Implant Health in Treated Periodontitis Patients: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
- Randomized clinical trials, case–control and cross-sectional studies and prospective and retrospective cohort studies in humans.
- Only studies published in English in an international peer-reviewed journal.
- The study population must be between 18 and 80 years old.
- All patients with periodontitis should have received prior nonsurgical or surgical periodontal treatment as needed. Periodontitis must have been inactive during the study. The subjects must also have been educated in correct and rigorous oral hygiene and continuing supportive periodontal maintenance. Clinically, implant follow-up after loading should have been performed for more than 2 years.
2.2. Exclusion Criteria
- Case report studies, systematic reviews and veterinary clinical trials
- Any study including patients with serious pathologies that could affect implant therapy
- Publications considering zygomatic, transmandibular implants and orthodontic temporary anchorage implants
- Studies in which the surgical intervention mentions immediate implantation or immediate prosthetic loading
- If the number of subjects included, N, was strictly less than 15, the power of the study was considered too weak.
- Concerning attrition, if the rate of loss to follow-up was greater than or equal to 20% according to the standards of the Cochrane collaboration, the bias was considered too great to select the study.
3. Results
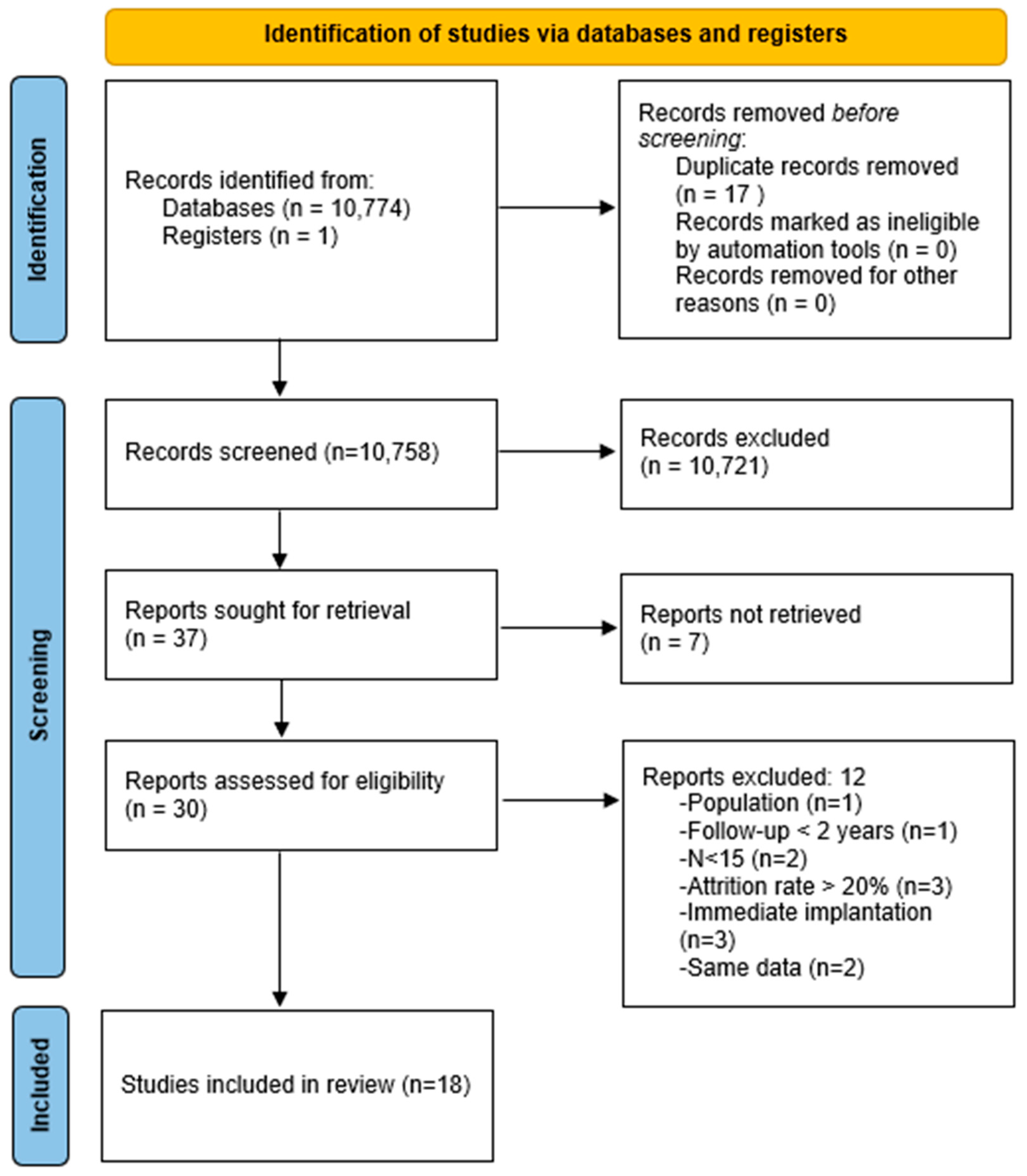
3.1. Study Selection
3.2. Study Characteristics
3.3. Quantitative Synthesis
3.3.1. Peri-Implantitis Rate
3.3.2. Survival Rate
3.3.3. Mean Peri-Implant Bone Loss
3.3.4. Mean Pocket Depth
3.4. Qualitative Synthesis
3.4.1. Peri-Implantitis Rate
3.4.2. Survival Rate
3.4.3. Mean Peri-Implant Bone Loss
3.4.4. Mean Pocket Depth
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sanz, M.; D’Aiuto, F.; Deanfield, J.; Fernandez-Avilés, F. European workshop in periodontal health and cardiovascular disease—Scientific evidence on the association between periodontal and cardiovascular diseases: A review of the literature. Eur. Heart J. Suppl. 2010, 12, B3–B12. [Google Scholar] [CrossRef]
- Janakiram, C.; Dye, B.A. A public health approach for prevention of periodontal disease. Periodontology 2020, 84, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Schou, S. Implant treatment in periodontitis-susceptible patients: A systematic review. J. Oral Rehabil. 2008, 35, 9–22. [Google Scholar] [CrossRef]
- Holm-Pedersen, P.; Lang, N.P.; Müller, F. What are the longevities of teeth and oral implants? Clin. Oral Implants Res. 2007, 18, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Papapanou, P.N. Periodontal diseases: Epidemiology. Ann. Periodontol. 1996, 1, 1–36. [Google Scholar] [CrossRef]
- Albrektsson, T.; Dahl, E.; Enbom, L.; Engevall, S.; Engquist, B.; Eriksson, A.R.; Feldmann, G.; Freiberg, N.; Glantz, P.O.; Kjellman, O.; et al. Osseointegrated Oral Implants: A Swedish Multicenter Study of 8139 Consecutively Inserted Nobelpharma Implants. J. Periodontol. 1988, 59, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Rasmusson, L.; Roos, J.; Bystedt, H. A 10-Year Follow-Up Study of Titanium Dioxide–Blasted Implants. Clin. Implant. Dent. Relat. Res. 2005, 7, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Berglundh, T.; Gislason, O.; Lekholm, U.; Sennerby, L.; Lindhe, J. Histopathological observations of human periimplantitis lesions. J. Clin. Periodontol. 2004, 31, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Araujo, M.G.; Lindhe, J. Peri-implant health. J. Clin. Periodontol. 2018, 45, S230–S236. [Google Scholar] [CrossRef]
- Lang, N.P.; Berglundh, T. Working Group 4 of Seventh European Workshop on Periodontology. Periimplant diseases: Where are we now?—Consensus of the Seventh European Workshop on Periodontology. J. Clin. Periodontol. 2011, 38, 178–181. [Google Scholar] [CrossRef]
- Buser, D.; Sennerby, L.; De Bruyn, H. Modern implant dentistry based on osseointegration: 50 years of progress, current trends and open questions. Periodontology 2000 2017, 73, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Duong, H.Y.; Roccuzzo, A.; Stähli, A.; Salvi, G.E.; Lang, N.P.; Sculean, A. Oral health-related quality of life of patients rehabilitated with fixed and removable implant-supported dental prostheses. Periodontology 2000 2022, 88, 201–237. [Google Scholar] [CrossRef] [PubMed]
- Roccuzzo, M.; De Angelis, N.; Bonino, L.; Aglietta, M. Ten-year results of a three-arm prospective cohort study on implants in periodontally compromised patients. Part 1: Implant loss and radiographic bone loss. Clin. Oral Implants Res. 2010, 21, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Casado, P.L.; Pereira, M.C.; Duarte, M.E.; Granjeiro, J.M. History of Chronic Periodontitis Is a High-Risk Indicator for Peri-Implant Disease. Braz. Dent. J. 2013, 24, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Graetz, C.; El-Sayed, K.F.; Geiken, A.; Plaumann, A.; Sälzer, S.; Behrens, E.; Wiltfang, J.; Dörfer, C.E. Effect of periodontitis history on implant success: A long-term evaluation during supportive periodontal therapy in a university setting. Clin. Oral Investig. 2018, 22, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Zorzano, L.A.; Estefanía-Fresco, R.; Telletxea, O.; Bravo, M. Prevalence of peri-implant inflammatory disease in patients with a history of periodontal disease who receive supportive periodontal therapy. Clin. Oral Implants Res. 2015, 26, 1338–1344. [Google Scholar] [CrossRef] [PubMed]
- Transparent Reporting of Systematic Reviews and Meta-Analyses. Available online: http://www.prisma-statement.org (accessed on 21 October 2023).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.; ter Riet, G.; Popay, J. Stage II: Conducting the review. Phase 5: Study quality assessment. In Undertaking Systematic Reviews of Research on Effectiveness [Internet], 2nd ed.; NHS Centre for Reviews and Dissemination; University of York: York, UK, 2001; pp. 1–20. Available online: http://www.york.ac.uk/inst/crd (accessed on 2 April 2024).
- Ma, L.L.; Wang, Y.Y.; Yang, Z.H.; Huang, D.; Weng, H.; Zeng, X.T. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: What are they and which is better? Mil. Med. Res. 2020, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Rasperini, G.; Siciliano, V.I.; Cafiero, C.; Salvi, G.E.; Blasi, A.; Aglietta, M. Crestal Bone Changes at Teeth and Implants in Periodontally Healthy and Periodontally Compromised Patients. A 10-Year Comparative Case-Series Study. J. Periodontol. 2014, 85, e152–e159. [Google Scholar] [CrossRef]
- Thöne-Mühling, M.; Kelm, D.; Mengel, R. Width of Keratinized Mucosa at Implant Sites in Patients Treated for Generalized Aggressive Periodontitis: A Cohort Study. Int. J. Oral Maxillofac. Implants 2016, 31, 392–397. [Google Scholar] [CrossRef]
- Levin, L.; Ofec, R.; Grossmann, Y.; Anner, R. Periodontal disease as a risk for dental implant failure over time: A long-term historical cohort study: Periodontal disease and implant survival. J. Clin. Periodontol. 2011, 38, 732–737. [Google Scholar] [CrossRef]
- Swierkot, K.; Lottholz, P.; Flores-de-Jacoby, L.; Mengel, R. Mucositis, Peri-Implantitis, Implant Success, and Survival of Implants in Patients With Treated Generalized Aggressive Periodontitis: 3- to 16-Year Results of a Prospective Long-Term Cohort Study. J. Periodontol. 2012, 83, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Lan, J.; Huang, H.; Liang, J.; Ma, X.; Huo, L.; Xu, X. A clinical study on the effectiveness of implant supported dental restoration in patients with chronic periodontal diseases. Int. J. Oral Maxillofac. Surg. 2013, 42, 256–259. [Google Scholar] [CrossRef]
- Roccuzzo, M.; Bonino, L.; Dalmasso, P.; Aglietta, M. Long-term results of a three arms prospective cohort study on implants in periodontally compromised patients: 10-year data around sandblasted and acid-etched (SLA) surface. Clin. Oral Implants Res. 2014, 25, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Cho-Yan Lee, J.; Mattheos, N.; Nixon, K.C.; Ivanovski, S. Residual periodontal pockets are a risk indicator for peri-implantitis in patients treated for periodontitis. Clin. Oral Implants Res. 2012, 23, 325–333. [Google Scholar] [CrossRef]
- Sayardoust, S.; Gröndahl, K.; Johansson, E.; Thomsen, P.; Slotte, C. Implant Survival and Marginal Bone Loss at Turned and Oxidized Implants in Periodontitis-Susceptible Smokers and Never-Smokers: A Retrospective, Clinical, Radiographic Case-Control Study. J. Periodontol. 2013, 84, 1775–1782. [Google Scholar] [CrossRef] [PubMed]
- Meyle, J.; Gersok, G.; Boedeker, R.H.; Gonzales, J.R. Long-term analysis of osseointegrated implants in non-smoker patients with a previous history of periodontitis. J. Clin. Periodontol. 2014, 41, 504–512. [Google Scholar] [CrossRef]
- Di Guarnieri, R.; Nardo, D.; Di Giorgio, G.; Miccoli, G.; Testarelli, L. Longevity of Teeth and Dental Implants in Patients Treated for Chronic Periodontitis Following Periodontal Maintenance Therapy in a Private Specialist Practice: A Retrospective Study with a 10-Year Follow-up. Int. J. Periodont. Restor. Dent. 2021, 41, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Pandolfi, A.; Rinaldo, F.; Pasqualotto, D.; Sorrentino, F.; La Torre, G.; Guerra, F. A retrospective cohort study on peri-implant complications in implants up to 10 years of functional loading in periodontally compromised patients. J. Periodontol. 2020, 91, 995–1002. [Google Scholar] [CrossRef]
- Vagia, P.; Papalou, I.; Burgy, A.; Tenenbaum, H.; Huck, O.; Davideau, J. Association between periodontitis treatment outcomes and peri-implantitis: A long-term retrospective cohort study. Clin. Oral Implants Res. 2021, 32, 721–731. [Google Scholar] [CrossRef]
- Raes, M.; D’hondt, R.; Teughels, W.; Coucke, W.; Quirynen, M. A 5-year randomized clinical trial comparing minimally with moderately rough implants in patients with severe periodontitis. J. Clin. Periodontol. 2018, 45, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, T.; Lin, M.; Xu, A.; Shen, X.; He, F. Risk factors associated with loss of variable-thread tapered implants: A retrospective observational study of 1–5 years. Clin. Oral Implants Res. 2023, 34, 662–674. [Google Scholar] [CrossRef] [PubMed]
- Aglietta, M.; Siciliano, V.I.; Rasperini, G.; Cafiero, C.; Lang, N.P.; Salvi, G.E. A 10-year retrospective analysis of marginal bone-level changes around implants in periodontally healthy and periodontally compromised tobacco smokers: Tobacco smoking and peri-implant bone loss. Clin. Oral Implants Res. 2011, 22, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Matarasso, S.; Rasperini, G.; Iorio Siciliano, V.; Salvi, G.E.; Lang, N.P.; Aglietta, M. A 10-year retrospective analysis of radiographic bone-level changes of implants supporting single-unit crowns in periodontally compromised vs. periodontally healthy patients. Clin. Oral Implants Res. 2010, 21, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, J.; Machtei, E.E. Immediate and Delayed Restoration of Dental Implants in Patients with a History of Periodontitis: A Prospective Evaluation up to 5 Years. Int. J. Oral Maxillofac. Implants 2012, 27, 1137–1143. [Google Scholar] [PubMed]
- Ormianer, Z.; Patel, A. The Use of Tapered Implants in the Maxillae of Periodontally Susceptible Patients: 10-Year Outcomes. Int. J. Oral Maxillofac. Implants 2012, 27, 442–448. [Google Scholar]
- Aguirre-Zorzano, L.A.; Vallejo-Aisa, F.J.; Estefania-Fresco, R. Supportive periodontal therapy and periodontal biotype as prognostic factors in implants placed in patients with a history of periodontitis. Med. Oral Patol. Oral Cir. Bucal 2013, 18, e786–e792. [Google Scholar] [CrossRef] [PubMed]
- Apatzidou, D.; Lappin, D.F.; Hamilton, G.; Papadopoulos, C.A.; Konstantinidis, A.; Riggio, M.P. Microbiome of peri-implantitis affected and healthy dental sites in patients with a history of chronic periodontitis. Arch. Oral Biol. 2017, 83, 145–152. [Google Scholar] [CrossRef]
- Correia, F.; Gouveia, S.; Felino, A.C.; Costa, A.L.; Almeida, R.F. Survival Rate of Dental Implants in Patients with History of Periodontal Disease: A Retrospective Cohort Study. Int. J. Oral Maxillofac. Implants 2017, 32, 927–934. [Google Scholar] [CrossRef]
- Zeza, B.; Pilloni, A.; Tatakis, D.N.; Mariotti, A.; Di Tanna, G.L.; Mongardini, C. Implant Patient Compliance Varies by Periodontal Treatment History. J. Periodontol. 2017, 88, 846–853. [Google Scholar] [CrossRef]
- Altay, M.A.; Tozoğlu, S.; Yıldırımyan, N.; Özarslan, M.M. Is History of Periodontitis a Risk Factor for Peri-implant Disease? A Pilot Study. Int. J. Oral Maxillofac. Implants 2018, 33, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Sung, C.E.; Chiang, C.Y.; Chiu, H.C.; Shieh, Y.S.; Lin, F.G.; Fu, E. Periodontal status of tooth adjacent to implant with peri-implantitis. J. Dent. 2018, 70, 104–109. [Google Scholar] [CrossRef]
- Gallego, L.; Sicilia, A.; Sicilia, P.; Mallo, C.; Cuesta, S.; Sanz, M. A retrospective study on the crestal bone loss associated with different implant surfaces in chronic periodontitis patients under maintenance. Clin. Oral Implants Res. 2018, 29, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, J.; Wang, L.; Li, J.; Zhou, F.; Ding, X.; Tao, A.; Lv, X. Clinical evaluation of dental implant rehabilitation in patients with chronic periodontitis. Int. J. Clin. Exp. Med. 2019, 12, 13831–13838. [Google Scholar]
- Armitage, G.C. Development of a Classification System for Periodontal Diseases and Conditions. Ann. Periodontol. 1999, 4, 1–6. [Google Scholar] [CrossRef]
- Jordana, F.; Susbielles, L.; Colat-Parros, J. Periimplantitis and Implant Body Roughness: A Systematic Review of Literature. Implant. Dent. 2018, 27, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, G.; Signoriello, A.; Pardo, A.; Serpa Romero, X.Z.; Vila Sierra, L.A.; Tovar, L.A.; Marincola, M.; Nocini, P.F. Short and ultra-short (<6-mm) locking-taper implants supporting single crowns in posterior areas (part II): A 5-year retrospective study on periodontally healthy patients and patients with a history of periodontitis. Clin. Implant. Dent. Relat. Res. 2022, 24, 455–467. [Google Scholar]
- Montenegro, S.C.L.; Retamal-Valdes, B.; Bueno-Silva, B.; Duarte, P.M.; Faveri, M.; Figueiredo, L.C.; Feres, M. Do patients with aggressive and chronic periodontitis exhibit specific differences in the subgingival microbial composition? A systematic review. J. Periodontol. 2020, 91, 1503–1520. [Google Scholar] [CrossRef]
- Cortelli, S.C.; Cortelli, J.R.; Romeiro, R.L.; Costa, F.O.; Aquino, D.R.; Orzechowski, P.R.; Araújo, V.C.; Duarte, P.M. Frequency of periodontal pathogens in equivalent peri-implant and periodontal clinical statuses. Arch. Oral Biol. 2013, 58, 67–74. [Google Scholar] [CrossRef]
- Rakic, M.; Grusovin, M.G.; Canullo, L. The Microbiologic Profile Associated with Peri-Implantitis in Humans: A Systematic Review. Int. J. Oral Maxillofac. Implants 2016, 31, 359–368. [Google Scholar] [CrossRef]
| Name of the Study | Follow-Up Time | Type of Study | Inclusion |
|---|---|---|---|
| CASADO et al., 2013 [14] | 8 years | Retrospective cohort studies | Quantitative synthesis |
| RASPERINI et al., 2014 [21] | 10 years | ||
| THÖNE-MÜHLING et al., 2016 [22] | 4 years | ||
| GRAETZ et al., 2017 [15] | 10 years | ||
| ROCCUZZO et al., 2010–2012 [13] | 10 years | Prospective cohort studies | |
| LEVIN et al., 2011 [23] | 12 years | ||
| SWIERKOT et al., 2012 [24] | 16 years | ||
| JIANG et al., 2013 [25] | 2 years | ||
| ROCCUZZO et al., 2014 [26] | 10 years | ||
| CHO-YAN LEE et al., 2011 [27] | 14 years | Retrospective case-control studies | |
| SAYARDOUST et al., 2013 [28] | 5 years | Transversal studies | Qualitative synthesis |
| AGUIRRE et al., 2015 [16] | 17 years | ||
| MEYLE et al., 2014 [29] | 10 years | ||
| DI GUARNIERI et al., 2020 [30] | 10 years | ||
| PANDOLFI et al., 2020 [31] | 10 years | ||
| VAGIA et al., 2021 [32] | 3 years | Retrospective study | |
| RAES et al., 2018 [33] | 5 years | Clinical randomized trials | |
| XU et al., 2023 [34] | 5 years | Retrospective study |
| Population Characteristics | Intervention Characteristics | Main Outcomes | Secondary Outcomes | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Inclusion | Name of the Study | Periodontal Diagnosis Groups | Patients Number (N) | Implants Number | Type of Implant: Surface | Peri-Implantitis Rate (%) | Survival Rate (%) | Mean of Peri-Implant Bone Loss (mm) | Mean of Pocket Depth (mm) |
| Quantitative synthesis | Roccuzzo et al., 2010–2012 [13] | MCP | 38 | 95 | Straumann: TPS | 27 | 92.8 | 1.14 ± 1.11 | 3.5 ± 0.9 |
| SCP | 42 | 90 | 47.2 | 90 | 0.98 ± 1.22 | 3.9 ± 0.7 | |||
| H | 32 | 61 | 10.7 | 96.6 | 0.75 ± 0.88 | 3.1 ± 0.5 | |||
| Cho-Yan Lee et al., 2011 [27] | CP | 30 | 56 | Straumann: SLA or TPS | 36.7% | 0.45 ± 0.94 | 2.83 ± 0.59 | ||
| H | 30 | 61 | 16.7% | 0.26 ± 0.72 | 2.81 ± 0.49 | ||||
| Levin et al., 2011 [23] | MCP | 149 | 447 | Unclear | 96.6 | ||||
| SCP | 285 | 747 | 94.8 | ||||||
| H | 283 | 747 | 96.9 | ||||||
| Swierkot et al., 2012 [24] | AP | 35 | 149 | Branemark MKII Nobel Biocare Osseotite Biomet 3i | 43 | 96 | |||
| H | 18 | 30 | 11 | 100 | |||||
| Casado et al., 2013 [14] | CP | 215 | 754 | EH/IH/CM | 59 | ||||
| H | 27 | ||||||||
| Jiang et al., 2013 [25] | CP | 30 | 149 | Unclear | 95.97 | ||||
| H | 30 | 127 | 97.6 | ||||||
| Rasperini et al., 2014 [21] | CP/Sm | 60 | 120 | Branemark: Machined | 85 | 3.47 ± 1.09 | |||
| Straumann: TPS | 3.77 ± 1.43 | ||||||||
| CP/NSm | MS and TPS | 90 | 2.32 ± 0.41 | ||||||
| H/Sm | 60 | MS | 95 | 2.65 ± 0.41 | |||||
| TPS | 2.51 ± 0.31 | ||||||||
| H/NSm | MS | 95 | 1.43 ± 0.38 | ||||||
| TPS | 1.95 ± 0.42 | ||||||||
| Roccuzzo et al., 2014 [26] | MCP | 46 | 96 | Straumann: SLA | 52.2 | 96.9 | 4.6 ± 3.1 | ||
| SCP | 45 | 102 | 66.7 | 97.1 | 4.8 ± 1.4 | ||||
| H | 32 | 54 | 18.8 | 100 | 4.4 ± 1.1 | ||||
| Thöne-Mühling et al., 2016 [22] | AP | 35 | 149 | Branemark MKII Nobel Biocare Osseotite Biomet 3i | 97.3 | 3.5 ± 0.7 | |||
| H | 18 | 30 | 100 | 3.42 ± 0.81 | |||||
| Graetz et al., 2017 [15] | CP | 29 | 69 | Unclear | 92.5 | 4.2 ± 1.6 | |||
| H | 29 | 76 | 91.4 | 2.9 ± 0.8 | |||||
| Total | 1571 | 4030 | |||||||
| Population Characteristics | Intervention Characteristics | Main Outcomes | Secondary Outcomes | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Inclusion | Name of the Study | Periodontal Diagnosis Groups | Patients Number | Implants Number | Type of Implant | Peri-Implantitis Rate (%) | Survival Rate (%) | Mean of Peri-Implant Bone Loss (mm) | Mean of Pocket Depth (mm) |
| Sayardoust et al., 2013 [28] | SCP/Sm | 80 | 252 | Branemark: Machined TiUite: Oxidized | - | 92.9 | 1.39 ± 1.57 | - | |
| SCP/NSm | 1.01 ± 1.09 | ||||||||
| Aguirre et al., 2015 [16] | CP | 170 | 786 | Astra Tech Nobel replace Steri-Oss | 5 years: 8 10 years: 9 17 years: 15 | - | - | - | |
| AP | 69 | ||||||||
| Meyle et al., 2014 [29] | CP | 20 | 54 | Frialit 2 Dentsply | 5 years: 8.9 10 years: 23.8 | 96.3 | 5 years: 0.23 ± 0.34 10 years: 0.63 ± 0.26 | 5 years: 2.9 ± 0.8 10 years: 3.3 ± 1.0 | |
| Raes et al., 2018 [33] | AP | 18 | 84 | Branemark: Machined | 5 years: 7.14 | 97.6 | 5 years: 1.00 ± 0.90 | 5 years: 3.1 ± 1.0 | |
| TiUnite: Oxidized | 5 years: 28.57 | 100 | 5 years: 1.65 ± 1.65 | 5 years: 4.2 ± 2.6 | |||||
| Di Guarnieri et al., 2020 [30] | CP | 58 | 127 | - | - | 90 | 3.1 ± 1.2 | 1–4 mm: 68.9 5–6 mm: 20.6 >6 mm: 10.5 | |
| Pandolfi et al., 2020 [31] | CP | 475 | 1991 | Straumann | 10 years: 12.9 | 96.1 | - | - | |
| Vagia et al., 2021 [32] | CP | 86 | 260 | Srtaumann | 10 years: 12,8 | - | - | - | |
| Xu et al., 2023 [34] | MCP | 1528 | 2998 | Nobel Biocare | - | 95,4% | - | - | |
| Total | 2504 | 6552 | |||||||
| Diagnosis of Periodontitis | Odds Ratio | p-Value | |
|---|---|---|---|
| Peri-implantitis rate | P group vs. H group | 4.80 | p < 0.05 |
| AP group vs. H group | 6.00 | p < 0.05 | |
| CP group vs. H group | 4.55 | p < 0.05 | |
| CP group vs. AP group | 1.59 | p < 0.05 | |
| Survival rate | P group vs. H group | 0.60 | p < 0.05 |
| CP group vs. H group | 0.60 | p < 0.05 | |
| CP group vs. AP group | 0.55 | p < 0.05 | |
| Std diff in means | |||
| Mean PI bone loss | P group vs. H group | 0.77 mm | p < 0.05 |
| Mean pocket depth | P group vs. H group | 0.56 mm | p < 0.05 |
| CP group vs. AP group | 0.44 mm | p > 0.05 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marty, L.; Hoornaert, A.; Enkel, B.; Penhoat, A.; Colat-Parros, J.; Soueidan, A.; Jordana, F. Implant Health in Treated Periodontitis Patients: A Systematic Review and Meta-Analysis. Dent. J. 2024, 12, 240. https://doi.org/10.3390/dj12080240
Marty L, Hoornaert A, Enkel B, Penhoat A, Colat-Parros J, Soueidan A, Jordana F. Implant Health in Treated Periodontitis Patients: A Systematic Review and Meta-Analysis. Dentistry Journal. 2024; 12(8):240. https://doi.org/10.3390/dj12080240
Chicago/Turabian StyleMarty, Léa, Alain Hoornaert, Bénédicte Enkel, Alan Penhoat, Jacques Colat-Parros, Assem Soueidan, and Fabienne Jordana. 2024. "Implant Health in Treated Periodontitis Patients: A Systematic Review and Meta-Analysis" Dentistry Journal 12, no. 8: 240. https://doi.org/10.3390/dj12080240
APA StyleMarty, L., Hoornaert, A., Enkel, B., Penhoat, A., Colat-Parros, J., Soueidan, A., & Jordana, F. (2024). Implant Health in Treated Periodontitis Patients: A Systematic Review and Meta-Analysis. Dentistry Journal, 12(8), 240. https://doi.org/10.3390/dj12080240






