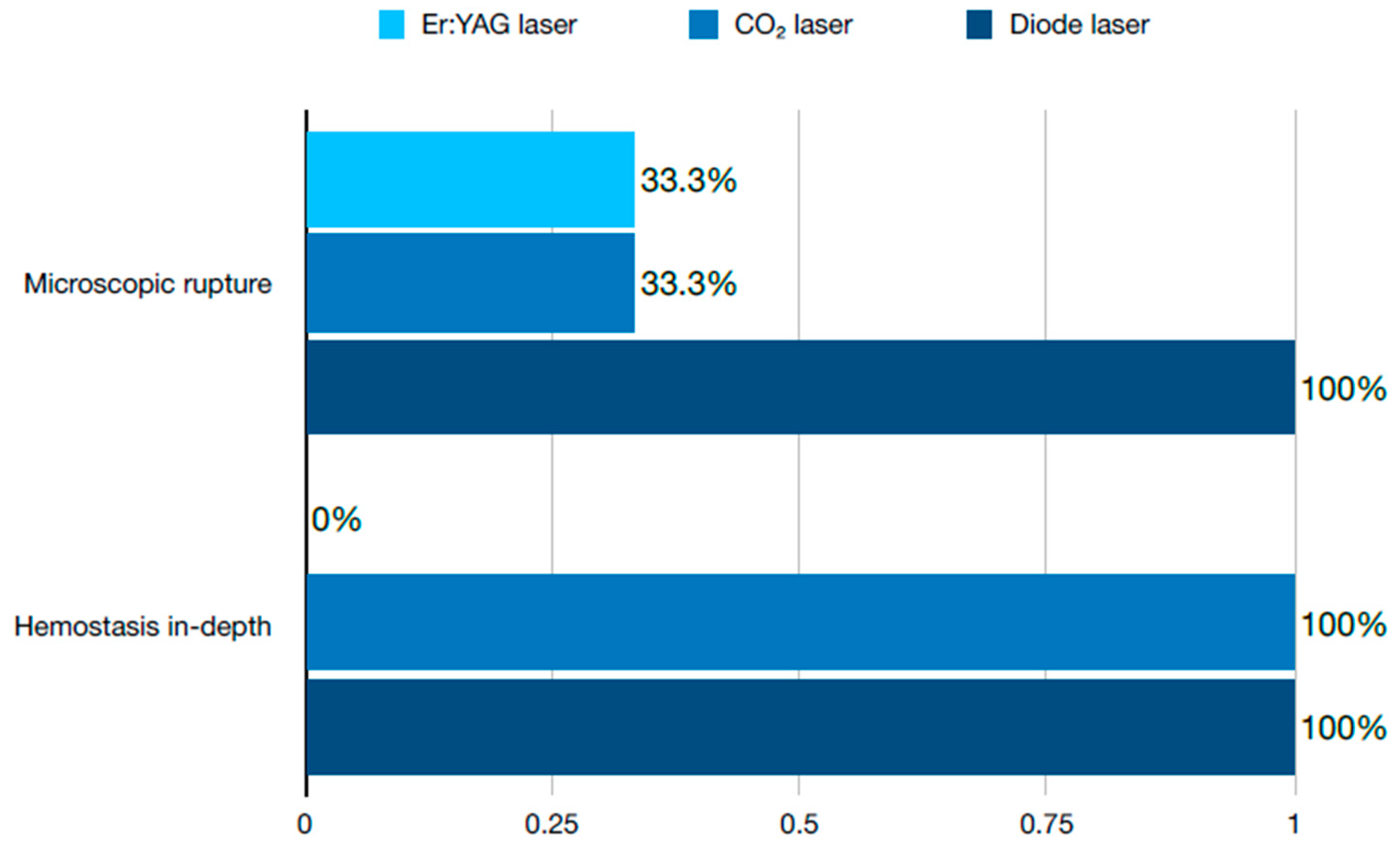
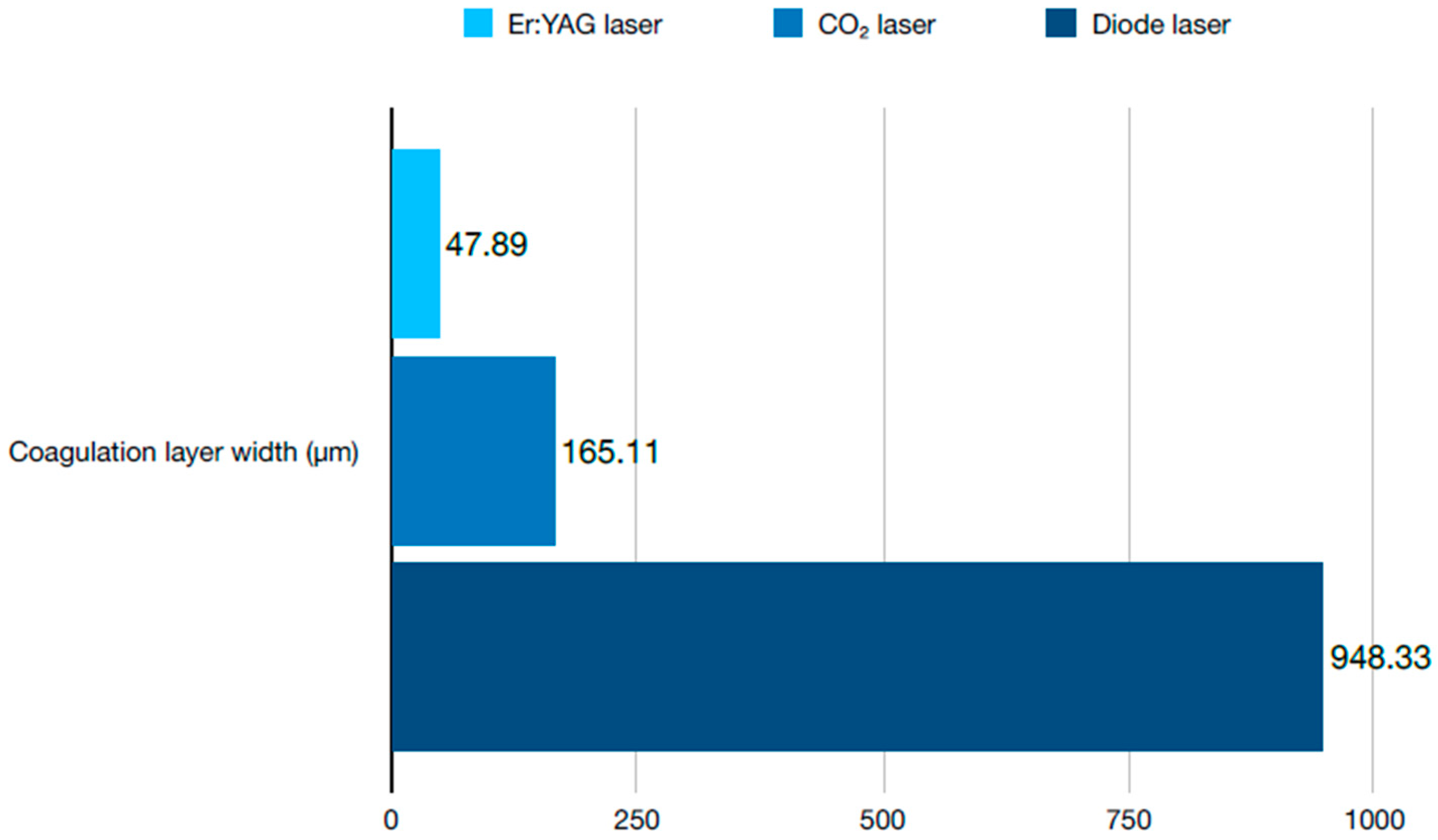
2.1. Soft Tissue Management
2.2. Preparation of Ovate Pontic Site
2.3. Tooth Whitening
2.4. Gingival Troughing
2.5. Surface Conditioning
2.6. Surface Decontamination
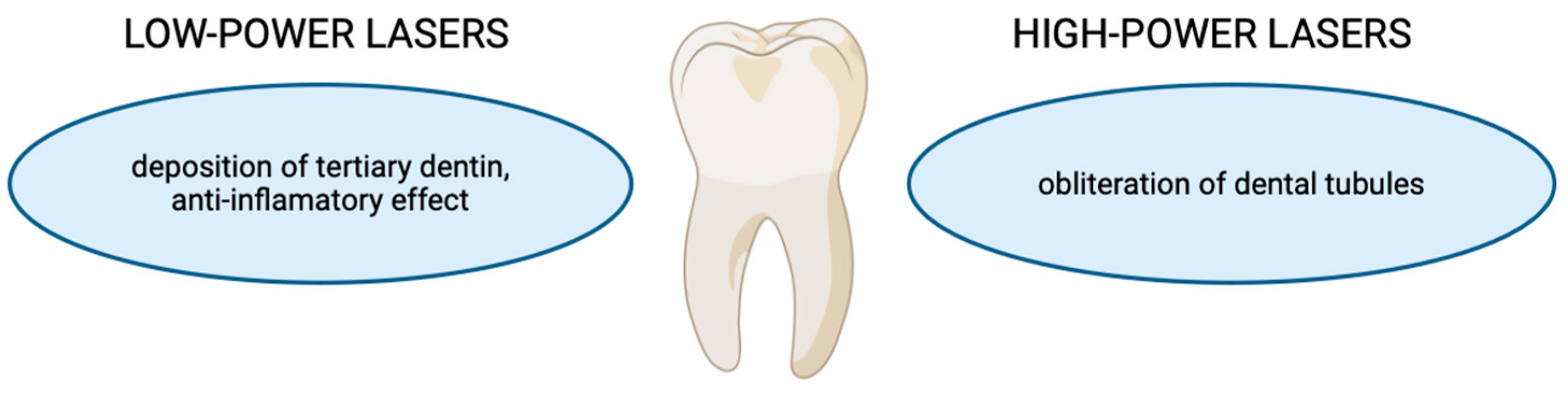
2.7. Dentine Hypersensitivity
2.8. Photobiomodulation and Tissue Regeneration
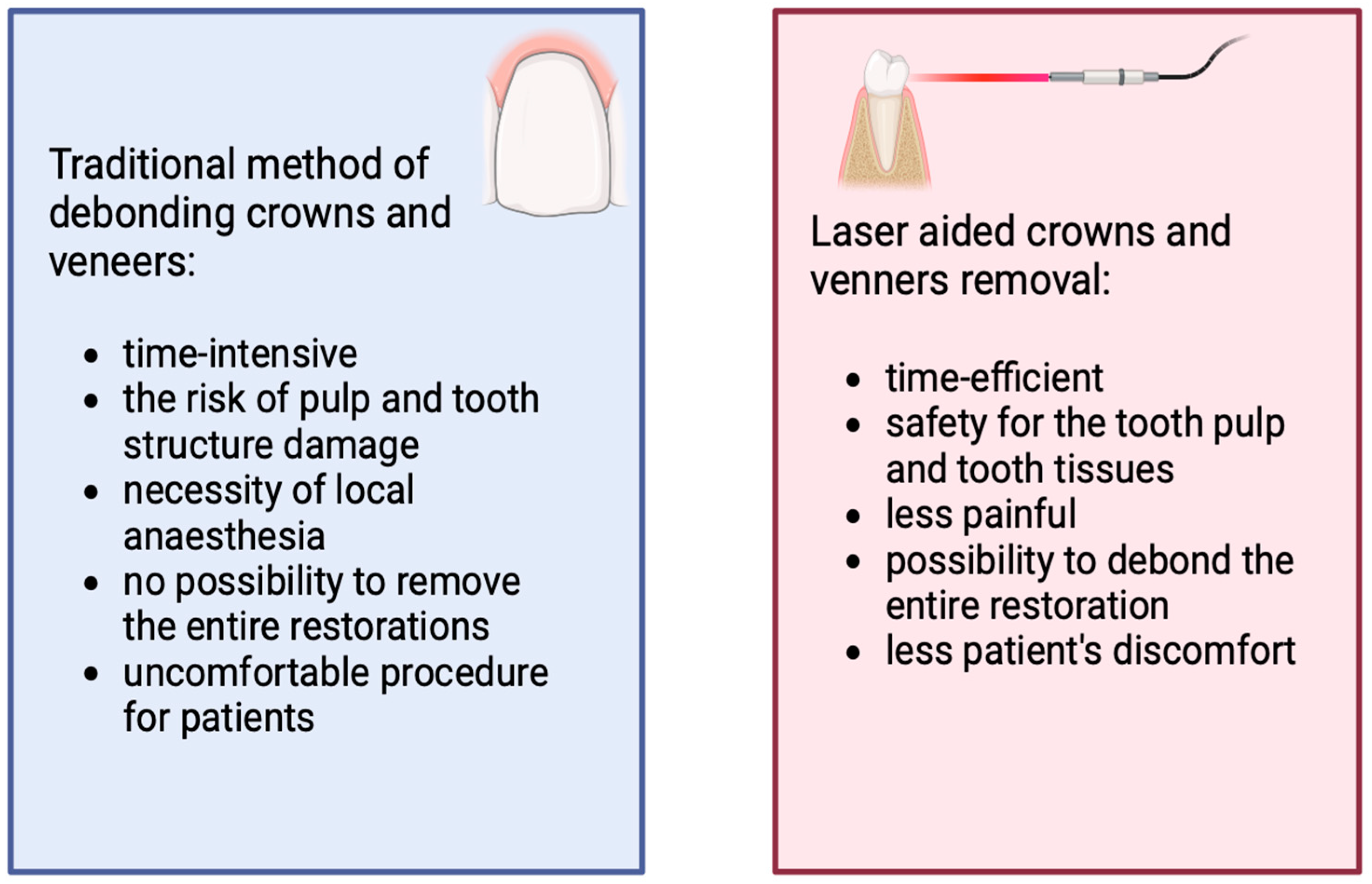
2.9. Removal of Ceramic Restorations
3. Limitations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdulsamee, N.; Elrefaey, M. Laser: Silent revolution in prosthetic dentistry bridging the gap to future. historical review. J. Dent. Health Oral Disord. Ther. 2022, 13, 9–19. [Google Scholar] [CrossRef]
- Devi, N.; Kumar, P.; Rakshna, M.; Rameshkumar, K. Application of lasers in prosthodontics: A review. J. Indian Acad. Dent. Spec. Res. 2018, 5, 42. [Google Scholar] [CrossRef]
- Gounder, R.; Gounder, S. Laser Science and its Applications in Prosthetic Rehabilitation. J. Lasers Med. Sci. 2016, 7, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Al Hussian, B.S.; Al Hammad, M.; Al Amri, N.; Al Markhan, F.; Asiri, A.; Al Deraibi, Z.; Alarnous, A. Application of Lasers in Various Procedures Performed in Prosthodontics; A Systemic Review. Pharmacophore 2022, 13, 129–134. [Google Scholar] [CrossRef]
- Varrak, A.; Kunt, G.E.; Ceylan, G. Lasers in Prosthodontics. ES J. Dent. Sci. 2020, 1, 1014. [Google Scholar]
- Coleton, S. Lasers in surgical periodontics and oral medicine. Dent. Clin. North Am. 2004, 48, 937–962. [Google Scholar] [CrossRef]
- Perveen, A.; Molardi, C.; Fornaini, C. Applications of Laser Welding in Dentistry: A State-of-the-Art Review. Micromachines 2018, 9, 209. [Google Scholar] [CrossRef] [PubMed]
- Pinero, J. Nd:YAG-assisted periodontal curettage to prevent bacteria before cardiovascular surgery. Dent. Today 1998, 17, 84–87. [Google Scholar] [PubMed]
- Convissar, R.A. Lasers in a hospital-based dental practice. Dent. Clin. North Am. 2000, 44, 875–887. [Google Scholar] [CrossRef]
- Chellappah, N.K.; Loh, H.S. Laser therapy for a haemophiliac. Case report. Aust. Dent. J. 1990, 35, 121–124. [Google Scholar] [CrossRef]
- Larionova, E.V.; Diachkova, E.Y.; Morozova, E.A.; Davtyan, A.A.; Tarasenko, S.V. Laser-assisted tooth extraction in patients with impaired hemostasis. Biomedicine 2021, 11, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Nanayakkara, L.; Yahaya, N.; Parreira, M.; Bajkin, B. Dental management of people with complex or rare inherited bleeding disorders. Haemophilia 2024, 30 (Suppl. S3), 128–134. [Google Scholar] [CrossRef] [PubMed]
- Kelkar, M.; Kadanthode, M.; Mohanty, S.; Verma, A. Effect of low-level laser therapy on post-extraction hemostasis in patients with hemophilia—A prospective cohort study. J. Craniomaxillofac. Surg. 2023, 51, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Ngoc, V.T.N.; Van Nga, T.D.; Chu, D.T.; Anh, L.Q. Pulpotomy management using laser diode in pediatric patient with severe hemophilia A under general anesthesia-A case report. Spec. Care Dent. 2018, 38, 155–159. [Google Scholar] [CrossRef]
- Dhayanidhi, A.; Mudiarasu, N.; Mathivanan, A.; Gopalkrishnan, J.R.; Nagarajan, S.K.K.; Bharathan, K. “Laser Dentistry”—The Need of the Hour: A Cross-sectional Study. J. Pharm. Bioallied Sci. 2020, 12 (Suppl. S1), S295–S298. [Google Scholar] [CrossRef]
- Bartlett, D.; O’Toole, S. Tooth wear and aging. Aust. Dent. J. 2019, 64 (Suppl. S1), S59–S62. [Google Scholar] [CrossRef]
- Yadav, R.; Meena, A.; Lee, H.-H.; Park, S.-J. Tribological behavior of dental resin composites: A comprehensive review. Tribol. Int. 2023, 190, 109017. [Google Scholar] [CrossRef]
- Blatz, M.B.; Chiche, G.; Bahat, O.; Roblee, R.; Coachman, C.; Heymann, H.O. Evolution of Aesthetic Dentistry. J. Dent. Res. 2019, 98, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- Purnapriya, B.; Sudheer, A.; Ramakrishna, M.; Navyadeepthi; Pooja, A.; Sudeepti, S. Principles for Establishment of Esthetics in Fixed Prosthodontics: A Review. Int. J. Dent. Med. Sci. Res. 2021, 3, 423–434. [Google Scholar]
- Edelhoff, D.; Spiekermann, H.; Yildirim, M. A review of esthetic pontic design options. Quintessence Int. 2002, 33, 736–746. [Google Scholar]
- Sadowsky, S.J. An overview of treatment considerations for esthetic restorations: A review of the literature. J. Prosthet. Dent. 2006, 96, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Meena, A.; Patnaik, A. Biomaterials for dental composite applications: A comprehensive review of physical, chemical, mechanical, thermal, tribological, and biological properties. Polym. Adv. Technol. 2022, 33, 1762–1781. [Google Scholar] [CrossRef]
- Elsayed, S.-H.; Mossaad, A.; Abdelrahman, M.; Kotb, A.; Alolayan, A. Gummy smile management using diode laser gingivectomy versus botulinum toxin injection—A prospective study. Ann. Maxillofac. Surg. 2021, 11, 70. [Google Scholar] [CrossRef] [PubMed]
- Tianmitrapap, P.; Srisuwantha, R.; Laosrisin, N. Flapless er,CR:YSGG laser versus traditional flap in crown lengthening procedure. J. Dent. Sci. 2022, 17, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Kazakova, R.T.; Tomov, G.T.; Kissov, C.K.; Vlahova, A.P.; Zlatev, S.C.; Bachurska, S.Y. Histological gingival assessment after conventional and laser gingivectomy. Folia Medica 2018, 60, 610–616. [Google Scholar] [CrossRef]
- Kayar, N.A.; Hatipoğlu, M. Can we determine an appropriate timing to avoid thermal pulp hazard during gingivectomy procedure? an in vitro study with diode laser. Photobiomodul. Photomed. Laser Surg. 2021, 39, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Gahan, M.J.; Nixon, P.J.; Robinson, S.; Chan, M.F. The ovate pontic for fixed bridgework. Dent. Update 2012, 39, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Manne, P.; Zakkula, S.; Atla, J.; Muvva, S.B.; Sampath, A. Redefining smile-a multidisciplinary approach. J. Clin. Diagn. Res. 2013, 7, 1527–1529. [Google Scholar] [CrossRef] [PubMed]
- Venkatasubramanyam, A.; Sigtia, S.; Sheth, E.; Hegde, R.; Muglikar, S. Laser-assisted natural gingival profile creation of an ovate pontic site. J. Dent. Lasers 2017, 11, 29–32. [Google Scholar] [CrossRef]
- Kripal, K.; Sirajuddin, S.; Rafiuddin, S.; Mp, R.; Chungkham, S. Iatrogenic Damage to the Periodontium Caused by Laser: An Overview. Open Dent. J. 2015, 9, 210–213. [Google Scholar] [CrossRef]
- Abbasi, M.; Pordel, E.; Chiniforush, N.; Firuzjaee, S.G.; Omrani, L.R. Hydrogen peroxide penetration into the pulp chamber during conventional in-office bleaching and diode laser-assisted bleaching with three different wavelengths. Laser Ther. 2019, 28, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Fekrazad, R.; Alimazandarani, S.; Kalhori, K.A.; Assadian, H.; Mirmohammadi, S.M. Comparison of laser and power bleaching techniques in tooth color change. J. Clin. Exp. Dent. 2017, 9, e511–e515. [Google Scholar] [CrossRef]
- Bloomquist, R.F.; Sword, R.J.; Londono, J.; Haywood, V.B. Bleaching: The initial treatment consideration for tetracycline-stained teeth. Br. Dent. J. 2021, 230, 807–812. [Google Scholar] [CrossRef]
- Hou, X.; Yuan, K.; Huang, Z.; Ma, R. Effects of Bleaching Associated with Er:YAG and Nd:YAG Laser on Enamel Structure and Bacterial Biofilm Formation. Scanning 2021, 2021, 6400605. [Google Scholar] [CrossRef] [PubMed]
- Mirzaie, M.; Yassini, E.; Ganji, S.; Moradi, Z.; Chiniforush, N. A Comparative Study of Enamel Surface Roughness After Bleaching with Diode Laser and Nd: YAG Laser. J. Lasers Med. Sci. 2016, 7, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Yusof, E.M.; Abdullah, S.A.; Mohamed, N.H. Influence of light and laser activation of tooth bleaching systems on enamel microhardness and surface roughness. J. Conserv. Dent. 2020, 23, 473–478. [Google Scholar] [CrossRef]
- Kiomars, N.; Azarpour, P.; Mirzaei, M.; Hashemi Kamangar, S.S.; Kharazifard, M.J.; Chiniforush, N. Evaluation of the Diode laser (810nm, 980 nm) on color change of teeth after external bleaching. Laser Ther. 2016, 25, 267–272. [Google Scholar] [CrossRef]
- Christensen, G.J. Simplifying and improving soft-tissue management for fixed-prosthodontic impressions. J. Am. Dent. Assoc. 2013, 144, 198–200. [Google Scholar] [CrossRef]
- Madaan, R.; Paliwal, J.; Sharma, V.; Meena, K.K.; Dadarwal, A.; Kumar, R. Comparative Evaluation of the Clinical Efficacy of Four Different Gingival Retraction Systems: An In Vivo Study. Cureus 2022, 14, e23923. [Google Scholar] [CrossRef]
- Kazakova, R.; Vlahova, A.; Tomov, G.; Dimitrova, M.; Kazakov, S.; Zlatev, S.; Forte, M.; Barile, G.; Corsalini, M.; Capodiferro, S. A Comparative Analysis of Post-Retraction Changes in Gingival Height after Conventional and Surgical Gingival Displacement: Rotary Curettage, Diode and Er:YAG Laser Troughing. Healthcare 2023, 11, 2262. [Google Scholar] [CrossRef]
- Tao, X.; Yao, J.W.; Wang, H.L.; Huang, C. Comparison of Gingival Troughing by Laser and Retraction Cord. Int. J. Periodontics Restor. Dent. 2018, 38, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Ashri, N.Y.; AlRifaiy, M.Q.; El Metwally, A. The Effect of Gingival Retraction Cord on Periodontal Health Compared to Other Gingival Retraction Procedures: A Systematic Review. Periodontics Prosthodont. 2016, 2. [Google Scholar] [CrossRef]
- Stuffken, M.; Vahidi, F. Preimpression troughing with the diode laser: A preliminary study. J. Prosthet. Dent. 2016, 115, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Labunet, A.; Tonea, A.; Kui, A.; Sava, S. The Use of Laser Energy for Etching Enamel Surfaces in Dentistry—A Scoping Review. Materials 2022, 15, 1988. [Google Scholar] [CrossRef] [PubMed]
- Feitosa, F.A.; de Araújo, R.M.; Tay, F.R.; Niu, L.; Pucci, C.R. Effect of high-power-laser with and without graphite coating on bonding of resin cement to lithium disilicate ceramic. Sci. Rep. 2017, 7, 17422. [Google Scholar] [CrossRef] [PubMed]
- Tzanakakis, E.; Kontonasaki, E.; Voyiatzis, G.; Andrikopoulos, K.; Tzoutzas, I. Surface characterization of monolithic zirconia submitted to different surface treatments applying optical interferometry and raman spectrometry. Dent. Mater. J. 2020, 39, 111–117. [Google Scholar] [CrossRef]
- Garófalo, S.A.; Wehner, M.; Dohrn, A.; Bilandžić, M.D.; Roos, C.; Wierichs, R.J.; Meyer-Lueckel, H.; Aranha, A.C.C.; Esteves-Oliveira, M. Increasing dental zirconia micro-retentive aspect through ultra-short pulsed laser microstructuring: Study on flexural strength and crystal phase characterization. Clin. Oral Investig. 2022, 26, 939–955. [Google Scholar] [CrossRef]
- Tzanakakis, E.C.; Beketova, A.; Papadopoulou, L.; Kontonasaki, E.; Tzoutzas, I.G. Novel Femto Laser Patterning of High Translucent Zirconia as an Alternative to Conventional Particle Abrasion. Dent. J. 2021, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Bourgi, R.; Cuevas-Suarez, C.E.; Devoto, W.; Monjaras-Avila, A.J.; Monteiro, P.; Kharma, K.; Lukomska-Szymanska, M.; Hardan, L. Effect of contamination and decontamination methods on the bond strength of adhesive systems to dentin: A systematic review. J. Esthet. Restor. Dent. 2023, 35, 1218–1238. [Google Scholar] [CrossRef]
- Azizi, A.; Shademan, S.; Rezai, M.; Rahimi, A.; Lawaf, S. Effect of photodynamic therapy with two photosensitizers on Streptococcus mutants: In vitro study. Photodiagnosis Photodyn. Ther. 2016, 16, 66–71. [Google Scholar] [CrossRef]
- Mishra, A.; Koul, M.; Abdullah, A.; Khan, N.; Dhawan, P.; Bhat, A. Comparative Evaluation of Antimicrobial Efficacy of Diode Laser (Continuous Mode), Diode Laser (Pulse Mode), and 5.25% of Sodium Hypochlorite in Disinfection of Root Canal: A Short Study. Int. J. Clin. Pediatr. Dent. 2022, 15, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Quinto, J., Jr.; Amaral, M.M.; Francci, C.E.; Ana, P.A.; Moritz, A.; Zezell, D.M. Evaluation of Intra Root Canal Er,Cr:YSGG Laser Irradiation on Prosthetic Post Adherence. J. Prosthodont. 2019, 28, e181–e185. [Google Scholar] [CrossRef] [PubMed]
- Wawrzyk, A.; Łobacz, M.; Adamczuk, A.; Sofińska-Chmiel, W.; Wilczyński, S.; Rahnama, M. The Use of a Diode Laser for Removal of Microorganisms from the Surfaces of Zirconia and Porcelain Applied to Superstructure Dental Implants. Microorganisms 2021, 9, 2359. [Google Scholar] [CrossRef] [PubMed]
- Pion, L.A.; Matos, L.L.M.; Gimenez, T.; Palma-Dibb, R.G.; Faraoni, J.J. Treatment outcome for dentin hypersensitivity with laser therapy: Systematic review and meta-analysis. Dent. Med. Probl. 2023, 60, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Biagi, R.; Cossellu, G.; Sarcina, M.; Pizzamiglio, I.T.; Farronato, G. Laser-assisted treatment of dentinal hypersensitivity: A literature review. Ann. Stomatol. 2016, 6, 75–80. [Google Scholar] [CrossRef]
- Rezazadeh, F.; Dehghanian, P.; Jafarpour, D. Laser Effects on the Prevention and Treatment of Dentinal Hypersensitivity: A Systematic Review. J. Lasers Med. Sci. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Liu, Q.; Yu, X.; Zeng, X. Laser therapy versus topical desensitising agents in the management of dentine hypersensitivity: A meta-analysis. Oral Dis. 2021, 27, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.T.; Huang, N.C.; Wang, H.L. Relationship between periodontics and prosthodontics: The two-way street. J. Prosthodont. Implantol. 2015, 4. [Google Scholar] [CrossRef]
- Heboyan, A.G.; Manrikyan, M.E.; Markaryan, M.M.; Vardanyan, I.F. Changes in the parameters of gingival crevicular fluid in masticatory function restoration by various prosthodontic constructions. Int. J. Pharm. Res. 2020, 12, 2088–2093. [Google Scholar] [CrossRef]
- Ramalho, K.M.; de Freitas, P.M.; Correa-Aranha, A.C.; Bello-Silva, M.S.; Lopes, R.M.d.G.; Eduardo, C.d.P. Lasers in esthetic dentistry: Soft tissue photobiomodulation, hard tissue decontamination, and ceramics conditioning. Case Rep. Dent. 2014, 2014, 927429. [Google Scholar] [CrossRef][Green Version]
- Chung, H.; Dai, T.; Sharma, S.K.; Huang, Y.Y.; Carroll, J.D.; Hamblin, M.R. The nuts and bolts of low-level laser (light) therapy. Ann. Biomed. Eng. 2012, 40, 516–533. [Google Scholar] [CrossRef] [PubMed]
- Parker, S.; Cronshaw, M.; Anagnostaki, E.; Bordin-Aykroyd, S.R.; Lynch, E. Systematic review of delivery parameters used in dental photobiomodulation therapy. Photobiomodul. Photomed. Laser Surg. 2019, 37, 784–797. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, P.V.; Setty, S.B.; Kulkarni, M.R. Photobiomodulation of human gingival fibroblasts with diode laser—A systematic review. J. Indian Soc. Periodontol. 2022, 26, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Dompe, C.; Moncrieff, L.; Matys, J.; Grzech-Leśniak, K.; Kocherova, I.; Bryja, A.; Bruska, M.; Dominiak, M.; Mozdziak, P.; Skiba, T.H.I.; et al. Photobiomodulation-Underlying Mechanism and Clinical Applications. J. Clin. Med. 2020, 9, 1724. [Google Scholar] [CrossRef] [PubMed]
- Cardoso Bezerra, S.J.; Fioranelli Vieira, G.; Eduardo, C.d.e.P.; de Freitas, P.M.; Aranha, A.C. Laser Phototherapy (660 nm) Can Be Beneficial for Reducing Gingival Inflammation in Prosthodontics. Case Rep. Dent. 2015, 2015, 132656. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.; Kotronia, E.; Ramsay, S.E. Frailty, aging, and periodontal disease: Basic biologic considerations. Periodontology 2000 2021, 87, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Pansani, T.N.; Basso, F.G.; Turrioni, A.P.; Soares, D.G.; Hebling, J.; de Souza Costa, C.A. Effects of low-level laser therapy and epidermal growth factor on the activities of gingival fibroblasts obtained from young or elderly individuals. Lasers Med. Sci. 2017, 32, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Zanin, F.; Moreira, M.S.; Pedroni, A.C.F.; Windlin, M.; Brugnera, A.P.; Brugnera Júnior, A.; Marques, M.M. Hemolasertherapy: A novel procedure for gingival papilla regeneration—Case report. Photomed. Laser Surg. 2018, 36, 221–226. [Google Scholar] [CrossRef]
- Zanin, F.; Brugnera, A. “In loco” gingival papilla regeneration with photobiomodulation: Is blood a natural biomaterial? Photobiomodul. Photomed. Laser Surg. 2020, 38, 653–655. [Google Scholar] [CrossRef]
- Kellesarian, S.V.; Ros Malignaggi, V.; Aldosary, K.M.; Javed, F. Laser-assisted removal of all ceramic fixed dental prostheses: A comprehensive review. J. Esthet. Restor. Dent. 2018, 30, 216–222. [Google Scholar] [CrossRef]
- Szewczyk, E. Removal of adhesively bonded all-ceramic restorations. Prosthodontics 2019, 69, 105–114. [Google Scholar] [CrossRef]
- Rechmann, P.; Buu, N.C.; Rechmann, B.M.; Finzen, F.C. Laser all-ceramic crown removal and pulpal temperature—A laboratory proof-of-principle study. Lasers Med. Sci. 2015, 30, 2087–2093. [Google Scholar] [CrossRef] [PubMed]
- Ghazanfari, R.; Azimi, N.; Nokhbatolfoghahaei, H.; Alikhasi, M. Laser Aided Ceramic Restoration Removal: A Comprehensive Review. J. Lasers Med. Sci. 2019, 10, 86–91. [Google Scholar] [CrossRef]
- Zhang, Y.; Rocca, J.P.; Fornaini, C.; Zhen, Y.; Zhao, Z.; Merigo, E. Erbium-Doped, Yttrium-Aluminum-Garnet Laser Debonding of Porcelain Laminate Veneers: An ex vivo Study. Contemp. Clin. Dent. 2018, 9, 570–573. [Google Scholar] [CrossRef]


| Laser Type | CO2 | Er:YAG | Er,Cr:YSGG | Nd:YAG | Argon | Diode | Helium Neon |
|---|---|---|---|---|---|---|---|
| Wavelength | 10,600 nm | 2940 nm | 2780 nm | 1064 nm | 488–514 nm | 655–980 nm | 637 nm |
| Mode | Pulse or continuous-wave | Pulse | Pulse | Pulse | Pulse or continuous-wave | Pulse or continuous-wave | Continuous-wave |
| Spectrum | Infrared | Infrared | Infrared | Infrared | Blue–blue/green | Red–Infrared | Red |
| Laser active material | Solid | Solid | Solid | Solid | Gas | Semiconductor | Gas |
| Level of energy emission | High | High | High | High | High | High and low | Low |
| Laser Type | Advantages | Disadvantages |
|---|---|---|
| CO2 |
|
|
| Erbium laser |
|
|
| Nd:YAG |
|
|
| Argon |
|
|
| Diode |
|
|
| Laser | Wavelength | Mode | Power (W) | Frequency | Tip (μm) | Cool |
|---|---|---|---|---|---|---|
| Diode | 810 | Continuous pulse | 2 | 20 | 320 | No |
| Nd:YAG | 1064 | Short pulse | 2 | 15 | 320 | No |
| Er:YAG | 2940 | Very long pulse | 2 | 15 | 500 | Air and water |
| Prosthodontic Applications | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Laser Type | Gingivectomy | Preparation of Ovate Pontic Site | Teeth Whitening | Gingival Troughing | Surface Conditioning | Dentine Hypersensitivity Treatment | PBM and Tissue Regeneration | Removal of Ceramic Dental Restorations | Surface Decontamination |
| Diode laser | + | + | + | + | + | + | |||
| Er:YAG | + | + | + | + | + | + | |||
| CO₂ | + | + | + | + | |||||
| Er,Cr:YSGG | + | + | + | + | |||||
| Argon laser | + | ||||||||
| Nd:YAG | + | + | + | + | |||||
| He-Ne | + | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwaśna, M.; Cłapińska, P.; Piosik, Z.; Barysz, K.; Dubiec, I.; Bęben, A.; Ordyniec-Kwaśnica, I. Intraoral Applications of Lasers in the Prosthetic Rehabilitation with Fixed Partial Dentures—A Narrative Review. Dent. J. 2024, 12, 164. https://doi.org/10.3390/dj12060164
Kwaśna M, Cłapińska P, Piosik Z, Barysz K, Dubiec I, Bęben A, Ordyniec-Kwaśnica I. Intraoral Applications of Lasers in the Prosthetic Rehabilitation with Fixed Partial Dentures—A Narrative Review. Dentistry Journal. 2024; 12(6):164. https://doi.org/10.3390/dj12060164
Chicago/Turabian StyleKwaśna, Magdalena, Paulina Cłapińska, Zuzanna Piosik, Kamila Barysz, Iga Dubiec, Adam Bęben, and Iwona Ordyniec-Kwaśnica. 2024. "Intraoral Applications of Lasers in the Prosthetic Rehabilitation with Fixed Partial Dentures—A Narrative Review" Dentistry Journal 12, no. 6: 164. https://doi.org/10.3390/dj12060164
APA StyleKwaśna, M., Cłapińska, P., Piosik, Z., Barysz, K., Dubiec, I., Bęben, A., & Ordyniec-Kwaśnica, I. (2024). Intraoral Applications of Lasers in the Prosthetic Rehabilitation with Fixed Partial Dentures—A Narrative Review. Dentistry Journal, 12(6), 164. https://doi.org/10.3390/dj12060164






