Abstract
Bioceramic materials for endodontic treatments have gradually transformed over the years into materials with enhanced biocompatibility and chemical and mechanical properties compared to earlier generations. In endodontics procedures, these materials are used as restorative material in applications such as root-end fillings, pulp capping, perforations repair, and apexification repair procedures. However, they have far from ideal mechanical and handling properties, biocompatibility issues, aesthetic concerns due to tooth discolouration, limited antibacterial activity, and affordability, which are amongst several key limitations. Notably, bioceramic materials are popular due to their biocompatibility, sealing ability, and durability, consequently surpassing traditional materials such as gutta-percha and zinc oxide–eugenol sealers. A lack of recent advancements in the field, combined with nanomaterials, has improved the formulations of these materials to overcome these limitations. The existing literature emphasises the benefits of bioceramics while underreporting their poor mechanical properties, handling difficulties, cost, and various other drawbacks. The key gaps identified in the literature are the insufficient coverage of emerging materials, narrow scope, limited insights into future developments, and underreporting of failures and complications of the existing materials. Consequently, this review aims to highlight the key limitations of various endodontic materials, primarily focusing on calcium silicate, calcium phosphate, and bioactive glass-based materials, which are the most abundantly used materials in dentistry. Based on the literature, bioceramic materials in endodontics have significantly improved over recent years, with different combinations of materials and technology compared to earlier generations while preserving many of their original properties, with some having affordable costs. This review also identified key innovations that could shape the future of endodontic materials, highlighting the ongoing evolution and advancements in endodontic treatments.
1. Introduction
Bioceramic materials are biocompatible ceramic materials designed to interact with biological systems, which are primarily used in medicine and dentistry to replace or regenerate damaged hard tissues [1]. In endodontics, bioceramic materials primarily interact with dental pulp and the surrounding tooth and root canal system tissues, which are optimally designed for biocompatibility and mechanical properties [2]. With the introduction of mineral trioxide aggregate (MTA) as a bioceramic in 1993, bioceramics have transformed endodontics by providing solutions for repairing and reconstructing damaged dental tissues to restore their original functionality [3,4]. As of 2023, the global bioceramics market was valued at USD 7.4 billion. Within this market, the segment for root canal sealers is expected to grow at a compound annual growth rate (CAGR) of 3.6%, increasing from USD 2.41 billion in 2024 to USD 3.25 billion by the end of 2032 [5,6,7]. The market growth is primarily driven by endodontic applications, such as root canal fillings, sealers, and regenerative procedures in endodontics. With new innovations, materials such as calcium silicates, including MTA, bioactive glasses, and other calcium silicates, have shown the ability to promote the healing and regeneration of damaged tooth tissue [7].
Although bioceramics are among the most popular materials used in most countries, some regions still heavily rely on non-bioceramic options, such as zinc oxide–eugenol-based cements, as well as gutta-percha [8,9,10]. They are used for various endodontic applications as sealers, obturation materials, and materials for repairing perforations, as well as for pulp capping, pulpotomies, managing resorption, apexification, and regenerative treatments (Figure 1). Traditional endodontic materials, such as gutta-percha and zinc oxide–eugenol (ZOE) cements, exhibit significant limitations compared to modern bioceramic materials, particularly in terms of biocompatibility, bioactivity, and tissue regeneration potential. While gutta-percha serves as a standard obturation material, it lacks the ability to promote healing or actively integrate with surrounding tissues. Similarly, ZOE cements suffer from poor solubility, prolonged setting times, and dimensional shrinkage upon curing, which can compromise root canal treatment sealing and long-term success. In contrast, bioceramic materials offer superior biological and physical properties, addressing these deficiencies and aligning with the demands of contemporary endodontic practice [11].
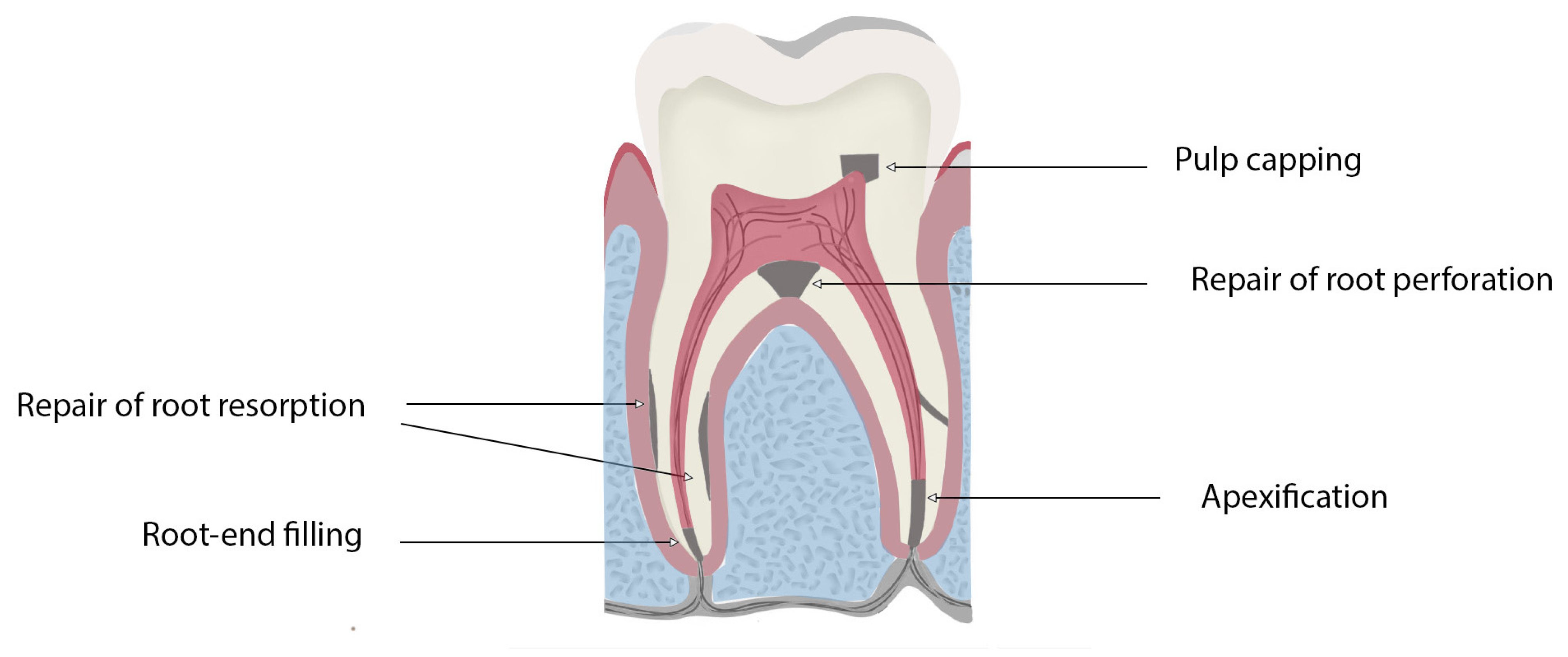
Figure 1.
Schematic diagram highlighting the clinical applications of bioceramics for use in endodontics procedures.
In restorative dentistry, bioceramic materials are used as dentin substitutes for pulp capping, treating dentin hypersensitivity, and enabling dentin remineralisation [12,13]. Despite their many properties, bioceramics still exhibit some limitations.
Bioceramic materials in endodontics have been classified in many ways based on their applications, bioactivity, and the primary component of the material [2,14,15]. These materials are widely employed in combination, and zirconia and alumina are primarily used in endodontic materials as filler materials and radiopacifiers in various formulations [2,16].
Despite recent advancements in nanotechnology, bioceramics designed for endodontic applications are still far from ideal, highlighting the need for continued innovation to address their inherent limitations. While nanoparticles enhance the properties of endodontic materials, they also introduce additional costs and manufacturing complexities. Common endodontic materials use silver, silica, zinc oxide, iron oxide, nanopolymers, and carbon-based nanoparticles such as carbon nanotubes. However, these nanoparticles may pose potential toxicity risks and require thorough evaluation for cytotoxicity and biocompatibility. As such, long-term clinical data will be necessary to assess the safety and effectiveness of these nanomaterials [17].
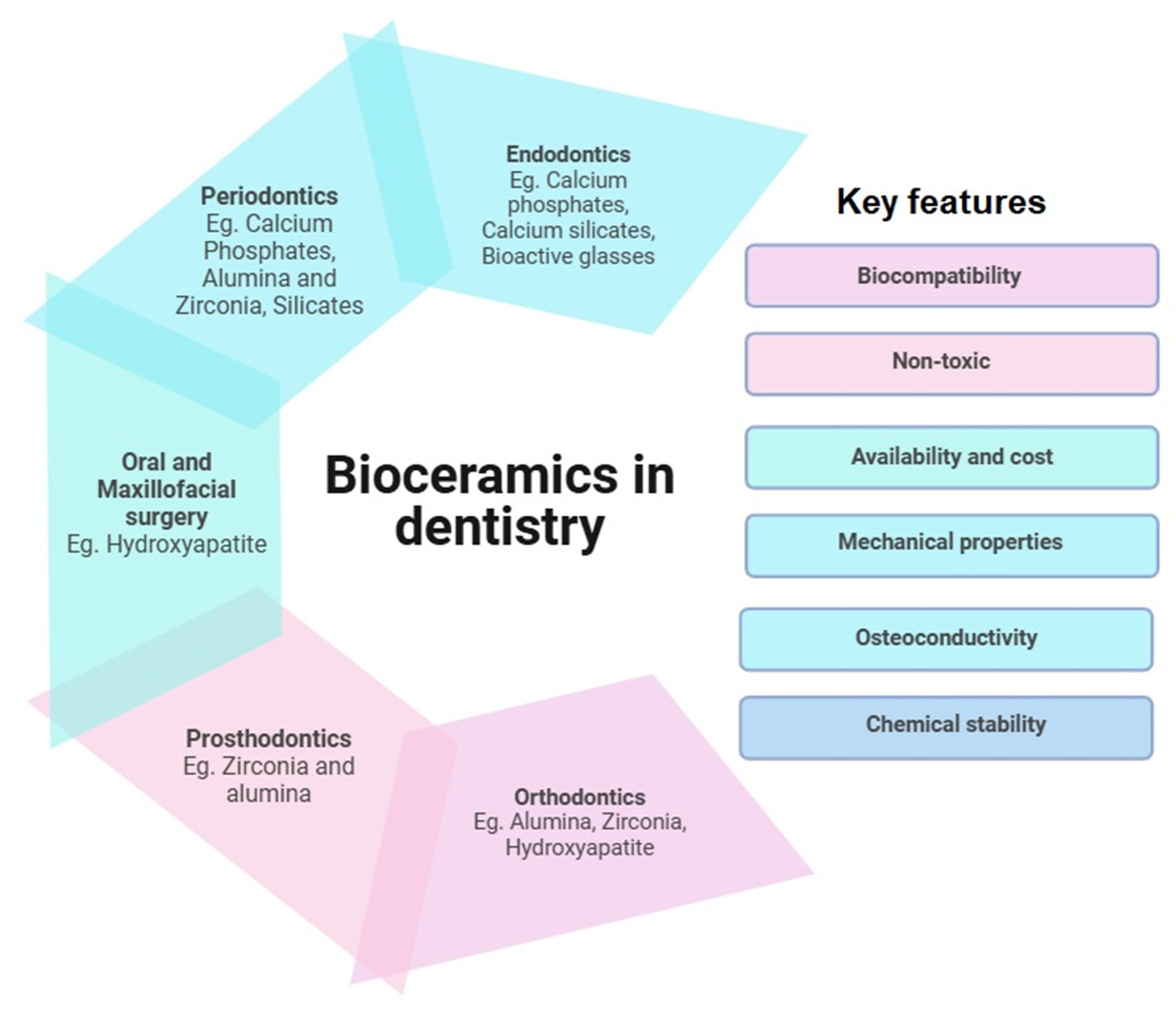
While most literature aims at discussing the properties and comparative studies of bioceramic materials [18,19], the main objective of this review is to identify the limitations of current endodontic materials, explore their development and improvement challenges, and examine the strategies used to address these issues. The insights gained could guide future researchers and developers in advancing the endodontic material arsenal. Figure 2 depicts the applications of bioceramics in dentistry alone, with examples and key features suitable for their applications.
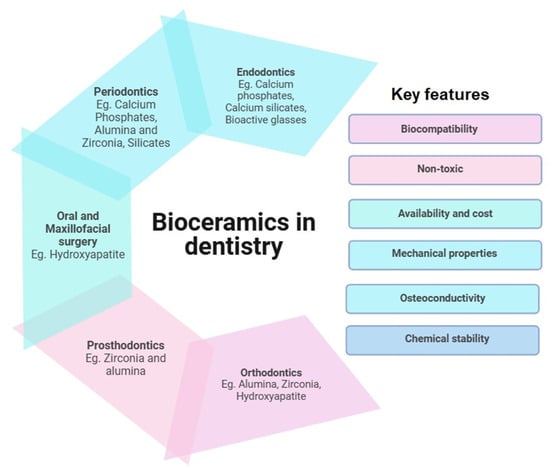
Figure 2.
Applications of bioceramics in dentistry: key component and features.
2. Bioceramic Materials
2.1. Calcium Silicates
Bioceramics used in endodontics are generally bioactive, amongst which calcium silicate-based cement (CSC) represents the most promising and frequently used due to its bioactivity, sealing ability, biocompatibility, and osteoinductivity [19,20,21]. In addition, calcium silicates also add value to endodontic materials, which can elevate pH and antibacterial properties [22]. CSCs can be classified based on their material chemistry and chemical composition. Among CSCs, tricalcium silicate and dicalcium silicate are the primary components; for example, mineral trioxide aggregate (MTA) combines tricalcium silicate and dicalcium silicates with other minerals. MTA is the first commercially available calcium silicate cement used in pulp capping. Its composition is almost identical to Portland cement, a material widely used in the construction industry, but adapted for endodontic therapy with refined materials and additives to improve its properties [23]. MTA is among the most widely used bioceramic in endodontics and is considered the gold standard due to its excellent biocompatibility, sealing ability, antimicrobial properties due to increased pH, and ability to promote healing peripheral tissues [21]. In addition, it has appropriate mechanical and bioactive properties that support its utility and make it one of the most versatile materials for use in endodontic procedures [24,25].
Endosequence (Brasseler, Savannah, GA, USA) and Biodentine (Septodont, Saint-Maur-des-Fossés, France) are two widely used endodontic materials, which also include tricalcium and dicalcium silicate. While these products have varying properties and applications, MTA remains the gold standard material for endodontics [26]. Table 1 highlights the most widely used calcium silicate materials for endodontic applications and their composition, applications, properties, and major drawbacks. Table 1 shows the calcium silicate-based bioceramics used in endodontic procedures.

Table 1.
Calcium silicate-based bioceramics used in endodontic procedures.
2.2. Calcium Phosphates
Calcium phosphate-based dental materials are pivotal in modern restorative and regenerative dentistry due to their biocompatibility and similarity to the natural tooth mineral content [43]. Amongst these materials, hydroxyapatite, tricalcium phosphate, and dicalcium phosphate stand out for their unique properties and applications.
2.2.1. Hydroxyapatite
Hydroxyapatite (HA) is the most abundant mineral in bone and teeth and is known for its excellent integration with natural bone, making it a popular choice for bone grafts, dental cements, and dental implants [44,45]. HA is a form of calcium phosphate that has the chemical formula Ca10(PO4)6(OH)2 and consequently consists of calcium (Ca2+), phosphate (PO43−), and hydroxide (OH−) ions [46]. Similar to other calcium phosphate-based materials, HA is biocompatible and induces osseointegration and osteoconduction [47]. HA has been traditionally used as a coating for dental and orthopaedic implants, and modern endodontic applications include periapical defect repair, pulp capping, formation of apical barriers, and reparation of mechanical bifurcation perforations [47]. HA has also been used as a component of root canal-filling material in animal studies, where subsequent evidence of osteoconduction and stability were demonstrated [48]. Although HA shows greater biocompatibility, incorporating it into endodontic materials presents challenges, as it has been reported to weaken the material’s mechanical properties [49]. Studies have shown that HA reduces compressive strength crack initiation and increases porosity due to the solubility of hydroxyapatite-based materials and challenges in fine-tuning degradation, which could ultimately lead to microleakage. These issues represent some of the challenges in adapting this material for use in clinical applications [44,50,51].
2.2.2. Dicalcium and Tricalcium Phosphates
With its varying solubility, tricalcium phosphate offers a controlled release of calcium and phosphate ions, enhancing bone regeneration and repair. Dicalcium phosphate, known for its rapid resorption rate, is often used in tooth-coloured filling materials and as a component in preventive dental treatment materials, aiding in remineralisation and preventing caries from forming [47]. Together, these calcium phosphate compounds provide a versatile toolkit for enhancing dental health and addressing a range of restorative needs.
Both tricalcium phosphate and dicalcium phosphate are equally valuable materials for use in bone regeneration and other dental applications. Tricalcium phosphate, in particular, acts as an osteoconductive material, promoting remineralisation and growth. This property has been utilised in dentistry for its use as a capping agent, apical barrier, and apexification treatments. It has also been tested in periodontal defect repair procedures. However, poor mechanical properties, low resistance to cracking, and unpredictable solubility may pose challenges when developing dental composite materials. Compared with Tricalcium phosphates, dicalcium phosphate dissolves relatively slowly and may be preferred where structural integrity is required [47].
In 1971, Hench developed a glass ceramic containing calcium and phosphate, known as bioglass, which demonstrated the formation of a chemical bond with host bone tissue through a calcium phosphate-rich layer [52]. Active restorative materials containing amorphous calcium phosphate (ACP) encapsulated in a polymer binder as a filler, were developed to promote tooth structure repair by sustaining significant amounts of calcium and phosphate ions [14]. In endodontics, calcium phosphate-based bioceramics are classified into calcium phosphate-based or mixtures of calcium silicate and phosphate [2]. Details of the most widely used calcium phosphate-based endodontic materials are provided in Table 2.

Table 2.
Details and properties of calcium phosphate-based bioceramic materials used in endodontics.
2.3. Bioactive Glasses
Bioactive glass (BG) is primarily composed of silicon dioxide (SiO2), calcium oxide (CaO), sodium oxide (Na2O), phosphate (P2O5), and borate (B2O3), which are distinguished by their ability to release ions and form a hydroxyapatite layer at tissue interfaces [62,63,64,65]. BGs are often incorporated into materials to overcome the limitations of commercially available bioceramic sealers. Due to their non-crystalline structure, these particles are expected to elicit improved bioactivity compared with crystalline bioceramics, such as MTA and iRoot BP Plus, in procedures such as pulp capping [66]. Experimental studies have demonstrated strong antimicrobial properties due to the ability of BGs to raise the pH and calcium levels in the surrounding local tissue environment, enabling disinfection [67]. Notably, BGs can be classified into silicate-based glass (SiO2), borate-based glass (B2O3), and phosphate-based glass (P2O5) [67]. BGs initially form a silica layer allowing for calcium and phosphate ions to react, forming a calcium phosphate layer and ultimately forming HA over time and stimulating osteoblasts to proliferate and differentiate, enabling the synthesis and deposition of bone matrix [67,68]. The first and most studied BG is Bioglass 45S5, which is known for releasing relatively high quantities of phosphate, calcium, silicon, and sodium ions, promoting hard tissue formation [69]. Bioglass appears in various forms, including particulate, powder, mesh, cones, and pellets. It is often used in clinical applications such as grafting materials, endosseous implants, remineralisation, antibacterial agents, and as a medium for drug delivery. Bioglass is extensively applied in dentistry and orthopaedic fields and is a key component in dental materials, especially in endodontic composites [70,71].
Incorporating bioactive particles Niobophosphate (NbG) or BG 45S5 into endodontic cement shows promise in neutralising acidic environments and promoting hydroxyapatite precursor formation [69]. Clinically, this could create a bactericidal cement that facilitates tissue healing. Improved radiopacity and flowability would also aid in visualising the material on radiographs and filling complex root canal anatomies. However, the drawbacks include excessive weight loss and post-setting cytotoxicity, which may lead to cement degradation and tissue irritation [69]. The details for current bioactive glass-based endodontic bioceramic materials and their properties are summarised in Table 3.

Table 3.
Details of current bioactive glass containing bioceramics used in endodontics procedures.
3. Limitations of Bioceramic Materials
3.1. Tooth Discolouration
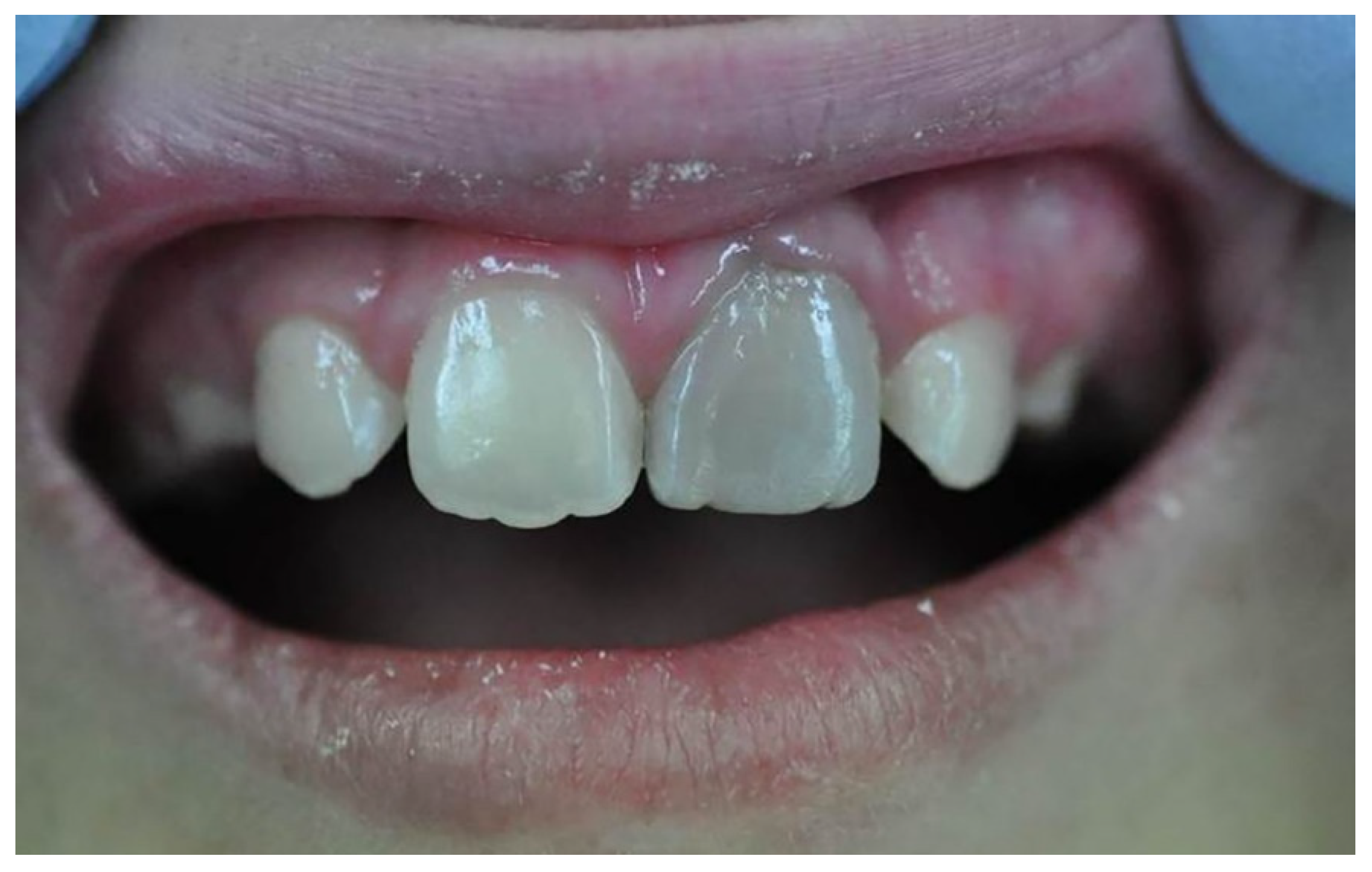
Tooth discolouration (Figure 3) is a common aesthetic complication, especially occurring in anterior teeth due to pathological conditions and the materials used in root canal treatments [84]. Remnants of the pulp, micro-leakage, internal absorption, and treatment failure are some of the key contributing factors to tooth discolouration. Tooth discolouration can also occur following endodontic treatment when components of endodontic materials, such as bismuth, silver, and iron, react with the surrounding environment. With respect to bismuth oxide, a common radiopacifier included in calcium silicate materials such as MTA reacts with irrigation fluids such as sodium hypochlorite, blood, and dentin collagen to form brown or greyish precipitates. Upon exposure, bismuth oxide in the MTA rapidly reacts with NaOCl to form a dark brown-black sodium bismuthate (NaBiO3). Bismuth oxide has also been reported to react with blood, resulting in dark-coloured products. When bismuth oxide reacts with blood components, haemoglobin and hematin via redox reactions, the resulting products can infiltrate dentinal tubules and the crown [85].
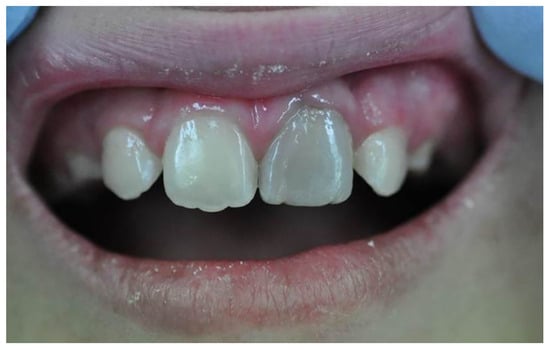
Figure 3.
Clinical image of tooth discolouration attributed to the use of MTA [86].
The tooth discolouration associated with calcium silicate-based materials can start to appear as early as 3 months after placement, and the gradual increase has been documented for up to 2 years [87]. Among the widely used MTA types, Angelus and ProProot MTA were reported to impart severe discolouration that considerably negatively affects the aesthetics of the tooth [87]. NeoMTA, EndoSequence bioceramic putty, and Biodentine did not normally exhibit clinically perceptible colour changes due to the usage of either tantalum oxide or zirconium dioxide and thus might be considered for use in the aesthetic zone [27,34].
However, some studies indicated that alternative radiopacifiers, such as zirconium oxide and calcium tungstate, can also show colour alterations, albeit at lower levels than bismuth oxide containing MTA [88]. Bioroot RCS and TotalFill BC Sealer, MTA Bio-C Pulpo, MTA Vitalcem, Medcem MTA, TMR MTA, EndocemZr, and Rootdent have been formulated with zirconium oxide, while MTA HP and PD MTA White with calcium tungstate are purported to be non-staining [89]. However, tooth discolouration was investigated in a three-year in vitro study, and it was concluded that the discolouration effect was within acceptable limits [90]. This study further indicated that irrigation with saline or distilled water following NaOCl irrigation resulted in lower tooth discolouration levels [91]. The usage of a radiopacifer has been illustrated in Table 1.
3.2. Relatively Long Setting Time of Bioceramic Materials
Long setting times associated with conventional endodontic cements are a significant drawback in endodontic treatments, where rapid sealing and bonding are essential to prevent reinfection and minimise treatment time, thereby reducing patient discomfort and treatment costs. The ideal setting time for many clinical endodontic applications ranges from 3 to 10 min [92]. For instance, apical surgery demands the shortest setting time, possibly due to the risk of wash-out caused by blood flow. Novel calcium silicate products, such as Biodentine, MTA Plus, and light-curable TheraCal, offer relatively shorter setting times, addressing these challenges effectively.
Calcium silicate-based hydraulic cements, primarily composed of calcium trisilicate and calcium disilicate, undergo a hydration reaction with moisture from the surrounding environment and tissues [93]. The setting of calcium trisilicates is the main contributor to setting time in calcium silicate-based materials, whereas a much slower dicalcium silicate reaction provides secondary strengthening [92]. The setting reaction of these materials depends on the composition of the material, the type of chemical reaction involved, and differences in particle size, crystallinity, pH, and temperature [89,93]. Out of all the calcium silicate-based endodontic materials, MTA has a relatively slow setting time of approximately 3–4 h and takes 21 days for the complete curing process under an ambient temperature [94]. The setting time also differs between the different MTA formulations and depends on the composition and powder-to-water ratio.
As highlighted, particle size is one of the major contributors to the longer MTA setting time. Contemporary MTAs contain larger particle sizes, which results in the disadvantage of slow setting time [89]. There have been many studies that have attempted to overcome the long setting time, which is a notable disadvantage. Since MTA root fillings are considered permanent, the fully set material becomes highly dense, making its removal difficult and time-consuming. [94]. Therefore, achieving a balance between hardness, improved setting time, and biocompatibility needs to be carefully considered, and this depends on the particular application.
3.3. Handling Properties of Bioceramic Materials
While bioceramics offer significant benefits in endodontics, different materials exhibit a range of handling challenges before, during, and after application, many of which remain unresolved. For instance, calcium silicate-based materials like mineral trioxide aggregate (MTA) have historically presented difficulties due to the consistency of the set material. However, newer formulations—such as MTA Angelus, MTA Plus, NeoMTA, and Biodentine—have addressed these issues through innovative modifications. The incorporation of additives like polymers, plasticisers, and hydrosoluble gels into these formulations has significantly improved their handling properties [89].
Key issues include placement and workability, prolonged setting times, and, critically, difficulty in removal during retreatment or procedural revisions [94,95].
Removal challenges are particularly pronounced with calcium silicate-based bioceramics like MTA and newer bioceramic sealers.
3.4. Mechanical Properties of Bioceramic Materials
Calcium silicate-based MTA materials currently in use have shown adequate compressive strength values. After 24 h of setting, the compressive strength is generally between 40 Mpa and 60–70 Mpa after 21 days [96]. However, different formulations of MTA exhibit a range of values depending on the composition of these materials [97]. The values of similar formulations depending on the particle size have also shown variations. MTA angelus, a second-generation MTA formulation, has shown lower compressive strength values [98]. Even though MTA angelus has shown lower mechanical properties due to the exclusion of calcium sulphate (gypsum) in its formulation, it has shown an improved setting time of less than 50 min, whereas ProRoot MTA was reported to take 2 h, which is clinically less desirable [99].
Standalone calcium phosphates, including HA, are not as well explored or clinically adopted as calcium silicates for endodontic applications, primarily due to their poor mechanical properties, such as lower compressive strength and longer setting times. Though they are not yet mainstream, there have been reports of calcium phosphate-based endodontic materials developed for clinical use. Although these materials are not yet widely adopted in clinical practice, notable developments have been in this field. One example is the Chitra-CPC sealer, a locally developed bioceramic material created by the Sree Chitra Tirunal Institute for Medical Sciences and Technology (SCTIMST) in India. Initially designed for use as a bone graft and perforation repair material, the Chitra-CPC sealer has demonstrated potential in endodontics by reinforcing root strength and exhibiting excellent biocompatibility [100]. However, modified endodontic cements that include hydroxyapatite, calcium silicates, or Portland cement have shown variable material properties [28,50,101].
Bioactive glass is frequently added to different formulations or composites used in endodontics to provide various functionalities. Glass ionomers often benefit from adding bioactive glasses. Incorporating bioactive glass into glass ionomers has demonstrated that it can induce precipitates on the surface of demineralised dentin—however, the mechanical properties, such as compressive strength, vary along with the different formulations employed [102].
3.5. Shrinkage Properties of Bioceramic Materials
Shrinkage with respect to bioceramics is the decrease in volume when a bioceramic material undergoes a chemical reaction leading to its hardening. The polymerisation shrinkage is particularly significant in the setting reaction of an endodontic cement utilised in root canal procedures that include polymers or resins, making it a critical aspect to consider [103].
Calcium silicates undergo hydration reactions to form calcium–silicate–hydrate (C–S–H) gels and calcium hydroxide. The process is water-dependent, and incomplete hydration or moisture loss can result in volumetric shrinkage. Shrinkage is exacerbated under certain environmental conditions, such as acidic pH, which accelerates material dissolution and disrupts the hydration reaction [104,105]. Notable examples include silicate-based materials such as NeoPUTTY (NeoMTA 2) and MTA HP, which exhibited significant volumetric reductions in acidic environments, as shown via micro-computed tomography (micro-CT) [105,106].
With respect to calcium phosphates, their setting relies on dissolution–precipitation to form hydroxyapatite. Calcium phosphate materials can exhibit negligible shrinkage or slight expansion depending on ion concentrations and hydration conditions [92,107]. However, desiccation may cause porosity or small shrinkage-related defects under poorly controlled hydration, affecting dimensional stability [92].
Bioactive glass, on the other hand, exhibits minimal shrinkage (<1%) due to its robust dimensional stability in hydrated environments [92]. In contrast, resin-modified glass ionomers experience polymerisation during the acid–base setting reaction, causing significant volumetric shrinkage (0.5–6%) [92,108]. This shrinkage is associated with marginal gaps and secondary failures in some endodontic applications [92,108]. Table 4 summarises the volumetric changes or the shrinkage of different types of materials and their impact on clinical settings.

Table 4.
Shrinkage properties of different bioceramic materials.
3.6. Biocompatibility and Cytotoxicity of Bioceramic Materials
Calcium silicate cements, often used in endodontics and restorative applications, are generally well regarded for their biocompatibility and ability to promote tooth structure repair. However, some cytotoxicity can occur due to the formulation, setting conditions, and the release of residual chemicals. The biocompatibility and cytotoxicity of these materials, primarily MTA, Biodentine and BioRoot RCS (Septodent, Saint-Maur-des-Fossés, France) in clinically relevant settings are well established. Systematic reviews, such as those by Oliveira et al. [111] and Maru et al. [112], have consolidated available data, demonstrating consistently favourable biocompatibility profiles, particularly when compared to traditional materials such as resin-based sealers. The data emphasises both in vitro and in vivo investigations, which support calcium silicate-based materials’ ability to promote cell viability and minimise inflammatory responses.
Mineral trioxide aggregate (MTA) exhibits transient cytotoxicity and localised inflammation primarily during its initial setting phase, attributed to its high pH and release of components like calcium hydroxide and bismuth oxide, with occasional concerns about systemic toxicity observed in animal models; however, its long-term biocompatibility remains favourable [113,114,115].
On the other hand, the use of bioactive glass types, such as Bioglass® 45S5 (NovaMin Technology, GlaxoSmithKline, Alachua, FL, USA), could lead to harmful effects on cells as a result of a significant increase in pH from the leakage of high levels of Na+ and Ca2+, which might also delay the formation of hydroxyapatite [116].
3.7. Microleakage
In endodontics, the passage of fluids, bacteria, or other substances in and out of the root canal system is called leakage. Microleakage is a known drawback of some endodontic materials. This process permits the entry of bacteria or fluids into the root canal system, leading to treatment failure and ongoing infection and inflammation. Leakage often occurs at a much smaller scale, which is often referred to as microleakage, and often at the interface between the endodontic material and the tooth structure. [117]. The movement of fluid, bacteria, and other substances, such as toxins, potentially results in long-term and short-term complications such as inflammation and reinfection. Modern endodontic treatments heavily rely on establishing an obturated root canal that is devoid of apical leakage.
Bioceramics that are used in endodontics undergo chemical degradation, particularly in a lower pH environment, are the main contributors to microleakage. Calcium silicate-based sealers undergo hydration-dependent setting reactions, which result in ion leaching over time under acidic pH conditions, and this leads to solubility complications affecting the mechanical integrity of the material [118]. However, the formation of HA on these materials may reduce this early stage degradation by inducing a mineral infiltration zone, promoting sealing [119].
In addition, the bond between endodontic sealers or root canal filling materials and the dentinal walls is crucial for proper sealing. This bond is essential for establishing a barrier against bacteria, thereby preventing reinfection. Studies have shown that resin-based sealers like AH Plus have better bonding strength than silicate-based bioceramic sealers [120].
While more stable in neutral conditions, calcium phosphate-based materials demonstrate increased solubility under acidic conditions and often show porosity or dissolution-related issues during the setting phase [118,121]. However, the mechanisms of degradation for calcium phosphate-based sealers remain comparatively understudied.
Endosequence BC Sealer is an endodontic material containing calcium silicates and calcium phosphates, and it has been shown to have more apical and coronal leakage than AH Plus, especially when used with the single cone technique (ref). Studies suggest that AH Plus forms a stronger bond due to its covalent interaction with dentin, resulting in less leakage.
Calcium phosphate monobasic present in the TotalFill® BC Sealer™ (FKG Dentaire Sàrl, Le Crêt-du-Locle, Switzerland) has been shown to reduce calcium ion release. Indeed, TotalFill® BC Sealer™ has a lower calcium ion release than BioRoot™ RCS. Other than the additives that limit the formation of the calcium hydroxide, the hydration of the premixed sealers depends on the water availability in the surroundings and the ion release through diffusion, which further delays or restricts the availability of calcium [108,122].
3.8. Solubility of Bioceramic Materials
The solubility of endodontic bioceramic materials can affect the materials’ overall clinical properties [123]. The solubility compromises the sealing ability, creating and increasing the space, potentially leading to treatment failure. It is recommended that a 3% maximum solubility be permitted in ISO 6876:2012 [124] root canal sealing materials to address this issue [123]. Notably, tricalcium phosphates have unpredictable fluid solubility [47]. BC-Endosequence (Brasseler USA, Savannah, GA, USA) bioceramic sealers, which have calcium phosphates as a component, have shown considerable (4.1%) solubility after 21 days when submerged in distilled water, and these values are much more favourable when compared with silicate-based endodontic materials such as ProRoot MTA [125].
3.9. Radiopacity of Bioceramic Materials
Radiopacity remains a critical concern in bioceramic-based endodontic materials due to variability in compliance with ISO 6876 and ADA Specification No. 57 standards [126]. A material is considered radiopaque if its ability to block X-rays matches or exceeds that of a 1 mm-thick aluminium (Al) sheet. The endodontic materials should exhibit minimum radiopacity requirements of 3 mm or 2 mm Al thickness, as the ISO and ADA standards specified. Insufficient radiopacity can lead to significant clinical challenges, including distinguishing materials from surrounding anatomic structures during radiographic evaluation, as observed with products like MTA Fillapex and Biodentine [1,2,5]. Several commonly used materials fail to meet these benchmarks under standard testing conditions. For example, MTA Fillapex often exhibits insufficient radiopacity due to its zirconium oxide-based formulation, which provides lower radiopacity than alternatives such as bismuth oxide [127,128,129]. Similarly, Biodentine has also shown mixed results; though compliant in some studies, it has failed to meet standards in others, with radiopacity as low as 2.8 mm Al under traditional test conditions, highlighting its borderline adequacy [130,131]. EndoSequence BC Sealer and Bio-C Sealer demonstrate radiopacity values slightly above the 3 mm Al ISO threshold but remain notably less radiopaque than epoxy-based sealers such as AH Plus, raising concerns regarding the clinical suitability under certain situations [128,132]. MTA Angelus (white/grey) meets or minimally exceeds ISO standards but demonstrates variability based on preparation techniques and testing methods, such as differences in powder–liquid ratios [129,133,134]. Conversely, formulations such as TotalFill BC Sealer HiFlow consistently achieve robust radiopacity beyond ISO benchmarks, attributed to the effective incorporation of bismuth oxide in optimal proportions [135,136,137]. Radiopacity inconsistencies are associated with factors such as radiopacifier type and concentration, variability in material dispersion, and testing protocol differences. For example, testing under real-world conditions, such as tissue-simulator models, has demonstrated that materials such as Biodentine may perform better than in standardised tests, suggesting limitations in the current ISO/ADA analysis methods [131]. However, the continued use of materials with borderline radiopacity values and limitations in current radiopacity standards underscores the need for improved formulations and more clinically relevant testing methods.
3.10. Antibacterial Properties of Bioceramic Materials
Calcium silicate-based endodontic materials’ antibacterial properties are mainly attributed to their increased pH, calcium ion release, and calcium hydroxide formation [138]. However, unlike calcium silicate (CS)-based materials, calcium phosphate cements do not impart antibacterial properties [139]. Providing antibacterial properties in most endodontic treatments would be recommended to eliminate bacteria from the root canal system, prevent reinfection, and offer a favourable healing environment. Antibacterial properties are particularly critical in combating persistent endodontic pathogens such as Enterococcus faecalis, Porphyromonas gingivalis, and Fusobacterium nucleatum, which are implicated in secondary infections and treatment failures [140,141]
Among endodontic materials, calcium silicate-based materials such as MTA are reported to have significant antibacterial properties. However, higher activity has been reported, particularly when they are freshly prepared and mixed. [141,142,143,144]. The change in activity has been attributed to the decrease in the pH of the media over time [145,146]. In addition, the activity has been reported to be dependent on the agar disk diffusion method employed. In contrast, direct methods have shown higher activity due to the easy diffusion of the ions [147].
Endodontic materials based on calcium silicates, such as MTA, Biodentine, NeoSealer Flo, and BioRoot RCS, have shown varying levels of antibacterial activity against different bacteria. Among these materials, Biodentine has consistently shown strong activity against Enterococcus faecalis and Streptococcus mutans, which is attributed to its ability to create a higher pH in the medium [148,149]. However, studies also suggest that the activity diminishes over time against biofilms. On the other hand, MTA has shown moderate activity against Enterococcus faecalis and Streptococcus mutans compared to Biodentine and newer endodontic sealers [150]. The literature suggests that silicate-based materials are effective against planktonic E. faecalis and S. mutans but show limited candida-causing Candida albicans [148]. Based on recent studies, emerging materials with modified nanoparticles, antimicrobial materials such as chitosan, or substitutions with antimicrobial elements show greater efficacy against managing bacterial strains that are resistant to conventional materials.
3.11. Affordability and Cost
Numerous bioceramic materials under different names are used for endodontic applications that incur varying costs. However, a direct comparative study of the cost of the materials or treatment has not been properly conducted in the recent past. The limited data (Table 5) suggest that Biodentine is more affordable and has a faster setting time; easiness of manipulation has become a popular choice among dentists. TheraCal LC, a resin-modified calcium silicate, also has a lower cost. However, a detailed analysis of effectiveness over cost requires in-depth analysis and more data for a proper analysis [151].

Table 5.
Cost and estimated treatment cost for most popular endodontic materials.
4. Future Advances in Bioceramics for Use in Endodontics
Future advances in bioceramics for use in endodontic treatment should involve enhancing the material’s properties, exploring combined applications, and tailoring treatments. Indeed, research needs to concentrate on refining these materials’ physical, chemical, and biological characteristics [152].
In the advancement of bioceramic materials, nanotechnology plays a pivotal role. The incorporation of nanomaterials into endodontic materials can enhance properties such as biocompatibility, bioactivity, mechanical strength, radiopacity, sealing ability, ease of handling, setting time, and, notably, antibacterial effects.
The mechanical properties of endodontic materials have been tested and enhanced through the incorporation of various nanomaterials. Carbon nanotubes; nanoclays such as montmorillonite, nano-calcium hydroxide, zinc oxide, aluminate, and silicon dioxide; and numerous other nanopowders have demonstrated improved mechanical properties when used as reinforcements [153,154,155]. In addition, nanoparticles have been shown to reduce leakage and improve the sealability of endodontic materials [156]. Antibacterial property is an important property of the endodontic materials for the prevention of reinfection of the treated tooth. Many nanoparticles, such as silver, zinc oxide, zirconium oxide, and titanium oxide, as well as many forms of nanomaterials, have been tested and incorporated to increase the antibacterial properties of endodontic materials [157,158]. Furthermore, some nanomaterials, such as montmorillonite have been tested for their ability to deliver drug molecules to specific targets, potentially increasing the efficacy of drugs designed for smart, targeted delivery [154]. Nano-hydroxyapatite, an important material and a primary component of dental structures, has been utilised in various ways to enhance the biocompatibility and osteogenicity of endodontic materials [159].
Employed nanomaterials have been incorporated as additives to improve and impart various properties. Materials such as silver oxide, zinc oxide, and many others can be synthesised separately and incorporated into endodontic materials. Some materials are incorporated using chemical methods [160], while others are incorporated through intercalation and doping [161], such as on the surface of nanoclays [154]. Additionally, these materials can be incorporated into polymer-based materials to enhance and impart various properties.
In addition to nanomaterials, numerous innovations have been explored to enhance the properties of endodontic materials. Several approaches that have been tested, implemented, or that show potential for improvement are outlined below.
4.1. Radiopacifiers
Tooth discolouration associated with endodontic material has been one of the major issues over the years. Calcium silicate-based MTA and related materials often included bismuth oxide as a radiopacifier, resulting in black or brown precipitation upon interaction with the surrounding environment. With the introduction of newer materials, tooth discolouration has been addressed with alternative radiopacifers such as zirconium oxide, zinc oxide, and tantalum oxide. Zirconium oxide-containing materials, such as EndocemZr [ECZ], are now available for clinical use, with low tooth discolouration levels; however, longer-term studies may be required to confirm this [162]. In addition, novel radiopacifiers have been studied that exhibit decreased tooth discolouration effects. Among these materials, MTA-like cement, created by incorporating 30% barium titanate and set with a 10% CaCl2 solution, demonstrated a radiopacity of 3.68 ± 0.24 mm Al, a diametral tensile strength (DTS) of 2.54 ± 0.28 MPa, and initial and final setting times of 55 and 23 min, respectively. Notably, the radiopacity was marginally increased when compared with the setting using deionised water. Additionally, this innovative MTA showed outstanding colour stability and excellent biocompatibility, making it suitable for future commercial use in endodontic treatments [83].
4.2. Handling Properties
In recent years, sol–gel methods for developing endodontic cement have gained attention with precise control over the material’s composition, microstructure, and texture, making it a highly promising avenue for advanced endodontic material in the upcoming years [163].
At the same time, various modifications have been introduced for calcium silicate-based materials, such as Generex A (Dentsply Tulsa Dental Specialties, Tulsa, OK, USA), which is one such formulation developed some time ago and is a calcium-silicate-based material similar to ProRoot MTA but mixed with gels instead of water. Generex A shows a dough-like consistency, making it easier to shape into a rope-like mass, which improves its handling properties. Generex A is also reported to have minimal tissue sensitivity, promote cell proliferation beside MTA, and facilitate nodule formation [164].
4.3. Biocompatibility
When discussing new developments in endodontic materials, HA has recently gained prominence in the development of bioceramic materials for endodontic applications. HA has excellent biocompatibility, bioactivity, osteoconductivity, and physical and chemical properties, making it well placed for use in dental bioceramics [165]. Recent studies have shown that natural HA derived from animal tissue, such as from bovine bone, has shown higher biocompatibility and is a viable, cheaper alternative to synthetic hydroxyapatite, which could easily be incorporated into dental bioceramics with lower associated costs and improved biocompatibility [166,167]. Bovine HA has already been tested in an experimental endodontic bioceramic material combined with ZrO2, and this Portland cement-based material demonstrated lower cytotoxicity and lower solubility, confirming that bovine or animal-derived HA has greater biocompatibility than synthetic hydroxyapatite-based endodontic cement [101]. As a waste product in the meat industry, this will further reduce the burden and environmental impact while providing cheaper and greener alternatives [166,167].
4.4. Mechanical Properties
Bioactive glass in dentistry can be incorporated into hydraulic ceramic and hydrophobic materials, making them clinically suitable novel materials with enhanced properties such as improved mechanical strength, sealing ability, remineralisation, and antibacterial activity [168]. Table 6 summarises the recent developments in endodontic bioceramics and addresses several limitations.
Studies investigating hydroxyapatite-modified endodontic cements have reported mixed outcomes regarding their mechanical properties, highlighting both potential benefits and trade-offs. Incorporating hydroxyapatite into calcium silicate-based cements, such as MTA or Portland cement, generally exhibit improved properties, such as increased compressive strength over extended curing periods [101], as well as a reduced setting time when Zn-doping of hydroxyapatite was used [28]. However, other modifications, such as nano-hydroxyapatite (10–20%), have seen reduced compressive strength [50] and have also resulted in increased porosity and solubility, suggesting that higher hydroxyapatite concentrations can negatively impact the mechanical properties. While hydroxyapatite-modified formulations demonstrated enhanced bioactivity and biocompatibility (e.g., improved cell viability and antibacterial activity) [101], these mechanical property compromises raise concerns regarding their long-term structural reliability. Importantly, studies in this area have focused mainly on compressive strength, with mechanical properties such as flexural strength and fracture toughness, while cyclic loading resistance remains underexplored [28,101]. While hydroxyapatite modification offers promising improvements in biological properties, further optimisation is required to address the current mechanical limitations and ensure their clinical applicability in endodontic treatments.
Calcium phosphate-based endodontic materials, such as tricalcium phosphate (TCP) or dicalcium phosphate (DCP), primarily aim to improve biocompatibility and ion release to support tissue healing and bioactivity. However, their mechanical properties, such as compressive strength, flexural strength, and fracture toughness, are consistently inferior to calcium silicate-based materials such as MTA or Biodentine. While doping with ions (e.g., strontium, copper, zinc) or combining with additives (e.g., PLA microspheres) can improve mechanical performance to some extent [1,3], these materials remain unsuitable for high-load or structurally demanding endodontic applications. Commercial examples like TheraCal LC and Calciblast highlight limited utility for specific cases such as in pulp capping or low-load sealing. Calcium silicate-based products with integrated calcium phosphate additives (e.g., Biodentine) often outperform purely calcium phosphate-based materials in mechanical and clinical metrics.
Bioactive glass is naturally prone to brittleness due to its glassy structure, which lacks the fracture toughness of certain ceramic materials. As a result, it is more vulnerable to fracturing when subjected to stress [169]. Future development should focus on hybrid materials or innovative composites that bridge the mechanical durability and bioactivity gap.
The broader implications of advancing endodontic materials with innovations like nanomaterials and by other means extend far beyond clinical efficacy, influencing cost, time efficiency, and accessibility in meaningful ways. While the initial development and integration of these materials may involve higher costs due to research and production, they promise long-term savings by reducing retreatment rates, minimising complications such as reinfections, and lowering the overall burden for patients and providers. From a clinical perspective, endodontic material advancement significantly enhances physicians’ procedural efficiency by providing optimal handling characteristics, rapid setting times, improved consistency, excellent radiopacity, and superior mechanical strength. These properties allow for precise application and adaptation within the complex root canal system, reducing chair time and increasing patient throughput in a busy practice. Moreover, their enhanced sealing capabilities and antimicrobial effects, which are often driven by nanomaterials, effectively eliminate residual bacteria and prevent microleakage, which are critical factors in minimising postoperative complications such as persistent infections or periapical pathology. This clinical reliability reduces the need for follow-up appointments, ensuring better treatment outcomes, streamlining patient management, and bolstering the overall success of endodontic therapy.
Patients benefit from accelerated healing, driven by bioactive nanomaterials that promote tissue regeneration, allowing for quicker recovery and fewer disruptions to daily life. Although adoption may initially be limited to specialists due to cost and training, as manufacturing scales and prices drop, these innovations could elevate the standard of care globally, particularly in underserved areas, balancing upfront investment with substantial long-term gains in efficiency, durability, and patient outcomes.

Table 6.
Summary of recent developments of endodontic bioceramic materials with the potential to advance endodontic treatment outcomes.
Table 6.
Summary of recent developments of endodontic bioceramic materials with the potential to advance endodontic treatment outcomes.
| Study | Limitations Addressed | Key Innovations/Modifications | Methods for Characterisation | Outcome | Reference |
|---|---|---|---|---|---|
| MTA, calcium silicates | Tooth discolouration, long setting time | Introduction of barium titanate (BTO) and calcium chloride | Radiopacity using X-ray device and setting time with Vicat needle | Increased radiopacity and shorter setting time | [83] |
| MTA, calcium silicates | Tooth discolouration | Addition of 5–45% zinc oxide | VITA Easyshade V digital spectrophotometer | Reduction in tooth discolouration without affecting other properties | [170] |
| MTA, calcium silicates, amoxicillin-loaded microspheres | Discolouration, long setting times, handling, antimicrobial properties | Amoxicillin loading, alternative radiopacifiers, improvements in handling tools | Handling evaluations, discolouration studies (alternative radiopacifiers), antimicrobial testing | Reduced discolouration; moderate antimicrobial effects; enhanced handling | [94] |
| MTA, calcium silicates, nanomaterials, hybrid calcium silicate–bioglass | Setting time, antimicrobial properties, brittleness | Nanoparticles (ZnO, TiO2, Ag), hybrid bioactive materials | Antibacterial testing, mechanical testing (brittleness), ion release, HA formation | Improved bioactivity and antimicrobial effects; moderate handling improvements | [20] |
| Generex A | Handling properties, improved osteogenic potential | MTA, mixed with gel material to create dough-like consistency that is easier to handle | NA | Improved handling properties | [2,164,171] |
| Sol–gel-derived MTA, bioceramics with ethanol post-treatment | Setting time, handling, bioactivity enhancement | Sol–gel synthesis, ethanol post-treatment for smaller particle size | Hydroxyapatite formation in SBF, SEM for surface analysis | Enhanced bioactivity (HA), reduced setting time, improved cohesion | [163] |
| Nanoparticle-modified MTA and bioglass | Long setting time, brittleness, antimicrobial properties | Zinc oxide (ZnO) particles, silver nanoparticles (AgNPs) | Antibacterial activity (disc diffusion), compression testing | Improved antimicrobial properties; brittleness reduced moderately; mechanical strength slightly improved | [172,173] |
| MTA, TotalFill, Biodentine | Discolouration, handling, setting times | Pre-mixed formulations, zirconium oxide as radiopacifier | Handling studies, discolouration observation | Reduced discolouration (zirconium oxide); faster setting times; enhanced usability | [174] |
| Sol–gel calcium silicate cements | Handling, setting times, bioactivity | Sol–gel method with post-synthesis ethanol treatment | Particle size analysis (XRD), bioactivity (HA formation), handling time comparisons | Finer particle size; faster setting times; enhanced bioactivity | [175] |
| MTA, Biodentine, Endobinder, Generex A | Setting time, biocompatibility | Modern alternatives to MTA; improved calcium silicate-based materials | Setting time evaluations, biocompatibility tests | Faster setting time; biocompatible formulations | [2,13] |
| Biodentine, TheraCal LC | Setting times, antibacterial properties, pulp capping | Light-curable formulations, pre-mixed formulations for easier handling | Antibacterial testing, cytotoxicity testing, setting-time measurement | Significantly faster setting time; reduced cytotoxicity | [175] |
| Calcium silicates, NeoMTA, Bio MTA+ | Setting time, discolouration | Nano-hydroxyapatite reinforcement, alternate radiopacifiers | Cytocompatibility tests, SEM imaging, HA formation | Improved bioactivity and cytocompatibility | [176] |
| Bioceramic sealers with silver nanoparticles | Antimicrobial properties, sealing capacity | AgNPs for bacterial inhibition | Push-out bond strength tests, antimicrobial assays | Enhanced antimicrobial properties; limited improvement in bond strength | [177] |
5. Conclusions
Bioceramics play a significant role in endodontic treatments, providing a biocompatible and bioactive solution that addresses many of the challenges of endodontic procedures. However, despite their beneficial biocompatibility and mechanical properties, none of the current products are ideal, and each has limitations. The literature has identified several key drawbacks, namely tooth discolouration, slower setting time, handling difficulties, and compatibility issues. Further work is therefore required in this field to develop improved bioceramic materials and address their current clinical limitations. As a narrative review, one of the main limitations identified is the overgeneralisation of the literature. Additionally, the existing studies have primarily focused on laboratory or in vitro findings, which may not fully translate to real-world clinical scenarios. In conclusion, while endodontic bioceramics have made significant progress in addressing specific drawbacks, the literature suggests that there is still room for improvement to increase their clinical utility.
Author Contributions
Conceptualization, P.A.A.S.P.K., J.R., P.R.C., P.C. and G.D.; methodology, P.A.A.S.P.K., J.R., P.R.C., P.C. and G.D.; software, P.A.A.S.P.K.; validation, P.A.A.S.P.K., J.R., P.R.C., P.C., G.D. and M.G.; formal analysis, P.A.A.S.P.K., J.R., P.R.C., P.C. and G.D.; writing—original draft preparation, P.A.A.S.P.K. and M.G.; writing—review and editing, P.A.A.S.P.K., J.R., P.R.C., P.C. and M.G.; visualization, P.A.A.S.P.K.; supervision, J.R., P.R.C., P.C. and G.D.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Panda, S.; Biswas, C.K.; Paul, S. A comprehensive review on the preparation and application of calcium hydroxyapatite: A special focus on atomic doping methods for bone tissue engineering. Ceram. Int. 2021, 47, 28122–28144. [Google Scholar] [CrossRef]
- Raghavendra, S.S.; Jadhav, G.R.; Gathani, K.M.; Kotadia, P. Bioceramics in endodontics—A review. J. Istanb. Univ. Fac. Dent. 2017, 51, S128–S137. [Google Scholar] [CrossRef]
- Yilmaz, B.; Alshemary, A.Z.; Evis, Z. Co-doped hydroxyapatites as potential materials for biomedical applications. Microchem. J. 2019, 144, 443–453. [Google Scholar] [CrossRef]
- Asgary, S.; Motazedian, H.R.; Parirokh, M.; Eghbal, M.J.; Kheirieh, S. Twenty years of research on mineral trioxide aggregate: A scientometric report. Iran. Endod. J. 2013, 8, 1–5. [Google Scholar]
- Report, M.R. Bioceramics Market by Type (Bio-Inert, Bio-Active, Bio-Resorbable), Material Type (Aluminium Oxide, Zirconia, Calcium Phosphate, Calcium Sulfate), Form (Powder, Liquid), Application (Orthopedics, Dental, Biomedical), and Region—Forcast to 2028; Markets and Markets: Pune, India, 2024. [Google Scholar]
- Network, T. Bioceramic Root Canal Sealer Market Analysis; Veryfied Market Research: Pune, India, 2024. [Google Scholar]
- Insights, F.B. Endodontics Market Size, Share & Industry Analysis, By Product Type and Consumables By End-user (Solo Practices, DSO/Group Practices, and Others), and Regional Forecast, 2025–2032. Available online: https://www.fortunebusinessinsights.com/endodontics-market-110481 (accessed on 3 March 2025).
- Safavi, K. Root end filling. Oral Maxillofac. Surg. Clin. N. Am. 2002, 14, 173–177. [Google Scholar] [CrossRef]
- McDonald, R.E.; Avery, D.R.; Dean, J.A. CHAPTER 19—Treatment of Deep Caries, Vital Pulp Exposure, and Pulpless Teeth. In McDonald and Avery Dentistry for the Child and Adolescent, 9th ed.; Dean, J.A., Avery, D.R., McDonald, R.E., Eds.; Mosby: St. Louis, MO, USA, 2011; pp. 343–365. [Google Scholar]
- Vishwanath, V.; Rao, H.M. Gutta-percha in endodontics—A comprehensive review of material science. J. Conserv. Dent. 2019, 22, 216–222. [Google Scholar] [CrossRef]
- Jain, P.; Ranjan, M. The rise of biocramics in endodontics: A review. Int. J. Pharm. Bio Sci. 2015, 6, 416–422. [Google Scholar]
- Prati, C.; Gandolfi, M.G. Calcium silicate bioactive cements: Biological perspectives and clinical applications. Dent. Mater. 2015, 31, 351–370. [Google Scholar] [CrossRef]
- Darade, L.; Ranjan, S.; Singh, G.B.; Gangadhar, B.V.M.; Vandekar, M.; Rathi, A.G. Bioceramic a futuristic boon in endodontics: A review. Int. J. Health Sci. 2022, 6, 9934–9942. [Google Scholar] [CrossRef]
- Zhekov, K.I.; Stefanova, V.P. Definition and Classification of Bioceramic Endodontic Sealers. Folia Medica 2021, 63, 901–904. [Google Scholar] [PubMed]
- Song, X.; Segura-Egea, J.J.; Díaz-Cuenca, A. Sol–Gel Technologies to Obtain Advanced Bioceramics for Dental Therapeutics. Molecules 2023, 28, 6967. [Google Scholar] [CrossRef] [PubMed]
- Bahadar, H.; Maqbool, F.; Niaz, K.; Abdollahi, M. Toxicity of Nanoparticles and an Overview of Current Experimental Models. Iran. Biomed. J. 2016, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Al-Haddad, A.; Che Ab Aziz, Z.A. Bioceramic-Based Root Canal Sealers: A Review. Int. J. Biomater. 2016, 2016, 9753210. [Google Scholar] [CrossRef]
- Asawaworarit, W.; Yachor, P.; Kijsamanmith, K.; Vongsavan, N. Comparison of the Apical Sealing Ability of Calcium Silicate-Based Sealer and Resin-Based Sealer Using the Fluid-Filtration Technique. Med. Princ. Pract. 2016, 25, 561–565. [Google Scholar] [CrossRef]
- Youness, R.A.; Tag El-deen, D.M.; Taha, M.A. A Review on Calcium Silicate Ceramics: Properties, Limitations, and Solutions for Their Use in Biomedical Applications. Silicon 2023, 15, 2493–2505. [Google Scholar] [CrossRef]
- Dong, X.; Xu, X. Bioceramics in Endodontics: Updates and Future Perspectives. Bioengineering 2023, 10, 354. [Google Scholar] [CrossRef] [PubMed]
- Kot, K.; Kucharski, Ł.; Marek, E.; Safranow, K.; Lipski, M. Alkalizing Properties of Six Calcium-Silicate Endodontic Biomaterials. Materials 2022, 15, 6482. [Google Scholar] [CrossRef]
- Torabinejad, M.; Hong, C.U.; McDonald, F.; Pitt Ford, T.R. Physical and chemical properties of a new root-end filling material. J. Endod. 1995, 21, 349–353. [Google Scholar] [CrossRef]
- Islam, I.; Kheng Chng, H.; Jin Yap, A.U. Comparison of the Physical and Mechanical Properties of MTA and Portland Cement. J. Endod. 2006, 32, 193–197. [Google Scholar] [CrossRef]
- Tsai, C.-L.; Ke, M.-C.; Chen, Y.-H.; Kuo, H.-K.; Yu, H.-J.; Chen, C.-T.; Tseng, Y.-C.; Chuang, P.-C.; Wu, P.-C. Mineral trioxide aggregate affects cell viability and induces apoptosis of stem cells from human exfoliated deciduous teeth. BMC Pharmacol. Toxicol. 2018, 19, 21. [Google Scholar] [CrossRef]
- Palczewska-Komsa, M.; Kinga, K.-W.; Nowicka, A. New Bioactive Calcium Silicate Cement Mineral Trioxide Aggregate Repair High Plasticity (MTA HP)—A Systematic Review. Materials 2021, 14, 4573. [Google Scholar] [CrossRef] [PubMed]
- Tanomaru-Filho, M.; Viapiana, R.; Guerreiro-Tanomaru, J. From MTA to New Biomaterials Based on Calcium Silicate. Odovtos Int. J. Dent. Sci. 2016, 18, 18–22. [Google Scholar] [CrossRef]
- Avram, A.; Gorea, M.; Balint, R.; Dumitrascu-Timis, L.; Jitaru, S.; Mocanu, A.; Tomoaia-Cotisel, M. Portland cement enriched with hydroxyapatite for endodontic applications. Stud. Univ. Babeș-Bolyai Chem. 2017, 62, 81–92. [Google Scholar] [CrossRef]
- Slaboseviciute, M.; Vasiliauskaite, N.; Drukteinis, S.; Martens, L.; Rajasekharan, S. Discoloration Potential of Biodentine: A Systematic Review. Materials 2021, 14, 6861. [Google Scholar] [CrossRef]
- Dioguardi, M.; Quarta, C.; Sovereto, D.; Troiano, G.; Zhurakivska, K.; Bizzoca, M.E.; Muzio, L.L.; Russo, L.L. Calcium Silicate Cements vs. Epoxy Resin Based Cements: Narrative Review. Oral 2021, 1, 23–35. [Google Scholar] [CrossRef]
- Zamparini, F.; Prati, C.; Taddei, P.; Spinelli, A.; Foggia, M.D.; Gandolfi, M. Chemical-Physical Properties and Bioactivity of New Premixed Calcium Silicate-Bioceramic Root Canal Sealers. Int. J. Mol. Sci. 2022, 23, 13914. [Google Scholar] [CrossRef] [PubMed]
- Reszka, P.; Nowicka, A.; Lipski, M.; Dura, W.; Droździk, A.; Woźniak, K. A Comparative Chemical Study of Calcium Silicate-Containing and Epoxy Resin-Based Root Canal Sealers. BioMed Res. Int. 2016, 2016, 9808432. [Google Scholar] [CrossRef]
- Jitaru, S.; Hodisan, I.; Lucia, T.; Anamaria, L.; Bud, M. The use of bioceramics in endodontics—Literature review. Clujul Med. 2016, 89, 470–473. [Google Scholar] [CrossRef]
- Duarte, M.; Marciano, M.; Vivan, R.; Filho, M.T.; Tanomaru, J.M.G.; Camilleri, J. Tricalcium silicate-based cements: Properties and modifications. Braz. Oral Res. 2018, 32 (Suppl. S1), e70. [Google Scholar] [CrossRef]
- Gandolfi, M.; Siboni, F.; Botero, T.; Bossù, M.; Riccitiello, F.; Prati, C. Calcium Silicate and Calcium Hydroxide Materials for Pulp Capping: Biointeractivity, Porosity, Solubility and Bioactivity of Current Formulations. J. Appl. Biomater. Funct. Mater. 2015, 13, 43–60. [Google Scholar] [CrossRef]
- De Siqueira, P.C.; de Alencar, A.H.G.; de Almeida Decurcio, D.; Silva, J.A.; Estrela, C. Characterization of chemical elements of calcium silicate-based cements. RSBO 2022, 19, 304‐12. [Google Scholar] [CrossRef]
- Guven, Y.; Tuna, E.B.; Dincol, M.E.; Aktoren, O. X-ray diffraction analysis of MTA-Plus, MTA-Angelus and DiaRoot BioAggregate. Eur. J. Dent. 2014, 8, 211–215. [Google Scholar] [CrossRef]
- Birant, S.; Gokalp, M.; Duran, Y.; Koruyucu, M.; Akkoc, T.; Seymen, F. Cytotoxicity of NeoMTA Plus, ProRoot MTA and Biodentine on human dental pulp stem cells. J. Dent. Sci. 2021, 16, 971–979. [Google Scholar] [CrossRef]
- Mora, A.; García-Bernal, D.; Rodríguez-Lozano, F.J.; Sanz, J.L.; Forner, L.; Ghilotti, J.; Lozano, A.; López-García, S. Biocompatibility, bioactivity and immunomodulatory properties of three calcium silicate-based sealers: An in vitro study on hPDLSCs. Clin. Oral Investig. 2024, 28, 416. [Google Scholar] [CrossRef]
- Borges, Á.H.; Orçati Dorileo, M.C.; Dalla Villa, R.; Borba, A.M.; Semenoff, T.A.; Guedes, O.A.; Estrela, C.R.; Bandeca, M.C. Physicochemical properties and surfaces morphologies evaluation of MTA FillApex and AH plus. Sci. World J. 2014, 2014, 589732. [Google Scholar] [CrossRef]
- Talabani, R.; Garib, B.T.; Reza, M. Bioactivity and Physicochemical Properties of Three Calcium Silicate-Based Cements: An In Vitro Study. BioMed Res. Int. 2020, 2020, 9576930. [Google Scholar] [CrossRef]
- Camilleri, J.; Sorrentino, F.; Damidot, D. Investigation of the hydration and bioactivity of radiopacified tricalcium silicate cement, Biodentine and MTA Angelus. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2013, 29, 580–593. [Google Scholar] [CrossRef]
- Alazrag, M.A.; Abu-Seida, A.M.; El-Batouty, K.M.; El Ashry, S.H. Marginal adaptation, solubility and biocompatibility of TheraCal LC compared with MTA-angelus and biodentine as a furcation perforation repair material. BMC Oral Health 2020, 20, 298. [Google Scholar] [CrossRef]
- Balhuc, S.; Campian, R.; Labunet, A.; Negucioiu, M.; Buduru, S.; Kui, A. Dental Applications of Systems Based on Hydroxyapatite Nanoparticles—An Evidence-Based Update. Crystals 2021, 11, 674. [Google Scholar] [CrossRef]
- Habibah, T.U.; Amlani, D.V.; Brizuela, M. Hydroxyapatite Dental Material; StatPearls Publishing: Treasure Island, FL, USA, 2018. [Google Scholar]
- Paraš, S.; Trišić, D.; Mitrović Ajtić, O.; Antonijević, Đ.; Čolović, B.; Drobne, D.; Jokanović, V. Biocompatibility study of a new dental cement based on hydroxyapatite and calcium silicates: Focus on liver, kidney, and spleen tissue effects. Int. J. Mol. Sci. 2021, 22, 5468. [Google Scholar] [CrossRef]
- Sawada, M.; Sridhar, K.; Kanda, Y.; Yamanaka, S. Pure hydroxyapatite synthesis originating from amorphous calcium carbonate. Sci. Rep. 2021, 11, 11546. [Google Scholar] [CrossRef]
- Al-Sanabani, J.S.; Madfa, A.A.; Al-Sanabani, F.A. Application of calcium phosphate materials in dentistry. Int. J. Biomater. 2013, 2013, 876132. [Google Scholar] [CrossRef]
- Mitić, A.; Živković, M.; Živković, D.; Popović, L.; Veličković, Z.; Miladinović, M.; Šubarić, L.; Marjanović, D.; Cvetković, A. Use of calcium hydroxyapatite and growth factors in endodontic therapy. Vojnosanit. Pregl. 2021, 78, 310–316. [Google Scholar] [CrossRef]
- Klimek, L.; Kopacz, K.; Śmielak, B.; Kula, Z. An Evaluation of the Mechanical Properties of a Hybrid Composite Containing Hydroxyapatite. Materials 2023, 16, 4548. [Google Scholar] [CrossRef]
- Guerreiro-Tanomaru, J.M.; Vazquez-Garcia, F.A.; Bosso-Martelo, R.; Bernardi, M.I.B.; Faria, G.; Tanomaru Filho, M. Effect of addition of nano-hydroxyapatite on physico-chemical and antibiofilm properties of calcium silicate cements. J. Appl. Oral Sci. 2016, 24, 204–210. [Google Scholar] [CrossRef]
- Saghiri, M.A.; Shabani, A.; Asatourian, A.; Sheibani, N. Storage Medium Affects the Surface Porosity of Dental Cements. J. Clin. Diagn. Res. 2017, 11, Zc116–Zc119. [Google Scholar] [CrossRef]
- Zhao, X. 6—Bioactive materials in orthopaedics. In Bioactive Materials in Medicine; Zhao, X., Courtney, J.M., Qian, H., Eds.; Woodhead Publishing: Cambridge, UK, 2011; pp. 124–154. [Google Scholar]
- Shon, W.; Bae, K.; Baek, S.; Kum, K.; Ah-Reum, H.; Lee, W. Effects of calcium phosphate endodontic sealers on the behavior of human periodontal ligament fibroblasts and MG63 osteoblast-like cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 2141–2147. [Google Scholar] [CrossRef]
- Jung Hee, K.; Baek, S.-H.; Bae, K.-S. Cytotoxicity and Genotoxicity of Newly Developed Calcium Phosphate-based Root Canal Sealers. Restor. Dent. Endod. 2006, 31, 36–49. [Google Scholar] [CrossRef]
- Chang, S.-W.; Lee, S.-Y.; Kang, S.-K.; Kum, K.-Y.; Kim, E.-C. In Vitro Biocompatibility, Inflammatory Response, and Osteogenic Potential of 4 Root Canal Sealers: Sealapex, Sankin Apatite Root Sealer, MTA Fillapex, and iRoot SP Root Canal Sealer. J. Endod. 2014, 40, 1642–1648. [Google Scholar] [CrossRef] [PubMed]
- Carlos Roberto Emerenciano, B.; Diego, V.; Marques, V.A.; Gomes-Filho, J.E.; Cintra, L.; Jacinto, R.; Dezan-Júnior, E. Biocompatibility and biomineralization assessment of bioceramic-, epoxy-, and calcium hydroxide-based sealers. Braz. Oral Res. 2016, 30, e81. [Google Scholar] [CrossRef]
- Komath, M.; Harikrishna, V. Fully injectable calcium phosphate cement--a promise to dentistry. Indian J. Dent. Res. Off. Publ. Indian Soc. Dent. Res. 2004, 15, 89–95. [Google Scholar]
- Mestieri, L.B.; Collares, F.; Zaccara, I.M.; Moreira, M.S.; Kopper, P.M.P.; Leitune, V.; Grecca, F. Biological Properties of Experimental Methacrylate-Based Sealers Containing Calcium Phosphates. Braz. Dent. J. 2021, 32, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Portella, F.; Collares, F.; Santos, L.A.D.d.; Santos, B.P.d.; Camassola, M.; Leitune, V.; Samuel, S. Glycerol salicylate-based containing α-tricalcium phosphate as a bioactive root canal sealer. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 1663–1669. [Google Scholar] [CrossRef] [PubMed]
- Yusuke, S.; Makoto, H.; Takuya, Y.; Hiroshi, K.; Makino, K.; Hirano, Y.; Takagi, S.; Chow, L.; Ogiso, B. Development of a novel fluorapatite-forming calcium phosphate cement with calcium silicate: In vitro and in vivo characteristics. Dent. Mater. J. 2015, 34, 263–269. [Google Scholar] [CrossRef]
- Ingrid, Z.; Gilbert Alfonso, M.; Jorge Iván, C.; Lina Marcela Ruiz, R.; Carlos-Humberto, V.-L.; José Herminsul Mina, H.; Mayra Eliana Valencia, Z.; Grande-Tovar, C. Chitosan (CS)/Hydroxyapatite (HA)/Tricalcium Phosphate (β-TCP)-Based Composites as a Potential Material for Pulp Tissue Regeneration. Polymers 2023, 15, 3213. [Google Scholar] [CrossRef]
- Washio, A.; Morotomi, T.; Shinji, Y.; Kitamura, C. Bioactive Glass-Based Endodontic Sealer as a Promising Root Canal Filling Material Without Semisolid Core Materials. Materials 2019, 12, 3967. [Google Scholar] [CrossRef]
- Gang, H.; Siyi, L.; Dong, Q.; Yanmei, D. Effect of a bioactive glass-based root canal sealer on root fracture resistance ability. J. Dent. Sci. 2022, 18, 27–33. [Google Scholar] [CrossRef]
- Alves, L.C.F.; Gomes, J.F.; Dantas, N.F.; Queiroz, M.N.; Portes, P.N.; Sato, F.; Fernandes, N.d.S.; Miyuki, K.; Nakamura, C.V.; Steimacher, A.; et al. Study of the influence of calcium fluoride on the bioactivity of boron-based glass. J. Non-Cryst. Solids 2024, 624, 122708. [Google Scholar] [CrossRef]
- Kaou, M.H.; Furkó, M.; Balázsi, K.; Balázsi, C. Advanced Bioactive Glasses: The Newest Achievements and Breakthroughs in the Area. Nanomaterials 2023, 13, 2287. [Google Scholar] [CrossRef]
- Long, Y.; Liu, S.; Zhu, L.; Liang, Q.; Chen, X.; Dong, Y. Evaluation of pulp response to novel bioactive glass pulp capping materials. J. Endod. 2017, 43, 1647–1650. [Google Scholar]
- Jafari, N.; Habashi, M.S.; Hashemi, A.; Shirazi, R.; Tanideh, N.; Tamadon, A. Application of bioactive glasses in various dental fields. Biomater. Res. 2022, 26, 31. [Google Scholar] [CrossRef] [PubMed]
- Murata, K.; Washio, A.; Morotomi, T.; Rojasawasthien, T.; Kokabu, S.; Kitamura, C. Physicochemical Properties, Cytocompatibility, and Biocompatibility of a Bioactive Glass Based Retrograde Filling Material. Nanomaterials 2021, 11, 1828. [Google Scholar] [CrossRef]
- Cardoso, O.S.; Meier, M.M.; Carvalho, E.M.; Ferreira, P.V.C.; Gavini, G.; Zago, P.M.W.; Grazziotin-Soares, R.; Menezes, A.S.d.; Carvalho, C.N.; Bauer, J. Synthesis and characterization of experimental endodontic sealers containing bioactive glasses particles of NbG or 45S5. J. Mech. Behav. Biomed. Mater. 2022, 125, 104971. [Google Scholar] [CrossRef]
- Krishnan, V.; Lakshmi, T. Bioglass: A novel biocompatible innovation. J. Adv. Pharm. Technol. Res. 2013, 4, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Raszewski, Z.; Chojnacka, K.; Mikulewicz, M. Investigating Bioactive-Glass-Infused Gels for Enamel Remineralization: An In Vitro Study. J. Funct. Biomater. 2024, 1, 119. [Google Scholar] [CrossRef]
- Hoikkala, N.; Xiaoju, W.; Hupa, L.; Smått, J.; Peltonen, J.; Vallittu, P. Dissolution and mineralization characterization of bioactive glass ceramic containing endodontic sealer Guttaflow Bioseal. Dent. Mater. J. 2018, 37, 988–994. [Google Scholar] [CrossRef]
- Paola, T.; Foggia, M.D.; Zamparini, F.; Carlo, P.; Gandolfi, M. Guttapercha Improves In Vitro Bioactivity and Dentin Remineralization Ability of a Bioglass Containing Polydimethylsiloxane-Based Root Canal Sealer. Molecules 2023, 28, 7088. [Google Scholar] [CrossRef]
- Taddei, P.; Foggia, M.D.; Zamparini, F.; Prati, C.; Gandolfi, M. The Influence of the Matrix on the Apatite-Forming Ability of Calcium Containing Polydimethylsiloxane-Based Cements for Endodontics. Molecules 2022, 2, 5750. [Google Scholar] [CrossRef]
- Al-Sabawi, N.; Sawsan, A.-J. Interfacial adaptation of newly prepared nano-tricalcium silicate-58s bioactive glass-based endodontic sealer. J. Dent. Res. Dent. Clin. Dent. Prospect. 2024, 18, 115–122. [Google Scholar] [CrossRef]
- Seung Bin, J.; Hyun Kyung, K.; Hae, L.; Yu-Jin, K.; Kapil Dev, P.; Jonathan Campbell, K.; Jung-Hwan, L.; Song, M. Physical Properties and Biofunctionalities of Bioactive Root Canal Sealers In Vitro. Nanomaterials 2020, 10, 1750. [Google Scholar] [CrossRef]
- Nagpal, R.; Taneja, S.; Bhalla, V.K. The effect of bioactive glass-based, bioceramic based and epoxy amine resin based root canal sealers on post-obturation pain: A double blinded randomized controlled trial. J. Conserv. Dent. Endod. 2024, 27, 591–597. [Google Scholar] [CrossRef]
- Song, W.; Shue, L.; Tang, Q.; Lili, C.; Zhenglin, Y. In vitro biocompatibility and bioactivity of calcium silicate-based bioceramics in endodontics (Review). Int. J. Mol. Med. 2021, 48, 128. [Google Scholar] [CrossRef] [PubMed]
- Cristina, R.-L.; Tanomaru-Filho, M.; Guerreiro-Tanomaru, J.; Marianella, B.-G.; Erick, H.-M.; Jessie, F.R.-C. Push-Out Bond Strength, Characterization, and Ion Release of Premixed and Powder-Liquid Bioceramic Sealers with or without Gutta-Percha. Scanning 2021, 2021, 6617930. [Google Scholar] [CrossRef]
- Song, W.; Wei, S.; Lili, C.; Zhenglin, Y. In vivo Biocompatibility and Bioactivity of Calcium Silicate-Based Bioceramics in Endodontics. Front. Bioeng. Biotechnol. 2020, 8, 580954. [Google Scholar] [CrossRef] [PubMed]
- Simila, H.; Karpukhina, N.; Hill, R. Physicomechanical properties of strontium and fluoride modified biodentine TM. East Afr. Med. J. 2017, 94, 923–934. [Google Scholar]
- Sekhar, V.; Shobana, S.; Kavitha, M. Comparative Evaluation of Fluoride Release and Compressive Strength of Biodentine Modified Using Sodium Fluorosilicate and Hydrofluoric Acid: An In-Vitro Study. Cureus 2023, 15, e45852. [Google Scholar] [CrossRef]
- Lin, H.-N.; Chen, W.-W.; Hsu, C.-C.; Chen, M.-S.; Chang, P.-J.; Chang, W.-M.; Zhang, F.-H.; Chen, C.-Y.; Lee, P.-Y.; Lin, C.-K. Endodontic Radiopacifying Application of Barium Titanate Prepared through a Combination of Mechanical Milling and Heat Treatment. Materials 2023, 16, 7270. [Google Scholar] [CrossRef]
- Kahler, B. Present status and future directions—Managing discoloured teeth. Int. Endod. J. 2022, 55 (Suppl. S4), 922–950. [Google Scholar] [CrossRef]
- Guimarães, B.M.; Tartari, T.; Marciano, M.A.; Vivan, R.R.; Mondeli, R.F.; Camilleri, J.; Duarte, M.A. Color stability, radiopacity, and chemical characteristics of white mineral trioxide aggregate associated with 2 different vehicles in contact with blood. J. Endod. 2015, 41, 947–952. [Google Scholar] [CrossRef]
- Camilleri, J.; Borg, J.; Damidot, D.; Salvadori, E.; Pilecki, P.; Zaslansky, P.; Darvell, B.W.J.P.O. Colour and chemical stability of bismuth oxide in dental materials with solutions used in routine clinical practice. PLoS ONE 2020, 15, e0240634. [Google Scholar] [CrossRef]
- Nagas, E.; Ertan, A.; Eymirli, A.; Uyanik, O.; Cehreli, Z.C. Tooth Discoloration Induced by Different Calcium Silicate-Based Cements: A Two-Year Spectrophotometric and Photographic Evaluation In Vitro. J. Clin. Pediatr. Dent. 2021, 45, 112–116. [Google Scholar] [CrossRef]
- Marques Junior, R.B.; Baroudi, K.; Santos, A.; Pontes, D.; Amaral, M. Tooth Discoloration Using Calcium Silicate-Based Cements For Simulated Revascularization in Vitro. Braz. Dent. J. 2021, 32, 53–58. [Google Scholar] [CrossRef]
- Tsanova- Tosheva, D.; Dimitrova, I. Major Changes in the Development of Calcium Silicate-Based Cements in Dentistry. J. IMAB—Annu. Proceeding 2022, 28, 4612–4617. [Google Scholar] [CrossRef]
- Llena, C.; Herrero, A.; Lloret, S.; Barraza, M.; Sanz, J.L. Effect of calcium silicate-based endodontic sealers on tooth color: A 3-year in vitro experimental study. Heliyon 2023, 9, e13237. [Google Scholar] [CrossRef] [PubMed]
- Voveraityte, V.; Gleizniene, S.; Lodiene, G.; Grabliauskiene, Z.; Machiulskiene, V. Spectrophotometric analysis of tooth discolouration induced by mineral trioxide aggregate after final irrigation with sodium hypochlorite: An in vitro study. Aust. Endod. J. 2017, 43, 11–15. [Google Scholar] [PubMed]
- Eskandari, F.; Razavian, A.; Hamidi, R.; Yousefi, K.; Borzou, S. An Updated Review on Properties and Indications of Calcium Silicate-Based Cements in Endodontic Therapy. Int. J. Dent. 2022, 2022, 6858088. [Google Scholar] [CrossRef]
- Altan, H.; Tosun, G. The setting mechanism of mineral trioxide aggregate. J. Istanb. Univ. Fac. Dent. 2016, 50, 65–72. [Google Scholar] [CrossRef]
- Pushpalatha, C.; Dhareshwar, V.; Sowmya, S.V.; Augustine, D.; Vinothkumar, T.S.; Renugalakshmi, A.; Shaiban, A.; Kakti, A.; Bhandi, S.H.; Dubey, A.; et al. Modified Mineral Trioxide Aggregate—A Versatile Dental Material: An Insight on Applications and Newer Advancements. Front. Bioeng. Biotechnol. 2022, 10, 941826. [Google Scholar] [CrossRef]
- Zhekov, K.I.; Stefanova, V.P. Retreatability of Bioceramic Endodontic Sealers: A Review. Folia Medica 2020, 62, 258–264. [Google Scholar]
- Cirstescu, I.; Rodriguez, M.-L. PRODUCT PROFILE: Mineral Trioxide Aggregate (MTA): An Updated Review—Oral Health Group. Available online: https://www.oralhealthgroup.com/features/product-profile-mineral-trioxide-aggregate-mta-an-updated-review/ (accessed on 10 February 2025).
- Rawtiya, M.; Verma, K.; Singh, S.; Munaga, S.; Khan, S. MTA-Based Root Canal Sealers. J. Orofac. Res. 2013, 3, 16–21. [Google Scholar] [CrossRef]
- Tabari, K.; Rahbar, M.; Safyari, L.; Safarvand, H. Comparison of Compressive Strength and Setting Time of Four Experimental Nanohybrid Mineral Trioxide Aggregates and Angelus Mineral Trioxide Aggregate. World J. Dent. 2017, 8, 386–392. [Google Scholar] [CrossRef]
- Camilleri, J. Mineral trioxide aggregate: Present and future developments. Endod. Top. 2015, 32, 31–46. [Google Scholar] [CrossRef]
- Ratnakumari, N.; Thomas, B. A Histopathological Comparison of Pulpal Response to Chitra-CPC and Formocresol used as Pulpotomy Agents in Primary Teeth: A Clinical Trial. Int. J. Clin. Pediatr. Dent. 2012, 5, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Yong, D.; Choi, J.J.E.; Cathro, P.; Cooper, P.R.; Dias, G.; Huang, J.; Ratnayake, J. Development and Analysis of a Hydroxyapatite Supplemented Calcium Silicate Cement for Endodontic Treatment. Materials 2022, 15, 1176. [Google Scholar] [CrossRef]
- Lile, I.E.; Freiman, P.; Hosszu, T.; Vasca, E.; Vasca, V.; Bungau, S.; Vaida, L. A subsidiary physical research of glass ionomers. Mater. Plast. 2015, 2, 175–179. [Google Scholar]
- Kim, Y.K.; Grandini, S.; Ames, J.M.; Gu, L.S.; Kim, S.K.; Pashley, D.H.; Gutmann, J.L.; Tay, F.R. Critical review on methacrylate resin-based root canal sealers. J. Endod. 2010, 36, 383–399. [Google Scholar] [CrossRef]
- Torres, F.F.E.; Zordan-Bronzel, C.L.; Guerreiro-Tanomaru, J.; Chávez-Andrade, G.; Pinto, J.C.; Tanomaru-Filho, M. Effect of immersion in distilled water or phosphate-buffered saline on the solubility, volumetric change and presence of voids of new calcium silicate-based root canal sealers. Int. Endod. J. 2020, 53, 385–391. [Google Scholar] [CrossRef]
- Giovanna da Cunha, M.; Tavares, K.I.M.C.; Santos-Junior, A.O.; Torres, F.F.E.; Pinto, J.C.; Guerreiro-Tanomaru, J.; Tanomaru-Filho, M. Volumetric change of calcium silicate-based repair materials in a simulated inflammatory environment: A micro-computed tomography study. J. Conserv. Dent. Endod. 2024, 27, 817–821. [Google Scholar] [CrossRef]
- Torres, F.F.E.; Pinto, J.C.; Gabriella Oliveira, F.; Guerreiro-Tanomaru, J.; Tanomaru-Filho, M. A micro-computed tomographic study using a novel test model to assess the filling ability and volumetric changes of bioceramic root repair materials. Restor. Dent. Endod. 2020, 46, e2. [Google Scholar] [CrossRef]
- Ortiz, F.G.; Jimeno, E.B. Analysis of the porosity of endodontic sealers through micro-computed tomography: A systematic review. J. Conserv. Dent. 2018, 21, 238–242. [Google Scholar] [CrossRef]
- Patcharachol, L.; Jeeraphat, J.; Srisatjaluk, R.; Komoltri, C. Bacterial leakage and marginal adaptation of various bioceramics as apical plug in open apex model. J. Investig. Clin. Dent. 2018, 10, e12371. [Google Scholar] [CrossRef]
- Torres, F.F.E.; Guerreiro-Tanomaru, J.; Chávez-Andrade, G.; Pinto, J.C.; Berbert, F.L.C.V.; Tanomaru-Filho, M. Micro-computed tomographic evaluation of the flow and filling ability of endodontic materials using different test models. Restor. Dent. Endod. 2020, 45, e11. [Google Scholar] [CrossRef] [PubMed]
- Vergaças, J.H.N.; de Lima, C.O.; Barbosa, A.F.A.; Vieira, V.T.L.; Antunes, H.d.S.; da Silva, E.J.N.L. Marginal gaps and voids of three root-end filling materials: A microcomputed tomographic study. Microsc. Res. Tech. 2021, 85, 617–622. [Google Scholar] [CrossRef]
- De Oliveira, N.G.; de Souza Araújo, P.R.; da Silveira, M.T.; Sobral, A.P.V.; Carvalho, M.V. Comparison of the biocompatibility of calcium silicate-based materials to mineral trioxide aggregate: Systematic review. Eur. J. Dent. 2018, 12, 317–326. [Google Scholar] [CrossRef]
- Maru, V.; Dixit, U.; Patil, R.; Rupanshi, P. Cytotoxicity and Bioactivity of Mineral Trioxide Aggregate and Bioactive Endodontic Type Cements: A Systematic Review. Int. J. Clin. Pediatr. Dent. 2021, 14, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Garcia, L.F.R.; Huck, C.; Magalhães, F.C.; Souza, P.D.d.; Costa, C.A.d.S. Systemic effect of mineral aggregate-based cements: Histopathological analysis in rats. J. Appl. Oral Sci. 2017, 25, 620–630. [Google Scholar] [CrossRef]
- Pelepenko, L.E.; Marciano, M.; Richard, M.S.; Camilleri, J. Leaching and cytotoxicity of bismuth oxide in ProRoot MTA—A laboratory investigation. Int. Endod. J. 2024, 57, 1293–1314. [Google Scholar] [CrossRef]
- Moretton, T.R.; Brown, C.E., Jr.; Legan, J.J.; Kafrawy, A.H. Tissue reactions after subcutaneous and intraosseous implantation of mineral trioxide aggregate and ethoxybenzoic acid cement. J. Biomed. Mater. Res. 2000, 52, 528–533. [Google Scholar] [CrossRef]
- Skallevold, H.E.; Rokaya, D.; Khurshid, Z.; Zafar, M.S. Bioactive Glass Applications in Dentistry. Int. J. Mol. Sci. 2019, 20, 5960. [Google Scholar] [CrossRef]
- Salem, A.S.; Saleh, A.R.M.; Elmasmari, H.A. In vitro assessment of apical leakage of bioceramic endodontic sealer with two obturation techniques. Open Dent. J. 2018, 12, 1162–1168. [Google Scholar]
- Violeta, P.; Vanja Opačić, G.; Dzeletovic, B.; Jokanović, V.; Živković, S. Marginal Microleakage of Newly Synthesized Nanostructured Biomaterials Based on Active Calcium Silicate Systems and Hydroxyapatite. Stomatol. Glas. Srb. 2015, 62, 109–116. [Google Scholar] [CrossRef]
- K.S., A.; Devadiga, D.; Hegde, M.N. Evaluation of Microleakage of Four Root Canal Sealers—A Fluorescent Microscope Study. J. Evol. Med. Dent. Sci. 2020, 9, 3800–3805. [Google Scholar] [CrossRef]
- Merfea, M.; Cimpean, S.I.; Chiorean, R.S.; Antoniac, A.; Delean, A.G.; Badea, I.C.; Badea, M.E. Comparative Assessment of Push-Out Bond Strength and Dentinal Tubule Penetration of Different Calcium-Silicate-Based Endodontic Sealers. Dent. J. 2024, 12, 397. [Google Scholar] [CrossRef] [PubMed]
- Shalina, R.; Marissa, C.; Usman, M.; Suprastiwi, E.; Renna Maulana, Y.; Meidyawati, R. Comparison of Three Bioceramic Sealers in Terms of Dentinal Sealing Ability in the Root Canal. Int. J. Appl. Pharm. 2020, 12, 4–7. [Google Scholar] [CrossRef]
- Camilleri, J. Will Bioceramics be the—Future Root Canal Filling Materials? Curr. Oral Health Rep. 2017, 4, 228–238. [Google Scholar] [CrossRef]
- Abu Zeid, S.T.; Alnoury, A. Characterisation of the Bioactivity and the Solubility of a New Root Canal Sealer. Int. Dent. J. 2023, 73, 760–769. [Google Scholar] [CrossRef]
- ISO 6876:2012; Dentistry—Root Canal Sealing Materials. International Organization for Standardization: Geneva, Switzerland, 2012.
- Kaup, M.; Schäfer, E.; Dammaschke, T. An in vitro study of different material properties of Biodentine compared to ProRoot MTA. Head Face Med. 2015, 11, 16. [Google Scholar] [CrossRef]
- ANSI/ADA Specification No. 57; Endodontic Sealing Materials. American Dental Association: Chicago, IL, USA, 2000.
- Malka, V.; Hochscheidt, G.L.; Larentis, N.L.; Grecca, F.; Fontanella, V.; Kopper, P.M.P. A new in vitro method to evaluate radio-opacity of endodontic sealers. Dento Maxillo Facial Radiol. 2015, 44, 20140422. [Google Scholar] [CrossRef]
- Dzeletovic, B.; Ivana, M.; Đorđe, A.; Jovan, B.; Zoran, P.; Antić, S.; Maja, L.-Z. Radiopacity of premixed and two-component Calcium silicate-based Root Canal sealers. Balk. J. Dent. Med. 2022, 26, 161–166. [Google Scholar] [CrossRef]
- Reszka, P.; Grocholewicz, K.; Droździk, A.; Lipski, M. Evaluation of the radiopacity of selected calcium-silicate root canal sealers. Pomeranian J. Life Sci. 2019, 65, 17–24. [Google Scholar]
- Tanalp, J.; Karapinar-Kazandag, M.; Semanur, D.; Mehmet Baybora, K. Comparison of the Radiopacities of Different Root-End Filling and Repair Materials. Sci. World J. 2013, 2013, 594950. [Google Scholar] [CrossRef]
- Grazziotin-Soares, R.; Felipe Barros, M.; Debora, D.; Alexander Pompermayer, J.; Vania Regina Camargo, F.; Patrícia Maria Poli, K. Bioceramic root repair materials appear more radiopaque in a radiopacity test simulating clinical reality than in a traditional circular-disks test. Rev. Da Fac. De Odontol. De Porto Alegre 2024, 65. [Google Scholar] [CrossRef]
- Candeiro, G.; Fabrícia Campelo, C.; Duarte, M.H.; Danieli Colaço, R.-S.; Gavini, G. Evaluation of radiopacity, pH, release of calcium ions, and flow of a bioceramic root canal sealer. J. Endod. 2012, 38, 842–845. [Google Scholar] [CrossRef]
- Filho, M.T.; Laitano, S.C.; Gonçalves, M.; Tanomaru, J.M.G. Evaluation of the radiopacity of root- end filling materials by digitization of radiographic images. Braz. J. Oral Sci. 2006, 5, 1018–1021. [Google Scholar] [CrossRef]
- Ocak, M. Radiopacity Evaluation of Three Calcium Silicate-Based Materials by Digital Radiography. 2013. Available online: https://www.semanticscholar.org/paper/RADIOPACITY-EVALUATION-OF-THREE-CALCIUM-MATERIALS-Ocak/a25d00f506725506fb083360621009b44f92bb68 (accessed on 10 February 2025).
- Goda, B.; Drukteinis, S.; Brukiene, V.; Rajasekharan, S. Immediate and Long-Term Radiopacity and Surface Morphology of Hydraulic Calcium Silicate-Based Materials. Materials 2022, 15, 6635. [Google Scholar] [CrossRef] [PubMed]
- Dana, H.; Chisnoiu, A.; Badea, M.; Moldovan, M.; Chisnoiu, R. Comparative radiographic assessment of a new bioceramic-based root canal sealer. Clujul Med. 2017, 90, 226–230. [Google Scholar] [CrossRef]
- Dzeletovic, B.; Ivana, B.; Djordje, A.; Jovan, B.; Zoran, P.; Vanja, O.-G. Radiopacity of calcium silicate-based endodontic sealers using digital imaging. Serbian Dent. J. 2021, 68, 189–196. [Google Scholar] [CrossRef]
- Janini, A.C.P.; Bombarda, G.F.; Pelepenko, L.E.; Marciano, M.A. Antimicrobial Activity of Calcium Silicate-Based Dental Materials: A Literature Review. Antibiotics 2021, 10, 865. [Google Scholar] [CrossRef]
- Shieh, T.M.; Hsu, S.M.; Chang, K.C.; Chen, W.C.; Lin, D.J. Calcium Phosphate Cement with Antimicrobial Properties and Radiopacity as an Endodontic Material. Materials 2017, 10, 1256. [Google Scholar] [CrossRef]
- Amjad Abu, H.; Ana Luisa, T.; Larissa Marques, P.; Ramos, L.P.; Campos, T.; Maisour Ala, R.; Talal, A.-N.; Oliveira, L.D.d.; Carvalho, C. Antimicrobial Action, Genotoxicity, and Morphological Analysis of Three Calcium Silicate-Based Cements. BioMed Res. Int. 2022, 2022, 2155226. [Google Scholar] [CrossRef]
- Koruyucu, M.; Topçuoğlu, N.; Tuna, E.; Ozel, S.; Gençay, K.; Kulekci, G.; Seymen, F. An assessment of antibacterial activity of three pulp capping materials on Enterococcus faecalis by a direct contact test: An in vitro study. Eur. J. Dent. 2015, 9, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Üstün, Y.; Sağsen, B.; Durmaz, S.; Perçin, D. In vitro antimicrobial efficiency of different root canal sealers against Enterecoccus faecalis. Eur. J. Gen. Dent. 2013, 2, 134. [Google Scholar] [CrossRef]
- Poojitha, S.; Tripuravaram Vinay Kumar, R.; Vijay, V.; Kingston, C.; Mahalakshmi, K. Evaluation of the pH and Antibacterial Efficacy of Mineral Trioxide Aggregate With and Without the Incorporation of Titanium Tetrafluoride. Cureus 2024, 16, e64385. [Google Scholar] [CrossRef]
- Komora, P.; Vámos, O.; Gede, N.; Hegyi, P.; Kelemen, K.; Galvács, A.; Varga, G.; Kerémi, B.; Vág, J. Comparison of bioactive material failure rates in vital pulp treatment of permanent matured teeth—A systematic review and network meta-analysis. Sci. Rep. 2024, 14, 18421. [Google Scholar] [CrossRef]
- Saya, H.R.; Bakr, D.; Urfa Muneer, A.; Bassam, K.A. In vitro evaluation of the antibacterial effects of MTA- Fillapex and BIO-C® sealer at different time intervals. Cell. Mol. Biol. 2023, 69, 116–119. [Google Scholar] [CrossRef]
- Rehan, A. Antibacterial Activity of Two Calcium Silicate-Based Root Canal Sealers Against Enterococcus Faecalis. Egypt. Dent. J. 2019, 65, 2723–2730. [Google Scholar] [CrossRef][Green Version]
- Mengzhen, J.; Yaqi, C.; Ye, W.; Kaixin, X.; Xuan, C.; Zou, L. Evaluation of Antimicrobial Activity of A Fast-Setting Bioceramic Endodontic Material. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Parinaz, E.; Jahromi, M.; Arezoo, T. In vitro antimicrobial activity of mineral trioxide aggregate, Biodentine, and calcium-enriched mixture cement against Enterococcus faecalis, Streptococcus mutans, and Candida albicans using the agar diffusion technique. Dent. Res. J. 2021, 18, 3. [Google Scholar] [CrossRef]
- Bhat, S.; Bhagat, R. Evaluation of antibacterial efficiency of different pulp capping materials method against E. faecalis and S. mutans: An in vitro study. Int. J. Appl. Dent. Sci. 2019, 5, 13–15. [Google Scholar]
- Aditi, J.; Gupta, A.; Rupika, A. Comparative evaluation of the antibacterial activity of two Biocompatible materials i.e., Biodentine and MTA when used as a direct pulp capping agent against streptococcus mutans and Enterococcus faecalis—An in vitro study. Endodontology 2018, 30, 66–68. [Google Scholar] [CrossRef]
- Kunert, M.; Lukomska-Szymańska, M. Bio-Inductive Materials in Direct and Indirect Pulp Capping—A Review Article. Materials 2020, 13, 1204. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xiao, Y.; Song, W.; Ye, L.; Yang, C.; Xing, Y.; Yuan, Z. Clinical application of calcium silicate-based bioceramics in endodontics. J. Transl. Med. 2023, 21, 853. [Google Scholar] [CrossRef]
- Baghdadi, I.; AbuTarboush, B.J.; Zaazou, A.; Skienhe, H.; Özcan, M.; Zakhour, M.; Salameh, Z. Investigation of the structure and compressive strength of a bioceramic root canal sealer reinforced with nanomaterials. J. Appl. Biomater. Funct. Mater. 2021, 19, 22808000211014747. [Google Scholar] [CrossRef]
- Prasad Kumara, P.A.A.S.; Deng, X.; Cooper, P.R.; Cathro, P.; Dias, G.; Gould, M.; Ratnayake, J. Montmorillonite in dentistry: A review of advances in research and potential clinical applications. Mater. Res. Express 2024, 11, 072001. [Google Scholar] [CrossRef]
- Naguib, G.; Maghrabi, A.A.; Mira, A.I.; Mously, H.A.; Hajjaj, M.; Hamed, M.T. Influence of inorganic nanoparticles on dental materials’ mechanical properties. A narrative review. BMC Oral Health 2023, 23, 897. [Google Scholar] [CrossRef]
- Desouky, A.; Negm, M.; Ali, M.M. Sealability of Different Root Canal Nanosealers: Nano Calcium Hydroxide and Nano Bioactive Glass. Open Dent. J. 2019, 13, 308–315. [Google Scholar]
- Oncu, A.; Huang, Y.; Amasya, G.; Sevimay, F.S.; Orhan, K.; Celikten, B. Silver nanoparticles in endodontics: Recent developments and applications. Restor. Dent. Endod. 2021, 46, e38. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, R.; Vatani, A.; Sabzi, A.; Safaei, M. A narrative review on application of metal and metal oxide nanoparticles in endodontics. Heliyon 2024, 10, e34673. [Google Scholar] [CrossRef]
- Mierzejewska, Ż.A.; Rusztyn, B.; Łukaszuk, K.; Borys, J.; Borowska, M.; Antonowicz, B. The Latest Advances in the Use of Nanoparticles in Endodontics. Appl. Sci. 2024, 14, 7912. [Google Scholar] [CrossRef]
- Abed, F.M.; Kotha, S.B.; AlShukairi, H.; Almotawah, F.N.; Alabdulaly, R.A.; Mallineni, S.K. Effect of Different Concentrations of Silver Nanoparticles on the Quality of the Chemical Bond of Glass Ionomer Cement Dentine in Primary Teeth. Front. Bioeng. Biotechnol. 2022, 10, 816652. [Google Scholar] [CrossRef]
- Ratnayake, J.; Ramesh, N.; Gould, M.L.; Mucalo, M.R.; Dias, G.J. Silicate-substituted bovine-derived hydroxyapatite as a bone substitute in regenerative dentistry. J. Appl. Biomater. Funct. Mater. 2025, 23, 22808000251314302. [Google Scholar] [CrossRef]
- Yun, D.A.; Park, S.J.; Lee, S.R.; Min, K.S. Tooth discoloration induced by calcium-silicate-based pulp-capping materials. Eur. J. Dent. 2015, 9, 165–170. [Google Scholar] [CrossRef]
- Song, X.; Díaz-Cuenca, A. Sol-Gel Synthesis of Endodontic Cements: Post-Synthesis Treatment to Improve Setting Performance and Bioactivity. Materials 2022, 15, 6051. [Google Scholar] [CrossRef]
- Washington, J.T.; Schneiderman, E.; Spears, R.; Fernandez, C.R.; He, J.; Opperman, L.A. Biocompatibility and Osteogenic Potential of New Generation Endodontic Materials Established by Using Primary Osteoblasts. J. Endod. 2011, 37, 1166–1170. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Prieto, S.J.; Fonseca, L.F.; Sequeda-Castañeda, L.G.; Díaz, K.J.; Castañeda, L.Y.; Leyva-Rojas, J.A.; Salcedo-Reyes, J.C.; Acosta, A.P. Elaboration and Biocompatibility of an Eggshell-Derived Hydroxyapatite Material Modified with Si/PLGA for Bone Regeneration in Dentistry. Int. J. Dent. 2019, 2019, 5949232. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, J. Development of a Novel Xenograft Material from New Zealand Sourced Bovine Cancellous Bone for Bone Tissue Regeneration. Ph.D. Thesis, University of Otago, Dunedin, New Zealand, 2017. [Google Scholar]
- Cai, M.; Ratnayake, J.; Cathro, P.; Gould, M.; Ali, A. Investigation of a Novel Injectable Chitosan Oligosaccharide—Bovine Hydroxyapatite Hybrid Dental Biocomposite for the Purposes of Conservative Pulp Therapy. Nanomaterials 2022, 12, 3925. [Google Scholar] [CrossRef]
- Raina, R. Bioactive Glass in Dentistry: A Review. J. Adv. Med. Dent. Sci. Res. 2023, 11, 121–124. [Google Scholar]
- Ataya, S.; Hannora, A. Structure and compression strength of hydroxyapatite/titania nanocomposites formed by high energy ball milling. J. Alloys Compd. 2016, 658, 222–233. [Google Scholar] [CrossRef]
- Marciano, M.A.; Camilleri, J.; Costa, R.M.; Matsumoto, M.A.; Guimarães, B.M.; Duarte, M.A.H. Zinc Oxide Inhibits Dental Discoloration Caused by White Mineral Trioxide Aggregate Angelus. J. Endod. 2017, 43, 1001–1007. [Google Scholar] [CrossRef]
- Bryan, T.E.; Khechen, K.; Brackett, M.G.; Messer, R.L.; El-Awady, A.; Primus, C.M.; Gutmann, J.L.; Tay, F.R. In vitro osteogenic potential of an experimental calcium silicate-based root canal sealer. J. Endod. 2010, 36, 1163–1169. [Google Scholar] [CrossRef]
- Saxena, P.; Gupta, S.K.; Newaskar, V. Biocompatibility of root-end filling materials: Recent update. Restor. Dent. Endod. 2013, 38, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Farzaneh, A.; Yuan, C.; Walsh, L.J.; Peters, O.; Chun, X. Application of Nanomaterials in Endodontics. BME Front. 2024, 5, 0043. [Google Scholar] [CrossRef]
- Duran, C.; Dra, L.G.-C.; González, V.V.; Losada, C.G. Push out bond strength of hydraulic cements used at different thicknesses. BMC Oral Health 2023, 23, 81. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, K.; Gomes, P.; Neppelenbroek, K.; Rodrigues, C.; Fernandes, M.H.; Grenho, L. Fast-Setting Calcium Silicate-Based Pulp Capping Cements—Integrated Antibacterial, Irritation and Cytocompatibility Assessment. Materials 2023, 16, 450. [Google Scholar] [CrossRef] [PubMed]
- Merve, E.; Guven, Y.; Seyhan, M.; Ersev, H.; Tuna-İnce, E.B. Evaluation of the genotoxicity, cytotoxicity, and bioactivity of calcium silicate-based cements. BMC Oral Health 2024, 24, 119. [Google Scholar] [CrossRef]
- Karla, N.-O.; Niño-Martínez, N.; Idania De, A.-M.; Nuria, P.-M.; Facundo, R.; Horacio, B.; Martínez-Castañón, G. The Push-Out Bond Strength, Surface Roughness, and Antimicrobial Properties of Endodontic Bioceramic Sealers Supplemented with Silver Nanoparticles. Molecules 2024, 29, 4422. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).