Osteoporosis and Apical Periodontitis Prevalence: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
- → Population/participants: Adult individuals (≥18 years old)
- → Intervention(s), exposure(s): Patients with OP
- → Comparator(s)/control: Healthy individuals (without OP)
- → Outcome: Prevalence of AP associated with or without root-filled teeth in patients diagnosed with OP
- → Inclusion criteria: Studies reporting the prevalence of AP from adult individuals with OP and healthy controls, in randomized clinical trials, cross-sectional, cohort, and case-control studies.
- → Exclusion criteria: Animal or laboratory investigations, studies not including a control group of healthy individuals, studies not reporting AP prevalence. Studies that did not address the specific research question were excluded. Repeated findings, meta-analyses, scoping, systematic, or narrative reviews, meeting abstracts, case series, and case reports, were excluded.
2.3. Search Strategy
2.4. Selection of the Studies
2.5. Data Extraction
2.6. Quality Assessment
3. Results
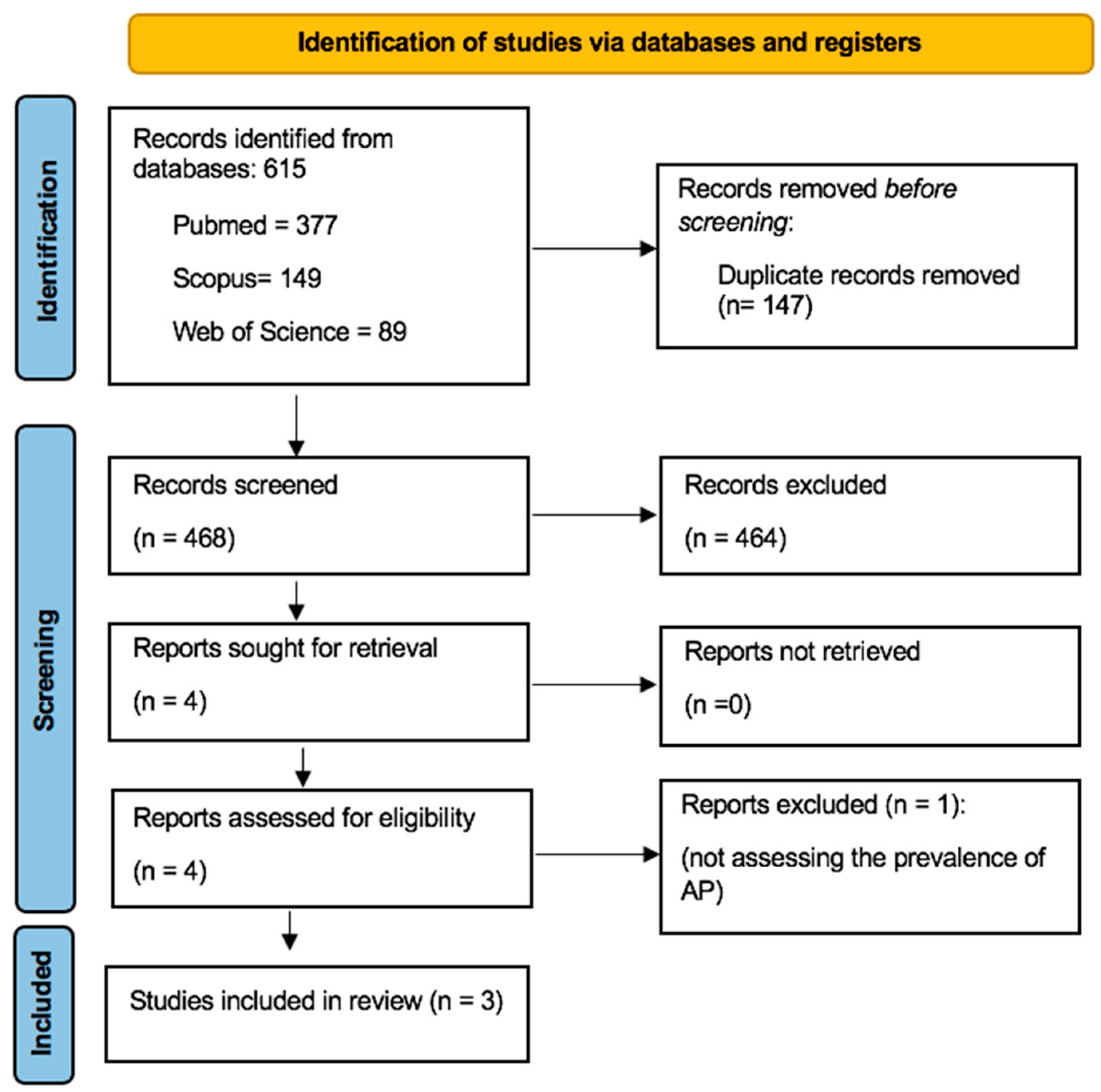
3.1. Literature Search Process
3.2. Characteristics of the Included Studies
3.3. Main Findings
3.3.1. AP Prevalence in OP Patients
3.3.2. The Impact of OP Medications on the Prevalence of AP
3.3.3. Progression of AP
3.3.4. Quality of the Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shrihari, K.; Kour, H. Narrative Review on Osteoporosis: A Silent Killer. J. Clin. Diagn. Res. 2024, 18, RE01–RE06. [Google Scholar]
- Xiao, P.L.; Cui, A.Y.; Hsu, C.J.; Peng, R.; Jiang, N.; Xu, X.H.; Ma, Y.G.; Liu, D.; Lu, H.D. Global, regional prevalence, and risk factors of osteoporosis according to the World Health Organization diagnostic criteria: A systematic review and meta-analysis. Osteoporos. Int. 2022, 33, 2137–2153. [Google Scholar] [CrossRef]
- Segura-Egea, J.J.; Cabanillas-Balsera, D.; Martín-González, J.; Cintra, L.T.A. Impact of systemic health on treatment outcomes in endodontics. Int. Endod. J. 2023, 56 (Suppl. 2), 219–235. [Google Scholar] [CrossRef]
- López-López, J.; Castellanos-Cosano, L.; Estrugo-Devesa, A.; Gómez-Vaquero, C.; Velasco-Ortega, E.; Segura-Egea, J.J. Radiolucent periapical lesions and bone mineral density in post-menopausal women. Gerodontology 2015, 32, 195–201. [Google Scholar] [CrossRef]
- Cadoni, E.; Ideo, F.; Marongiu, G.; Mezzena, S.; Frigau, L.; Mela, Q.; Capone, A.; Duncan, H.F.; Cotti, E. Periapical status in patients affected by osteoporosis: A retrospective clinical study. Clin. Exp. Dent. Res. 2022, 8, 1068–1075. [Google Scholar] [CrossRef]
- Ye, Z.; Lu, H.; Liu, P. Association between essential hypertension and bone mineral density: A systematic review and meta-analysis. Oncotarget 2017, 8, 68916–68927. [Google Scholar] [CrossRef]
- Yi, K.J.; Kang, R.M.; Zhang, Y.Y.; Li, Q. Causal relationship between circulating inflammatory factors and osteoporosis: A bidirectional Mendelian randomization study. Eur. Rev. Med. Pharmacol. Sci. 2024, 28, 2237–2249. [Google Scholar] [CrossRef]
- Sözen, T.; Özışık, L.; Başaran, N. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef]
- Trémollieres, F. Assessment and hormonal management of osteoporosis. Climacteric 2019, 22, 122–126. [Google Scholar] [CrossRef]
- Siqueira, J.F., Jr.; Rôças, I.N. Clinical implications and microbiology of bacterial persistence after treatment procedures. J. Endod. 2008, 34, 1291–1301.e3. [Google Scholar] [CrossRef]
- Siqueira, J.F., Jr. Endodontic infections: Concepts, paradigms, and perspectives. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2002, 94, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F.; Rôças, I.N.; Ricucci, D. Biofilms in endodontic infection. Endod. Top. 2010, 22, 33–49. [Google Scholar] [CrossRef]
- Jakovljevic, A.; Ideo, F.; Jacimovic, J.; Aminoshariae, A.; Nagendrababu, V.; Azarpazhooh, A.; Cotti, E. The Link Between Apical Periodontitis and Gastrointestinal Diseases-A Systematic Review. J. Endod. 2023, 49, 1421–1431. [Google Scholar] [CrossRef]
- Tibúrcio-Machado, C.S.; Michelon, C.; Zanatta, F.B.; Gomes, M.S.; Marin, J.A.; Bier, C.A. The global prevalence of apical periodontitis: A systematic review and meta-analysis. Int. Endod. J. 2021, 54, 712–735. [Google Scholar] [CrossRef]
- Segura-Egea, J.J.; Martin-Gonzalez, J.; Castellanos-Cosano, L. Endodontic medicine: Connections between apical periodontitis and systemic diseases. Int. Endod. J. 2015, 48, 933–951. [Google Scholar] [CrossRef] [PubMed]
- Jakovljevic, A.; Nikolic, N.; Jacimovic, J.; Pavlovic, O.; Milicic, B.; Beljic-Ivanovic, K.; Miletic, M.; Andric, M.; Milasin, J. Prevalence of Apical Periodontitis and Conventional Nonsurgical Root Canal Treatment in General Adult Population: An Updated Systematic Review and Meta-analysis of Cross-sectional Studies Published between 2012 and 2020. J. Endod. 2020, 46, 1371–1386.e8. [Google Scholar] [CrossRef]
- Ye, L.; Cao, L.; Song, W.; Yang, C.; Tang, Q.; Yuan, Z. Interaction between apical periodontitis and systemic disease (Review). Int. J. Mol. Med. 2023, 52, 60. [Google Scholar] [CrossRef]
- Xiong, H.; Peng, B.; Wei, L.; Zhang, X.; Wang, L. Effect of an estrogen-deficient state and alendronate therapy on bone loss resulting from experimental periapical lesions in rats. J. Endod. 2007, 33, 1304–1308. [Google Scholar] [CrossRef]
- Wayama, M.T.; Yoshimura, H.; Ohba, S.; Yoshida, H.; Matsuda, S.; Kobayashi, J.; Kobayashi, M.; Gomes Filho, J.E.; Sano, K. Diminished Progression of Periapical Lesions with Zoledronic Acid in Ovariectomized Rats. J. Endod. 2015, 41, 2002–2007. [Google Scholar] [CrossRef][Green Version]
- Silva, R.A.B.; Sousa-Pereira, A.P.; Lucisano, M.P.; Romualdo, P.C.; Paula-Silva, F.W.G.; Consolaro, A.; Silva, L.A.B.; Nelson-Filho, P. Alendronate inhibits osteocyte apoptosis and inflammation via IL-6, inhibiting bone resorption in periapical lesions of ovariectomized rats. Int. Endod. J. 2020, 53, 84–96. [Google Scholar] [CrossRef]
- Ikeda, M.; Karakawa, A.; Takizawa, H.; Azetsu, Y.; Sakai, N.; Chatani, M.; Suzuki, N.; Takami, M. Effects of Anti-Receptor Activator of Nuclear Factor Kappa B Ligand Antibody and Zoledronic Acid on Periapical Lesion Development in Mice. J. Endod. 2022, 48, 632–640. [Google Scholar] [CrossRef]
- Pauli, M.A.; Bordignon, N.C.T.; Martini, G.R.; Minamisako, M.C.; Gondak, R. Prevalence of dental alterations in patients under bisphosphonates therapy: A systematic review. Oral. Maxillofac. Surg. 2023, 27, 399–409. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Dereci, Ö.; Orhan, E.O.; Irmak, Ö.; Ay, S. The effect of the duration of intravenous zolendronate medication on the success of non-surgical endodontic therapy: A retrospective study. BMC Oral. Health 2016, 16, 9. [Google Scholar] [CrossRef][Green Version]
- Katz, J.; Rotstein, I. Prevalence of Periapical Lesions in Patients with Osteoporosis. J. Endod. 2021, 47, 234–238. [Google Scholar] [CrossRef]
- Halse, A.; Molven, O. A strategy for the diagnosis of periapical pathosis. J. Endod. 1986, 12, 534–538. [Google Scholar] [CrossRef]
- Song, J.W.; Chung, K.C. Observational studies: Cohort and case-control studies. Plast. Reconstr. Surg. 2010, 126, 2234–2242. [Google Scholar] [CrossRef]
- Gutmann, J.L.; Baumgartner, J.C.; Gluskin, A.H.; Hartwell, G.R.; Walton, R.E. Identify and define all diagnostic terms for periapical/periradicular health and disease states. J. Endod. 2009, 35, 1658–1674. [Google Scholar] [CrossRef]
- De Moor, R.J.; Hommez, G.M.; De Boever, J.G.; Delmé, K.I.; Martens, G.E. Periapical health related to the quality of root canal treatment in a Belgian population. Int. Endod. J. 2000, 33, 113–120. [Google Scholar] [CrossRef]
- López-López, J.; Jané-Salas, E.; Estrugo-Devesa, A.; Castellanos-Cosano, L.; Martín-González, J.; Velasco-Ortega, E.; Segura-Egea, J.J. Frequency and distribution of root-filled teeth and apical periodontitis in an adult population of Barcelona, Spain. Int. Dent. J. 2012, 62, 40–46. [Google Scholar] [CrossRef]
- López-López, J.; Jané-Salas, E.; Estrugo-Devesa, A.; Velasco-Ortega, E.; Martín-González, J.; Segura-Egea, J.J. Periapical and endodontic status of type 2 diabetic patients in Catalonia, Spain: A cross-sectional study. J. Endod. 2011, 37, 598–601. [Google Scholar] [CrossRef] [PubMed]
- Heuchert, J.; Kozieł, S.; Spinek, A.E. Radiomorphometric indices of the mandible as indicators of decreased bone mineral density and osteoporosis—Meta-analysis and systematic review. Osteoporos. Int. 2024, 35, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Gaur, A.; Dhillon, M.; Puri, N.; Sethi Ahuja, U.; Rathore, A. Questionable accuracy of CBCT in determining bone density: A comparative CBCT-CT in vitro study. Dent. Med. Probl. 2022, 59, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Orstavik, D.; Kerekes, K.; Eriksen, H.M. The periapical index: A scoring system for radiographic assessment of apical periodontitis. Endod. Dent. Traumatol. 1986, 2, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Poyato-Borrego, M.; Segura-Sampedro, J.J.; Martín-González, J.; Torres-Domínguez, Y.; Velasco-Ortega, E.; Segura-Egea, J.J. High Prevalence of Apical Periodontitis in Patients with Inflammatory Bowel Disease: An Age- and Gender- matched Case-control Study. Inflamm. Bowel Dis. 2020, 26, 273–279. [Google Scholar] [CrossRef]
- Sánchez-Domínguez, B.; López-López, J.; Jané-Salas, E.; Castellanos-Cosano, L.; Velasco-Ortega, E.; Segura-Egea, J.J. Glycated hemoglobin levels and prevalence of apical periodontitis in type 2 diabetic patients. J. Endod. 2015, 41, 601–606. [Google Scholar] [CrossRef]
- Rizzoli, R.; Burlet, N.; Cahall, D.; Delmas, P.D.; Eriksen, E.F.; Felsenberg, D.; Grbic, J.; Jontell, M.; Landesberg, R.; Laslop, A.; et al. Osteonecrosis of the jaw and bisphosphonate treatment for osteoporosis. Bone 2008, 42, 841–847. [Google Scholar] [CrossRef]
- Hsiao, A.; Glickman, G.; He, J. A retrospective clinical and radiographic study on healing of periradicular lesions in patients taking oral bisphosphonates. J. Endod. 2009, 35, 1525–1528. [Google Scholar] [CrossRef] [PubMed]
- Gilles, J.A.; Carnes, D.L.; Dallas, M.R.; Holt, S.C.; Bonewald, L.F. Oral bone loss is increased in ovariectomized rats. J. Endod. 1997, 23, 419–422. [Google Scholar] [CrossRef]
- Soliman, T.; Ali, Z.; Zayed, M.; Sabry, D.; AbuBakr, N. Assessing the bone-healing potential of bone marrow mesenchymal stem cells in jawbone osteoporosis in albino rats. Dent. Med. Probl. 2022, 59, 75–83. [Google Scholar] [CrossRef]
| Database | Search Strategy | Findings |
|---|---|---|
| PubMed | #1 (osteoporosis OR bisphosphonates OR menopause) | 216,172 |
| #2 (Endodontics OR Periapical Periodontitis OR Periapical Diseases OR Apical Periodontitis OR Periradicular Lesion OR Periapical Radiolucency OR Radiolucent Periapical Lesion) | 65,113 | |
| #1 and #2 | 377 | |
| Scopus | #1 (osteoporosis OR bisphosphonates OR menopause) | 249,224 |
| #2 (Endodontics OR “Periapical Periodontitis” OR “Periapical Diseases” OR “Apical Periodontitis” OR “Periradicular Lesion” OR “Periapical Radiolucency” OR “Radiolucent Periapical Lesion”) | 40,924 | |
| #1 and #2 | 149 | |
| Web of Science | #1 (osteoporosis OR bisphosphonates OR menopause) | 192,512 |
| #2 (Endodontics OR “Periapical Periodontitis” OR “Periapical Diseases” OR “Apical Periodontitis” OR “Periradicular Lesion” OR “Periapical Radiolucency” OR “Radiolucent Periapical Lesion”) | 27,151 | |
| #1 and #2 | 89 |
| Authors, Year | Study Design | Number of Participants/Age (Mean ± Standard Deviation Range) | Population Characteristics | Investigated Outcomes of Interest | Exposure Evaluation Method/AP Definition | Main Results |
|---|---|---|---|---|---|---|
| Lopez-Lopez et al. 2015 [4] | Cross-sectional study | 75 (62.5 ± 1.7)/years Ostoporotic (12) Osteopenic (36) Control (27) Greater than 50 years old | Post-menopausal women recruited at the Dental Clinic of the University of Barcelona, Spain | - Number of teeth present - Number and location of root-filled teeth - Number and location of teeth having coronal restorations - Number and location of teeth having AP | Panoramic radiograph (AP was defined as periodontal ligament space larger than the normal width) | A marginally significant association was evident between low bone mineral density (BMD) and the presence of AP (OR = 1.9; CI 95% = 1.0–3.8; p = 0.050) |
| Katz et al. 2021 [25] | Cross-sectional study | 1.644.953 Age not specified | Integrated data from the total hospital patient’s population of the University of Florida (USA) Health Office for the period of 2011–2020 were used | - Prevalence of AP - Prevalence of OP - Prevalence of AP in patients with OP - Prevalence of AP in patients treated with alendronate and risedronate | Panoramic radiograph (AP was defined as radiographic evidence of apical rarefying osteitis) | The prevalence AP was significantly higher in OP patients (OR = 3.36; p < 0.0001) OP patients treated with BPs showed a marked reduction in the prevalence of AP (p < 0.0001) |
| Cadoni et al. 2022 [5] | Case-control study | Cases: 76/64.61 ± 8.09 years D (9) BPs (31) BPs + D (11) NM (25) Control: 76/59.67 ± 9.88 years | Patients diagnosed with OP without systemic conditions, referred to the University Dental Clinic from the Departments of Rheumatology and Orthopedics at the Cagliari University Hospital (Italy), from February 2015 to October 2020 | - Number of teeth present - Number of caries and AP - Prevalence of AP - Decayed, missing, filled teeth index - Prevalence of AP in root canal-treated teeth - Quality of root-filled teeth and restoration | Periapical radiograph Panoramic radiograph PAI score | Primary OP does not appear to be associated with the prevalence of AP regardless of whether the condition is untreated or treated with therapeutic agents like BPs and denosumab The prevalence of AP was higher in root-filled teeth in the OP group (p = 0.03) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pestana de Vasconcelos, N.; Martins, I.S.; Afonso, A.S.; Braga, A.C.; Pina-Vaz, I. Osteoporosis and Apical Periodontitis Prevalence: A Systematic Review. Dent. J. 2024, 12, 272. https://doi.org/10.3390/dj12080272
Pestana de Vasconcelos N, Martins IS, Afonso AS, Braga AC, Pina-Vaz I. Osteoporosis and Apical Periodontitis Prevalence: A Systematic Review. Dentistry Journal. 2024; 12(8):272. https://doi.org/10.3390/dj12080272
Chicago/Turabian StylePestana de Vasconcelos, Natália, Isabel Silva Martins, Américo Santos Afonso, Ana Cristina Braga, and Irene Pina-Vaz. 2024. "Osteoporosis and Apical Periodontitis Prevalence: A Systematic Review" Dentistry Journal 12, no. 8: 272. https://doi.org/10.3390/dj12080272
APA StylePestana de Vasconcelos, N., Martins, I. S., Afonso, A. S., Braga, A. C., & Pina-Vaz, I. (2024). Osteoporosis and Apical Periodontitis Prevalence: A Systematic Review. Dentistry Journal, 12(8), 272. https://doi.org/10.3390/dj12080272










