Biomaterials for Guided Tissue Regeneration and Guided Bone Regeneration: A Review
Abstract
1. Introduction
2. Methods
3. Membranes (Barriers)
3.1. Non-Absorbable Membranes
3.2. Absorbable Membranes
3.2.1. Collagen-Based Membranes
3.2.2. Synthetic Membranes
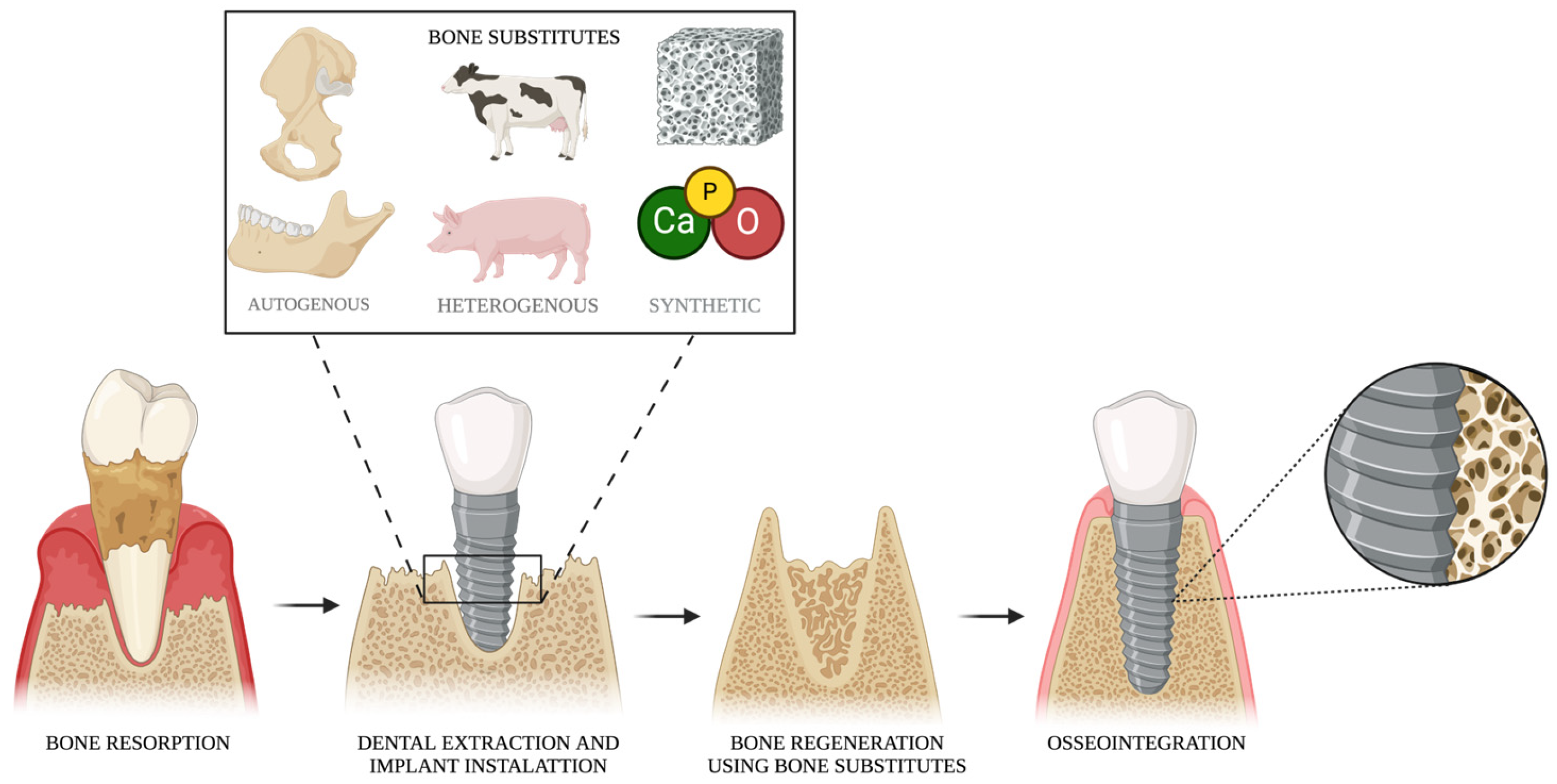
4. Bone Substitutes
4.1. Autogenous Bone
4.2. Heterogenous Bone Substitutes
4.3. Synthetic Bone Substitutes
| Commercial Name | Manufacturer | Composition | Reabsorption Rate | Reference |
|---|---|---|---|---|
| Perioglas® | NovaBone Products | Bioactive glass SiO2 (45%)/Na2O (24.5%) CaO (24.5%)/P2O5 (6%) | 6–12 months | [106] |
| Biogran® | Zimmer Biomet | Bioactive glass SiO2 (45%)/Na2O (24.5%) CaO (24.5%)/P2O5 (6%) | 6 months to 2 years | [107] |
| BoneCeramic® | Straumann | 60% HA/40% β-TCP | 6 months to 2 years | [108] |
| MBCP+® | Biomatlante | 20% HA/80% β-TCP | 3–12 months | [109] |
| Cerasorb® M | Curasan Inc. | 100% β-TCP | 12 months | [110] |
5. Mucosal Substitutes
5.1. Autogenous Soft Tissue
5.2. Allogenous Mucosal Substitutes
5.3. Heterogenous Mucosal Substitutes
6. Challenges, Innovations, and Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, H.; Bai, X.; Wang, X.; Qiang, J.; Sha, T.; Shi, Y.; Zheng, K.; Yang, Z.; Shi, C. Development and regeneration of periodontal supporting tissues. Genesis 2022, 60, e23491. [Google Scholar] [CrossRef] [PubMed]
- Manresa, C.; Sanz-Miralles, E.C.; Twigg, J.; Bravo, M. Supportive periodontal therapy (SPT) for maintaining the dentition in adults treated for periodontitis. Cochrane Database Syst. Rev. 2018, 1, CD009376. [Google Scholar] [CrossRef] [PubMed]
- Araujo, M.G.; Lindhe, J. Peri-implant health. J. Clin. Periodontol. 2018, 45, S230–S236. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, M.; Hernández-Lemus, E. Periodontal Inflammation and Systemic Diseases: An Overview. Front. Physiol. 2021, 12, 709438. [Google Scholar] [CrossRef]
- Nibali, L.; Sultan, D.; Arena, C.; Pelekos, G.; Lin, G.H.; Tonetti, M. Periodontal infrabony defects: Systematic review of healing by defect morphology following regenerative surgery. J. Clin. Periodontol. 2021, 48, 100–113. [Google Scholar] [CrossRef]
- Ziccardi, V.B.; Buchbinder, D. Guided tissue regeneration in dentistry. N. Y. State Dent. J. 1996, 62, 48–51. [Google Scholar]
- Melcher, A.H. On the repair potential of periodontal tissues. J. Periodontol. 1976, 47, 256–260. [Google Scholar] [CrossRef]
- Gottlow, J.; Nyman, S.; Karring, T.; Lindhe, J. New attachment formation as the result of controlled tissue regeneration. J. Clin. Periodontol. 1984, 11, 494–503. [Google Scholar] [CrossRef]
- Nyman, S.; Lindhe, J.; Karring, T.; Rylander, H. New attachment following surgical treatment of human periodontal disease. J. Clin. Periodontol. 1982, 9, 290–296. [Google Scholar] [CrossRef]
- Simoni, E.M.; Isufi, R.; Kadaifciu, D. Guided Bone Regeneration Effects on Bone Quantity and Outcomes of Dental Implants in Patients With Insufficient Bone Support: A Single-Center Observational Study. Cureus 2023, 15, e38988. [Google Scholar]
- Nyman, S. Bone regeneration using the principle of guided tissue regeneration. J. Clin. Periodontol. 1991, 18, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Retzepi, M.; Donos, N. Guided Bone Regeneration: Biological principle and therapeutic applications. Clin. Oral Implants Res. 2010, 21, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Childs, D.R.; Murthy, A.S. Overview of Wound Healing and Management. Surg. Clin. N. Am. 2017, 97, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Reinke, J.M.; Sorg, H. Wound repair and regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar] [CrossRef]
- Eldeeb, A.E.; Salah, S.; Elkasabgy, N.A. Biomaterials for Tissue Engineering Applications and Current Updates in the Field: A Comprehensive Review. AAPS Pharm. Sci. Tech. 2022, 23, 267. [Google Scholar] [CrossRef]
- Misch, C.E.; Dietsh, F. Bone-grafting materials in implant dentistry. Implant Dent. 1993, 2, 158–167. [Google Scholar] [CrossRef]
- Deng, Y.; Liang, Y.; Liu, X. Biomaterials for Periodontal Regeneration. Dent. Clin. N. Am. 2022, 66, 659–672. [Google Scholar] [CrossRef]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—A scale for the quality assessment of narrative review articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef]
- Frigério, P.B.; de Moura, J.; Pitol-Palin, L.; Monteiro, N.G.; Mourão, C.F.; Shibli, J.A.; Okamoto, R. Combination of a Synthetic Bioceramic Associated with a Polydioxanone-Based Membrane as an Alternative to Autogenous Bone Grafting. Biomimetics 2024, 9, 284. [Google Scholar] [CrossRef]
- Mizraji, G.; Davidzohn, A.; Gursoy, M.; Gursoy, U.; Shapira, L.; Wilensky, A. Membrane barriers for guided bone regeneration: An overview of available biomaterials. Periodontol. 2000 2023, 93, 56–76. [Google Scholar] [CrossRef]
- Ji, J.G.; Yu, A.; Choi, S.H.; Lee, D.W. Clinical, Radiographic, and Histomorphometric Evaluation of a Vertical Ridge Augmentation Procedure Using a Titanium-Reinforced Microporous Expanded Polytetrafluoroethylene Membrane: A Prospective Case Series with 1-Year Follow-Up. Materials 2021, 14, 3828. [Google Scholar] [CrossRef] [PubMed]
- Carbonell, J.M.; Martín, I.S.; Santos, A.; Pujol, A.; Sanz-Moliner, J.D.; Nart, J. High-density polytetrafluoroethylene membranes in guided bone and tissue regeneration procedures: A literature review. Int. J. Oral Maxillofac. Surg. 2014, 43, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Machtei, E.E. The effect of membrane exposure on the outcome of regenerative procedures in humans: A meta-analysis. J. Periodontol. 2001, 72, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Vroom, M.G.; Gründemann, L.J.; Gallo, P. Clinical Classification of Healing Complications and Management in Guided Bone Regeneration Procedures with a Nonresorbable d-PTFE Membrane. Int. J. Periodontics Restor. Dent. 2022, 42, 419–427. [Google Scholar] [CrossRef]
- Xie, Y.; Li, S.; Zhang, T.; Wang, C.; Cai, X. Titanium mesh for bone augmentation in oral implantology: Current application and progress. Int. J. Oral Sci. 2020, 12, 37. [Google Scholar] [CrossRef]
- Felice, P.; Pistilli, R.; Pellegrino, G.; Bonifazi, L.; Tayeb, S.; Simion, M.; Barausse, C. A randomized controlled trial comparing the effectiveness of guided bone regeneration with polytetrafluoroethylene titanium-reinforced membranes, CAD/CAM semi-occlusive titanium meshes and CAD/CAM occlusive titanium foils in partially atrophic arches. Int. J. Oral Implantol. 2024, 17, 285–296. [Google Scholar]
- Pistilli, R.; Simion, M.; Barausse, C.; Gasparro, R.; Pistilli, V.; Bellini, P.; Felice, P. Guided Bone Regeneration with Nonresorbable Membranes in the Rehabilitation of Partially Edentulous Atrophic Arches: A Retrospective Study on 122 Implants with a 3- to 7-Year Follow-up. Int. J. Periodontics Restor. Dent. 2020, 40, 685–692. [Google Scholar] [CrossRef]
- Abtahi, S.; Chen, X.; Shahabi, S.; Nasiri, N. Resorbable Membranes for Guided Bone Regeneration: Critical Features, Potentials, and Limitations. ACS Mater. Au 2023, 3, 394–417. [Google Scholar] [CrossRef]
- Sbricoli, L.; Guazzo, R.; Annunziata, M.; Gobbato, L.; Bressan, E.; Nastri, L. Selection of Collagen Membranes for Bone Regeneration: A Literature Review. Materials 2020, 13, 786. [Google Scholar] [CrossRef]
- Vallecillo, C.; Osorio, M.T.; Infante, N.; Ávalos, M.J.; Vallecillo-Rivas, M.; Lynch, C.D.; Toledano, M. In Vitro Degradation of Collagen-Based Membranes for Guided Bone Regeneration After Zn-Ions or Doxycycline Functionalization. Polymers 2024, 16, 3109. [Google Scholar] [CrossRef]
- Bunyaratavej, P.; Wang, H.L. Collagen membranes: A review. J. Periodontol. 2001, 72, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Janjić, K.; Agis, H.; Moritz, A.; Rausch-Fan, X.; Andrukhov, O. Effects of collagen membranes and bone substitute differ in periodontal ligament cell microtissues and monolayers. J. Periodontol. 2022, 93, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Wang, X.; Chen, Y.; Yue, O.; Bai, Z.; Cui, B.; Jiang, H.; Liu, X. A Review of Recent Progress on Collagen-Based Biomaterials. Adv. Healthc. Mater. 2023, 12, e2202042. [Google Scholar] [CrossRef] [PubMed]
- Sela, M.N.; Kohavi, D.; Krausz, E.; Steinberg, D.; Rosen, G. Enzymatic degradation of collagen-guided tissue regeneration membranes by periodontal bacteria. Clin. Oral Implants Res. 2003, 14, 263–268. [Google Scholar] [CrossRef]
- Madsen, D.H.; Leonard, D.; Masedunskas, A.; Moyer, A.; Jürgensen, H.J.; Peters, D.E.; Amornphimoltham, P.; Selvaraj, A.; Yamada, S.S.; Brenner, D.A.; et al. M2-like macrophages are responsible for collagen degradation through a mannose receptor-mediated pathway. J. Cell Biol. 2013, 202, 951–966. [Google Scholar] [CrossRef]
- Sela, M.N.; Babitski, E.; Steinberg, D.; Kohavi, D.; Rosen, G. Degradation of collagen-guided tissue regeneration membranes by proteolytic enzymes of Porphyromonas gingivalis and its inhibition by antibacterial agents. Clin. Oral Implants Res. 2009, 20, 496–502. [Google Scholar] [CrossRef]
- Tal, H.; Kozlovsky, A.; Artzi, Z.; Nemcovsky, C.E.; Moses, O. Long-term biodegradation of cross-linked and non-cross-linked collagen barriers in human guided bone regeneration. Clin. Oral Implants Res. 2008, 19, 295–302. [Google Scholar] [CrossRef]
- Lauer-Fields, J.L.; Juska, D.; Fields, G.B. Matrix metalloproteinases and collagen catabolism. Biopolymers 2002, 66, 19–32. [Google Scholar] [CrossRef]
- Moses, O.; Eliezer, M.; Nemcovsky, C.; Tal, H.; Weinreb, M. Accelerated degradation of collagen membranes in diabetic rats is associated with increased infiltration of macrophages and blood vessels. Clin. Oral Investig. 2016, 20, 1589–1596. [Google Scholar] [CrossRef]
- Sculean, A.; Nikolidakis, D.; Schwarz, F. Regeneration of periodontal tissues: Combinations of barrier membranes and grafting materials—Biological foundation and preclinical evidence: A systematic review. J. Clin. Periodontol. 2008, 35, 106–116. [Google Scholar] [CrossRef]
- Jiménez Garcia, J.; Berghezan, S.; Caramês, J.M.M.; Dard, M.M.; Marques, D.N.S. Effect of cross-linked vs non-cross-linked collagen membranes on bone: A systematic review. J. Periodontal Res. 2017, 52, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, A.; Apel, C.; Sellhaus, B.; van Neerven, S.; Wessing, B.; Hilgers, R.D.; Pallua, N. Differences in degradation behavior of two non-cross-linked collagen barrier membranes: An in vitro and in vivo study. Clin. Oral Implants Res. 2014, 25, 1403–1411. [Google Scholar] [CrossRef] [PubMed]
- An, Y.Z.; Song, Y.W.; Thoma, D.S.; Strauss, F.J.; Lee, J.S. Enhancing guided bone regeneration with cross-linked collagen-conjugated xenogeneic bone blocks and membrane fixation: A preclinical in vivo study. Clin. Oral Implants Res. 2024, 35, 1226–1239. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Rothamel, D.; Herten, M.; Sager, M.; Becker, J. Angiogenesis pattern of native and cross-linked collagen membranes: An immunohistochemical study in the rat. Clin. Oral Implants Res. 2006, 17, 403–409. [Google Scholar] [CrossRef]
- Jin, X.; Park, J.Y.; Lee, J.S.; Jung, U.W.; Choi, S.H.; Cha, J.K. Tissue integration patterns of non-crosslinked and crosslinked collagen membranes: An experimental in vivo study. J. Periodontal Implant Sci. 2023, 53, 207–217. [Google Scholar] [CrossRef]
- Dogan Kaplan, A.; Cinar, I.C.; Gultekin, B.A.; Avci Kupeli, Z.; Ozfirat, E.C.; Yalcin, S. The Effect of Different Types of Collagen Membranes on Peri-Implant Dehiscence Defects. J. Craniofac. Surg. 2023, 34, 2479–2484. [Google Scholar] [CrossRef]
- Grassi, A.; Bizzoca, M.E.; De Biasi, L.; Padula, R.; Annicchiarico, C.; Cervino, G.; Lo Muzio, L.; Mastrangelo, F. Management of Vestibular Bone Fenestration with Periosteal Inhibition (PI) Technique During Alveolar Socket Preservation: A Case Report. Medicina 2024, 60, 1912. [Google Scholar] [CrossRef]
- Ramos, E.U.; Leandro, M.N.C.; Criales, J.O.C.; Buitron, M.R.O.; Verástegui, E.S.; Carbajal, W.M.; Adrianzén, R.C.S.; Grijalva, A.E.E.; Baylon, A.A.B.; Bassi, A.P.F. Evaluation of Porcine Collagen Membranes Used with Guided Bone Regeneration for Critical Defects: A Histological, Histomorphometric, Immunohistochemical, and Inflammatory Profile Analysis. Eur. J. Dent. 2024, 18, 898–906. [Google Scholar] [CrossRef]
- Menceva, Z.; Dimitrovski, O.; Popovska, M.; Spasovski, S.; Spirov, V.; Petrushevska, G. Free Gingival Graft versus Mucograft: Histological Evaluation. Open Access Maced. J. Med. Sci. 2018, 6, 675–679. [Google Scholar]
- Gao, Y.; Wang, S.; Shi, B.; Wang, Y.; Chen, Y.; Wang, X.; Lee, E.S.; Jiang, H.B. Advances in Modification Methods Based on Biodegradable Membranes in Guided Bone/Tissue Regeneration: A Review. Polymers 2022, 14, 871. [Google Scholar] [CrossRef]
- Geevarghese, R.; Sajjadi, S.S.; Hudecki, A.; Sajjadi, S.; Jalal, N.R.; Madrakian, T.; Ahmadi, M.; Włodarczyk-Biegun, M.K.; Ghavami, S.; Likus, W.; et al. Biodegradable and Non-Biodegradable Biomaterials and Their Effect on Cell Differentiation. Int. J. Mol. Sci. 2022, 23, 6185. [Google Scholar] [CrossRef] [PubMed]
- Quirino, L.C.; de Azambuja Carvalho, P.H.; Neto, R.T.A.; Comachio, C.A.; Monteiro, N.G.; Ervolino-Silva, A.C.; Okamoto, R.; Pereira-Filho, V.A. Polydioxanone Membrane Compared with Collagen Membrane for Bone Regeneration. Polymers 2023, 15, 868. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, L.; Zhou, Z.; Lai, H.; Xu, P.; Liao, L.; Wei, J. Biodegradable Polymer Membranes Applied in Guided Bone/Tissue Regeneration: A Review. Polymers 2016, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, M.P. Bone Grafts in Dental Medicine: An Overview of Autografts, Allografts and Synthetic Materials. Materials 2023, 16, 4117. [Google Scholar] [CrossRef]
- Janicki, P.; Schmidmaier, G. What should be the characteristics of the ideal bone graft substitute? Combining scaffolds with growth factors and/or stem cells. Injury 2011, 42, S77–S81. [Google Scholar] [CrossRef]
- Jensen, S.S.; Gruber, R.; Buser, D.; Bosshardt, D.D. Osteoclast-like cells on deproteinized bovine bone mineral and biphasic calcium phosphate: Light and transmission electron microscopical observations. Clin. Oral Implants Res. 2015, 26, 859–864. [Google Scholar] [CrossRef]
- Miron, R.J.; Hedbom, E.; Saulacic, N.; Zhang, Y.; Sculean, A.; Bosshardt, D.D.; Buser, D. Osteogenic potential of autogenous bone grafts harvested with four different surgical techniques. J. Dent. Res. 2011, 90, 1428–1433. [Google Scholar] [CrossRef]
- Miron, R.J.; Zhang, Y.F. Osteoinduction: A review of old concepts with new standards. J. Dent. Res. 2012, 91, 736–744. [Google Scholar] [CrossRef]
- Weiland, A.J.; Moore, J.R.; Daniel, R.K. Vascularized bone autografts. Experience with 41 cases. Clin. Orthop. Relat. Res. 1983, 174, 87–95. [Google Scholar] [CrossRef]
- Hutchings, G.; Moncrieff, L.; Dompe, C.; Janowicz, K.; Sibiak, R.; Bryja, A.; Jankowski, M.; Mozdziak, P.; Bukowska, D.; Antosik, P.; et al. Bone Regeneration, Reconstruction and Use of Osteogenic Cells: From Basic Knowledge, Animal Models to Clinical Trials. J. Clin. Med. 2020, 9, 139. [Google Scholar] [CrossRef]
- Cricchio, G.; Lundgren, S. Donor site morbidity in two different approaches to anterior iliac crest bone harvesting. Clin. Implant Dent. Relat. Res. 2003, 5, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Khojasteh, A.; Nazeman, P.; Tolstunov, L. Tuberosity-alveolar block as a donor site for localized augmentation of the maxilla: A retrospective clinical study. Br. J. Oral Maxillofac. Surg. 2016, 54, 950–955. [Google Scholar] [CrossRef] [PubMed]
- Reininger, D.; Cobo-Vázquez, C.; Rosenberg, B.; López-Quiles, J. Alternative intraoral donor sites to the chin and mandibular body-ramus. J. Clin. Exp. Dent. 2017, 9, e1474–e1481. [Google Scholar] [CrossRef] [PubMed]
- Fakhry, A. The mandibular retromolar area as a donor site in maxillofacial bone grafting: Surgical notes. Int. J. Periodontics Restor. Dent. 2011, 31, 275–283. [Google Scholar]
- Schmidt, A.H. Autologous bone graft: Is it still the gold standard? Injury 2021, 52, S18–S22. [Google Scholar] [CrossRef]
- Zhao, R.; Yang, R.; Cooper, P.R.; Khurshid, Z.; Shavandi, A.; Ratnayake, J. Bone Grafts and Substitutes in Dentistry: A Review of Current Trends and Developments. Molecules 2021, 26, 3007. [Google Scholar] [CrossRef]
- Pallesen, L.; Schou, S.; Aaboe, M.; Hjørting-Hansen, E.; Nattestad, A.; Melsen, F. Influence of particle size of autogenous bone grafts on the early stages of bone regeneration: A histologic and stereologic study in rabbit calvarium. Int. J. Oral Maxillofac. Implants 2002, 17, 498–506. [Google Scholar]
- Pabst, A.; Becker, P.; Götz, W.; Heimes, D.; Thiem, D.G.E.; Blatt, S.; Kämmerer, P.W. A comparative analysis of particulate bovine bone substitutes for oral regeneration: A narrative review. Int. J. Implant Dent. 2024, 10, 26. [Google Scholar] [CrossRef]
- Dutta, S.R.; Passi, D.; Singh, P.; Bhuibhar, A. Ceramic and non-ceramic hydroxyapatite as a bone graft material: A brief review. Ir. J. Med. Sci. 2015, 184, 101–106. [Google Scholar] [CrossRef]
- Rouahi, M.; Gallet, O.; Champion, E.; Dentzer, J.; Hardouin, P.; Anselme, K. Influence of hydroxyapatite microstructure on human bone cell response. J. Biomed. Mater. Res. A 2006, 78, 222–235. [Google Scholar] [CrossRef]
- Bracey, D.N.; Jinnah, A.H.; Willey, J.S.; Seyler, T.M.; Hutchinson, I.D.; Whitlock, P.W.; Smith, T.L.; Danelson, K.A.; Emory, C.L.; Kerr, B.A. Investigating the Osteoinductive Potential of a Decellularized Xenograft Bone Substitute. Cells Tissues Organs 2019, 207, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Ganey, T.; Hutton, W.; Meisel, H.J. Osteoconductive carriers for integrated bone repair. SAS J. 2009, 3, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Hannink, G.; Arts, J.J. Bioresorbability, porosity and mechanical strength of bone substitutes: What is optimal for bone regeneration? Injury 2011, 42, S22–S25. [Google Scholar] [CrossRef] [PubMed]
- Fernandez de Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9, 2041731418776819. [Google Scholar] [CrossRef]
- Yang, S.; Lan, L.; Miron, R.J.; Wei, L.; Zhang, M.; Zhang, Y. Variability in Particle Degradation of Four Commonly Employed Dental Bone Grafts. Clin. Implant Dent. Relat. Res. 2015, 17, 996–1003. [Google Scholar] [CrossRef]
- Zhang, Q.; Jing, D.; Zhang, Y.; Miron, R.J. Histomorphometric Study of New Bone Formation Comparing Defect Healing with Three Bone Grafting Materials: The Effect of Osteoporosis on Graft Consolidation. Int. J. Oral Maxillofac. Implants 2018, 33, 645–652. [Google Scholar] [CrossRef]
- Abdelmoneim, D.; Coates, D.E.; Schmidlin, P.; Botter, S.; Li, K.C.; Porter, G.C.; Seo, B.; Duncan, W.J. In vivo healing of low temperature deproteinized bovine bone xenograft in a rabbit cranial model. J. Biomed. Mater. Res. A 2024, 112, 1436–1450. [Google Scholar] [CrossRef]
- Gallo, P.; Díaz-Báez, D.; Perdomo, S.; Aloise, A.C.; Tattan, M.; Saleh, M.H.A.; Pelegrine, A.A.; Ravidà, A.; Wang, H.L. Comparative analysis of two biomaterials mixed with autogenous bone graft for vertical ridge augmentation: A histomorphometric study in humans. Clin. Implant Dent. Relat. Res. 2022, 24, 709–719. [Google Scholar] [CrossRef]
- Sogal, A.; Tofe, A.J. Risk assessment of bovine spongiform encephalopathy transmission through bone graft material derived from bovine bone used for dental applications. J. Periodontol. 1999, 70, 1053–1063. [Google Scholar] [CrossRef]
- Wenz, B.; Oesch, B.; Horst, M. Analysis of the risk of transmitting bovine spongiform encephalopathy through bone grafts derived from bovine bone. Biomaterials 2001, 22, 1599–1606. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Liu, K.; Liu, R.X.; Xu, B.H. Safety and Efficacy of Midface Augmentation Using Bio-Oss Bone Powder and Bio-Gide Collagen Membrane in Asians. J. Clin. Med. 2023, 12, 959. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Nowzari, H.; Rich, S.K. Risk of prion disease transmission through bovine-derived bone substitutes: A systematic review. Clin. Implant Dent. Relat. Res. 2013, 15, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.E.; Nowzari, H. The long-term risks and complications of bovine-derived xenografts: A case series. J. Indian Soc. Periodontol. 2019, 23, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Sartori, S.; Silvestri, M.; Forni, F.; Icaro, A.C.; Tesei, P.; Cattaneo, V. Ten-year follow-up in a maxillary sinus augmentation using anorganic bovine bone (Bio-Oss). A case report with histomorphometric evaluation. Clin. Oral Implants Res. 2003, 14, 369–372. [Google Scholar] [CrossRef]
- Pereira, R.d.S.; de Carvalho, M.V.N.B.; Hochuli-Vieira, E.; Statkievicz, C.; Pereira Santos, D.L.; Augusto Neto, R.T.; Pinto, C.d.F.S.; Bennardo, F.; Mourão, C.F. Histomorphometric and Micro-CT Evaluation of Cerabone and Bio-Oss in Maxillary Sinus Lifting: A Randomized Clinical Trial. Medicina 2024, 60, 1834. [Google Scholar] [CrossRef]
- Polymeri, A.; Anssari-Moin, D.; van der Horst, J.; Wismeijer, D.; Laine, M.L.; Loos, B.G. Surgical treatment of peri-implantitis defects with two different xenograft granules: A randomized clinical pilot study. Clin. Oral Implants Res. 2020, 31, 1047–1060. [Google Scholar] [CrossRef]
- Romasco, T.; Tumedei, M.; Inchingolo, F.; Pignatelli, P.; Montesani, L.; Iezzi, G.; Petrini, M.; Piattelli, A.; Di Pietro, N. A Narrative Review on the Effectiveness of Bone Regeneration Procedures with OsteoBiol® Collagenated Porcine Grafts: The Translational Research Experience over 20 Years. J. Funct. Biomater. 2022, 13, 121. [Google Scholar] [CrossRef]
- Fukuba, S.; Okada, M.; Nohara, K.; Iwata, T. Alloplastic Bone Substitutes for Periodontal and Bone Regeneration in Dentistry: Current Status and Prospects. Materials 2021, 14, 1096. [Google Scholar] [CrossRef]
- Romanos, G.E.; Romanos, E.B.; Alqahtani, F.; Alqahtani, M.; Javed, F. “Religious Belief”: An Undervalued Ethical Inclusion Criterion for Clinical Trials on Bone Grafting Procedures. J. Relig. Health 2020, 59, 2928–2934. [Google Scholar] [CrossRef]
- Assari, A.; Hani, M.; Qaid, H.; Omar, B.; Aleid, L. Effect of religious beliefs on bone graft selection for oral and maxillofacial surgery in Saudi Arabia. J. Stomatol. Oral Maxillofac. Surg. 2022, 123, e563–e568. [Google Scholar] [CrossRef]
- Güngörmüş, Z.; Güngörmüş, M. Effect of Religious Belief on Selecting of Graft Materials Used in Oral and Maxillofacial Surgery. J. Oral Maxillofac. Surg. 2017, 75, 2347–2353. [Google Scholar] [CrossRef] [PubMed]
- Ashfaq, R.; Kovács, A.; Berkó, S.; Budai-Szűcs, M. Developments in Alloplastic Bone Grafts and Barrier Membrane Biomaterials for Periodontal Guided Tissue and Bone Regeneration Therapy. Int. J. Mol. Sci. 2024, 25, 7746. [Google Scholar] [CrossRef] [PubMed]
- Eppley, B.L.; Pietrzak, W.S.; Blanton, M.W. Allograft and alloplastic bone substitutes: A review of science and technology for the craniomaxillofacial surgeon. J. Craniofac. Surg. 2005, 16, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Valtanen, R.S.; Yang, Y.P.; Gurtner, G.C.; Maloney, W.J.; Lowenberg, D.W. Synthetic and Bone tissue engineering graft substitutes: What is the future? Injury 2021, 52, S72–S77. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphates (CaPO4): Occurrence and properties. Prog. Biomater. 2016, 5, 9–70. [Google Scholar]
- Dorozhkin, S.V. Calcium orthophosphate-based bioceramics. Materials 2013, 6, 3840–3942. [Google Scholar] [CrossRef]
- Onodera, J.; Kondo, E.; Omizu, N.; Ueda, D.; Yagi, T.; Yasuda, K. Beta-tricalcium phosphate shows superior absorption rate and osteoconductivity compared to hydroxyapatite in open-wedge high tibial osteotomy. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 2763–2770. [Google Scholar] [CrossRef]
- Clark, D.; Nakamura, M.; Miclau, T.; Marcucio, R. Effects of Aging on Fracture Healing. Curr. Osteoporos. Rep. 2017, 15, 601–608. [Google Scholar] [CrossRef]
- Gorter, E.A.; Reinders, C.R.; Krijnen, P.; Appelman-Dijkstra, N.M.; Schipper, I.B. The effect of osteoporosis and its treatment on fracture healing a systematic review of animal and clinical studies. Bone Rep. 2021, 15, 101117. [Google Scholar] [CrossRef]
- Skallevold, H.E.; Rokaya, D.; Khurshid, Z.; Zafar, M.S. Bioactive Glass Applications in Dentistry. Int. J. Mol. Sci. 2019, 20, 5960. [Google Scholar] [CrossRef]
- Madival, H.; Rajiv, A.; Muniraju, C.; Reddy, M.S. Advancements in Bioactive Glasses: A Comparison of Silicate, Borate, and Phosphate Network Based Materials. Biomed. Mater. Dev. 2025. [Google Scholar] [CrossRef]
- Kaou, M.H.; Furkó, M.; Balázsi, K.; Balázsi, C. Advanced Bioactive Glasses: The Newest Achievements and Breakthroughs in the Area. Nanomaterials 2023, 13, 2287. [Google Scholar] [CrossRef] [PubMed]
- Goretti, M.P.; Alon, U.S. Phosphate homeostasis and its role in bone health. Pediatr. Nephrol. 2012, 27, 2039–2048. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Liu, W.; Xiong, Z.; Hu, Y.; Xiao, J. Effects of biomimetic hydroxyapatite coatings on osteoimmunomodulation. Biomater. Adv. 2022, 134, 112640. [Google Scholar] [CrossRef]
- Hu, Y.C.; Zhong, J.P. Osteostimulation of bioglass. Chin. Med. J. 2009, 122, 2386–2389. [Google Scholar]
- Johnson, M.W.; Sullivan, S.M.; Rohrer, M.; Collier, M. Regeneration of peri-implant infrabony defects using PerioGlas: A pilot study in rabbits. Int. J. Oral Maxillofac. Implants 1997, 12, 835–839. [Google Scholar]
- Tadjoedin, E.S.; de Lange, G.L.; Lyaru, D.M.; Kuiper, L.; Burger, E.H. High concentrations of bioactive glass material (Biogran) vs autogenous bone for sinus floor elevation. Clin. Oral Implants Res. 2002, 13, 428–436. [Google Scholar] [CrossRef]
- Frenken, J.W.; Bouwman, W.F.; Bravenboer, N.; Zijderveld, S.A.; Schulten, E.A.; ten Bruggenkate, C.M. The use of Straumann Bone Ceramic in a maxillary sinus floor elevation procedure: A clinical, radiological, histological and histomorphometric evaluation with a 6-month healing period. Clin. Oral Implants Res. 2010, 21, 201–208. [Google Scholar] [CrossRef]
- Le Guehennec, L.; Goyenvalle, E.; Aguado, E.; Pilet, P.; Bagot D’Arc, M.; Bilban, M.; Spaethe, R.; Daculsi, G. MBCP biphasic calcium phosphate granules and tissucol fibrin sealant in rabbit femoral defects: The effect of fibrin on bone ingrowth. J. Mater. Sci. Mater. Med. 2005, 16, 29–35. [Google Scholar] [CrossRef]
- Meyer, C.; Chatelain, B.; Benarroch, M.; Garnier, J.F.; Ricbourg, B.; Camponovo, T. Massive sinus-lift procedures with beta-tricalcium phosphate: Long-term results. Rev. Stomatol. Chir. Maxillofac. 2009, 110, 69–75. [Google Scholar] [CrossRef]
- Rotundo, R.; Pancrazi, G.L.; Grassi, A.; Ceresoli, L.; Di Domenico, G.L.; Bonafede, V. Soft Tissue Substitutes in Periodontal and Peri-Implant Soft Tissue Augmentation: A Systematic Review. Materials 2024, 17, 1221. [Google Scholar] [CrossRef] [PubMed]
- Suchenski, M.; McCarthy, M.B.; Chowaniec, D.; Hansen, D.; McKinnon, W.; Apostolakos, J.; Arciero, R.; Mazzocca, A.D. Material properties and composition of soft-tissue fixation. Arthroscopy 2010, 26, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Camargo, P.M.; Melnick, P.R.; Kenney, E.B. The use of free gingival grafts for aesthetic purposes. Periodontol. 2000 2001, 27, 72–96. [Google Scholar] [CrossRef] [PubMed]
- Agudio, G.; Nieri, M.; Rotundo, R.; Cortellini, P.; Pini Prato, G. Free gingival grafts to increase keratinized tissue: A retrospective long-term evaluation (10 to 25 years) of outcomes. J. Periodontol. 2008, 79, 587–594. [Google Scholar] [CrossRef]
- Bouchard, P.; Etienne, D.; Ouhayoun, J.P.; Nilvéus, R. Subepithelial connective tissue grafts in the treatment of gingival recessions. A comparative study of 2 procedures. J. Periodontol. 1994, 65, 929–936. [Google Scholar] [CrossRef]
- Patel, M.; Nixon, P.J.; Chan, M.F. Gingival recession: Part 2. Surgical management using pedicle grafts. Br. Dent. J. 2011, 211, 315–319. [Google Scholar] [CrossRef]
- Montero, E.; Molina, A.; Matesanz, P.; Monje, A.; Sanz-Sánchez, I.; Herrera, D. Efficacy of soft tissue substitutes, in comparison with autogenous grafts, in surgical procedures aiming to increase the peri-implant keratinized mucosa: A systematic review. Clin. Oral Implants Res. 2022, 33, 32–46. [Google Scholar] [CrossRef]
- Tavelli, L.; Barootchi, S.; Stefanini, M.; Zucchelli, G.; Giannobile, W.V.; Wang, H.L. Wound healing dynamics, morbidity, and complications of palatal soft-tissue harvesting. Periodontol. 2000 2023, 92, 90–119. [Google Scholar] [CrossRef]
- Gapski, R.; Parks, C.A.; Wang, H.L. Acellular dermal matrix for mucogingival surgery: A meta-analysis. J. Periodontol. 2005, 76, 1814–1822. [Google Scholar] [CrossRef]
- Maia, L.P., Jr.; Novaes, A.B.; Souza, S.L.; Grisi, M.F.; Taba, M.; Palioto, D.B. In vitro evaluation of acellular dermal matrix as a three-dimensional scaffold for gingival fibroblasts seeding. J. Periodontol. 2011, 82, 293–301. [Google Scholar] [CrossRef]
- Shanmugam, M.; Sivakumar, V.; Anitha, V.; Sivakumar, B. Clinical evaluation of AlloDerm for root coverage and colour match. J. Indian Soc. Periodontol. 2012, 16, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Balderrama, Í.F.; Ferreira, R.; Rezende, D.R.B.; Nogueira, A.L.R.N.; Greghi, S.L.A.; Zangrando, M.S.R. Root coverage stability with acellular dermal matrix in multiple gingival recessions in esthetic zone: A clinical case report with 12-year follow-up. J. Indian Soc. Periodontol. 2019, 23, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, C.; Tarun-Kumar, A.B.; Mehta, D.S. Comparative evaluation of free gingival graft and AlloDerm® in enhancing the width of attached gingival: A clinical study. Contemp. Clin. Dent. 2015, 6, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Rokn, A.; Zare, H.; Haddadi, P. Use of Mucograft Collagen Matrix® versus Free Gingival Graft to Augment Keratinized Tissue around Teeth: A Randomized Controlled Clinical Trial. Front. Dent. 2020, 17, 1–8. [Google Scholar] [CrossRef]
- Fathiazar, A.; Shariatmadar Ahmadi, R.; Sayar, F. A Comparison between Mucoderm® and Connective Tissue Graft for Root Coverage. J. Dent. 2022, 23, 402–409. [Google Scholar]
- Ibrahim, A.; Saymeh, R. Alveolar Ridge Preservation with Fibro-Gide or Connective Tissue Graft: A Randomized Controlled Trial of Soft and Hard Tissue Changes. Clin. Exp. Dent. Res. 2024, 10, e929. [Google Scholar] [CrossRef]
- Menezes, K.M.; Borges, S.B.; Medeiros, I.; Gomes, G.E.D.S.; Roncalli, A.G.; Gurgel, B.C.V. Efficacy of xenogeneic collagen matrix in the treatment of gingival recessions: A controlled clinical trial. Braz. Oral Res. 2024, 38, e111. [Google Scholar] [CrossRef]
- Tommasato, G.; Del Fabbro, M.; Oliva, N.; Khijmatgar, S.; Grusovin, M.G.; Sculean, A.; Canullo, L. Autogenous graft versus collagen matrices for peri-implant soft tissue augmentation. A systematic review and network meta-analysis. Clin. Oral Investig. 2024, 28, 300. [Google Scholar] [CrossRef]
- Hammarström, L. Enamel matrix, cementum development and regeneration. J. Clin. Periodontol. 1997, 24, 658–668. [Google Scholar] [CrossRef]
- Haruyama, N.; Hatakeyama, J.; Moriyama, K.; Kulkarni, A.B. Amelogenins: Multi-Functional Enamel Matrix Proteins and Their Binding Partners. J. Oral Biosci. 2011, 53, 257–266. [Google Scholar] [CrossRef]
- Kunimatsu, R.; Tanimoto, K.; Tanne, Y.; Kamiya, T.; Ohkuma, S.; Huang, Y.C.; Yoshimi, Y.; Miyauchi, M.; Takata, T.; Tanne, K. Amelogenin enhances the proliferation of cementoblast lineage cells. J. Periodontol. 2011, 82, 1632–1638. [Google Scholar] [CrossRef] [PubMed]
- Kasaj, A.; Meister, J.; Lehmann, K.; Stratul, S.I.; Schlee, M.; Stein, J.M.; Willershausen, B.; Schmidt, M. The influence of enamel matrix derivative on the angiogenic activity of primary endothelial cells. J. Periodontal Res. 2012, 47, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Carinci, F.; Piattelli, A.; Guida, L.; Perrotti, V.; Laino, G.; Oliva, A.; Annunziata, M.; Palmieri, A.; Pezzetti, F. Effects of Emdogain on osteoblast gene expression. Oral Dis. 2006, 12, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Grusovin, M.G.; Papanikolaou, N.; Coulthard, P.; Worthington, H.V. Enamel matrix derivative (Emdogain) for periodontal tissue regeneration in intrabony defects. A Cochrane systematic review. Eur. J. Oral Implantol. 2009, 2, 247–266. [Google Scholar]
- Dec, P.; Modrzejewski, A.; Pawlik, A. Existing and Novel Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2022, 24, 529. [Google Scholar] [CrossRef]
- Shuai, C.; Liu, G.; Yang, Y.; Qi, F.; Peng, S.; Yang, W.; He, C.; Wang, G.; Qian, G. A strawberry-like Ag-decorated barium titanate enhances piezoelectric and antibacterial activities of polymer scaffold. Nano Energy 2020, 74, 104825. [Google Scholar] [CrossRef]
- Li, H.; Shuai, X.; Chen, Y.; Xiong, J.; Zou, Z.; Peng, S.; Qi, F.; Shuai, C. Engineering a Wirelessly Self-Powered Neural Scaffold Based on Primary Battery Principle to Accelerate Nerve Cell Differentiation. Colloids Surf. B Biointerfaces 2025, 249, 114521. [Google Scholar] [CrossRef]
- Bu, Y.; Ma, J.; Bei, J.; Wang, S. Surface Modification of Aliphatic Polyester to Enhance Biocompatibility. Front. Bioeng. Biotechnol. 2019, 7, 98. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, S.; Luo, P.; Deng, S.; Shan, Z.; Fang, J.; Liu, X.; Xie, J.; Liu, R.; Wu, S.; et al. Optimizing the bio-degradability and biocompatibility of a biogenic collagen membrane through cross-linking and zinc-doped hydroxyapatite. Acta Biomater. 2022, 143, 159–172. [Google Scholar] [CrossRef]
- Thomas, M.V.; Puleo, D.A. Infection, inflammation, and bone regeneration: A paradoxical relationship. J. Dent. Res. 2011, 90, 1052–1061. [Google Scholar] [CrossRef]
- Zelikman, H.; Slutzkey, G.; Rosner, O.; Levartovsky, S.; Matalon, S.; Beitlitum, I. Bacterial Growth on Three Non-Resorbable Polytetrafluoroethylene (PTFE) Membranes-An In Vitro Study. Materials 2022, 15, 5705. [Google Scholar] [CrossRef] [PubMed]
- Bueno, J.; Sánchez, M.C.; Toledano-Osorio, M.; Figuero, E.; Toledano, M.; Medina-Castillo, A.L.; Osorio, R.; Herrera, D.; Sanz, M. Antimicrobial effect of nanostructured membranes for guided tissue regeneration: An in vitro study. Dent Mater. 2020, 36, 1566–1577. [Google Scholar] [CrossRef] [PubMed]
- Nardo, T.; Chiono, V.; Carmagnola, I.; Fracchia, L.; Ceresa, C.; Tabrizian, M.; Ciardelli, G. Mussel-inspired antimicrobial coating on PTFE barrier membranes for guided tissue regeneration. Biomed. Mater. 2021, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Adamuz-Jiménez, A.; Manzano-Moreno, F.-J.; Vallecillo, C. Regeneration Membranes Loaded with Non-Antibiotic Anti-2 Microbials: A Review. Polymers 2024, 16, 95. [Google Scholar] [CrossRef]
- Leitner, A.; Walzthoeni, T.; Kahraman, A.; Herzog, F.; Rinner, O.; Beck, M.; Aebersold, R. Probing native protein structures by chemical cross-linking, mass spectrometry, and bioinformatics. Mol. Cell Proteom. 2010, 9, 1634–1649. [Google Scholar] [CrossRef]
- Pistilli, R.; Barausse, C.; Simion, M.; Bonifazi, L.; Karaban, M.; Ferri, A.; Felice, P. Simultaneous GBR and Implant Placement with Resorbable Membranes in the Rehabilitation of Partially Edentulous and Horizontally Atrophic Dental Arches: A Retrospective Study on 97 Implants with a 3- to 7-Year Follow-up. Int. J. Periodontics Restor. Dent. 2022, 42, 371–379. [Google Scholar] [CrossRef]
- Eliezer, M.; Sculean, A.; Miron, R.J.; Nemcovsky, C.; Bosshardt, D.D.; Fujioka-Kobayashi, M.; Weinreb, M.; Moses, O. Cross-linked hyaluronic acid slows down collagen membrane resorption in diabetic rats through reducing the number of macrophages. Clin. Oral Investig. 2022, 26, 2401–2411. [Google Scholar] [CrossRef]
- Yamaguchi, J.; Kondo, E.; Yasuda, K.; Onodera, J.; Yabuuchi, K.; Kaibara, T.; Takami, K.; Iwasaki, N.; Yagi, T. Improvement of absorbability, osteoconductivity, and strength of a β-tricalcium phosphate spacer for opening wedge high tibial osteotomy: Clinical evaluations with 106 patients. BMC Musculoskelet. Disord. 2024, 25, 441. [Google Scholar] [CrossRef]
- Dos Santos, C.P.C.; Cruel, P.T.E.; Buchaim, D.V.; da Cunha, M.R.; Ervolino, E.; Issa, J.P.M.; Miglino, M.A.; Buchaim, R.L. Calcium Hydroxyapatite Combined with Photobiomodulation for Bone Tissue Repair: A Systematic Review. Materials 2025, 18, 1120. [Google Scholar] [CrossRef]
- Freitas, R.M.; Spin-Neto, R.; Marcantonio, E.; Pereira, L.A.; Wikesjö, U.M.; Susin, C. Alveolar ridge and maxillary sinus augmentation using rhBMP-2: A systematic review. Clin. Implant Dent. Relat. Res. 2015, 17, e192–e201. [Google Scholar] [CrossRef]
- Kazimierczak, P.; Przekora, A. Osteoconductive and Osteoinductive Surface Modifications of Biomaterials for Bone Regeneration: A Concise Review. Coatings 2020, 10, 971. [Google Scholar] [CrossRef]
- Uribe, F.; Vásquez, B.; Alister, J.P.; Olate, S. Comparison of rhBMP-2 in Combination with Different Biomaterials for Regeneration in Rat Calvaria Critical-Size Defects. BioMed Res. Int. 2022, 2022, 6281641. [Google Scholar] [CrossRef]
- Cai, F.; Liu, Y.; Liu, K.; Zhao, R.; Chen, W.; Yusufu, A.; Liu, Y. Diabetes mellitus impairs bone regeneration and biomechanics. J. Orthop. Surg. Res. 2023, 18, 169. [Google Scholar] [CrossRef] [PubMed]
- Mulinari-Santos, G.; Batista, F.R.S.; Kirchweger, F.; Tangl, S.; Gruber, R.; Okamoto, R. Losartan reverses impaired osseointegration in spontaneously hypertensive rats. Clin. Oral Implants Res. 2018, 29, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Lemos, C.A.A.; de Oliveira, A.S.; Faé, D.S.; Oliveira, H.F.F.E.; Rosa, C.D.D.R.D.; Bento, V.A.A.; Verri, F.R.; Pellizzer, E.P. Do dental implants placed in patients with osteoporosis have higher risks of failure and marginal bone loss compared to those in healthy patients? A systematic review with meta-analysis. Clin. Oral Investig. 2023, 27, 2483–2493. [Google Scholar] [CrossRef]
- Zhao, Z.H.; Ma, X.L.; Ma, J.X.; Kang, J.Y.; Zhang, Y.; Guo, Y. Sustained release of naringin from silk-fibroin-nanohydroxyapatite scaffold for the enhancement of bone regeneration. Mater. Today Bio 2022, 13, 100206. [Google Scholar] [CrossRef]
- Duarte, N.D.; Mulinari-Santos, G.; Batista, F.R.d.S.; Gomes, M.B.; Monteiro, N.G.; Silva, A.C.E.; Gruber, R.; Lisboa-Filho, P.N.; Gomes-Ferreira, P.H.S.; Okamoto, R. Sonification of Deproteinized Bovine Bone Functionalized with Genistein Enhances Bone Repair in Peri-Implant Bone Defects in Ovariectomized Rats. J. Funct. Biomater. 2024, 15, 328. [Google Scholar] [CrossRef]
- Gomes-Ferreira, P.H.S.; de Oliveira, D.; Frigério, P.B.; de Souza Batista, F.R.; Grandfield, K.; Okamoto, R. Teriparatide improves microarchitectural characteristics of peri-implant bone in orchiectomized rats. Osteoporos. Int. 2020, 31, 1807–1815. [Google Scholar] [CrossRef]
- Gomes-Ferreira, P.H.S.; Frigério, P.B.; de Moura, J.; Duarte, N.D.; de Oliveira, D.; Deering, J.; Grandfield, K.; Okamoto, R. Evaluation of Vitamin D isolated or Associated with Teriparatide in Peri-Implant Bone Repair in Tibia of Orchiectomized Rats. Biology 2023, 12, 228. [Google Scholar] [CrossRef]
- Ogueri, K.S.; Jafari, T.; Escobar Ivirico, J.L.; Laurencin, C.T. Polymeric biomaterials for scaffold-based bone regenerative engineering. Regen. Eng. Transl. Med. 2019, 5, 128–154. [Google Scholar] [CrossRef]
- Athanasiadou, D.; Meshry, N.; Monteiro, N.G.; Ervolino-Silva, A.C.; Chan, R.L.; McCulloch, C.A.; Okamoto, R.; Carneiro, K.M.M. DNA hydrogels for bone regeneration. Proc. Natl. Acad. Sci. USA 2023, 120, e2220565120. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.S.; Dos Santos, A.B.; Carvalho, M.S. New Insights in Hydrogels for Periodontal Regeneration. J. Funct. Biomater. 2023, 14, 545. [Google Scholar] [CrossRef] [PubMed]
- Lange, M.; Babczyk, P.; Tobiasch, E. Exosomes: A New Hope for Angiogenesis-Mediated Bone Regeneration. Int. J. Mol. Sci. 2024, 25, 5204. [Google Scholar] [CrossRef] [PubMed]
- Santos, N.C.C.D.; Cotrim, K.C.; Achôa, G.L.; Kalil, E.C.; Kantarci, A.; Bueno, D.F. The Use of Mesenchymal Stromal/Stem Cells (MSC) for Periodontal and Peri-implant Regeneration: Scoping Review. Braz. Dent. J. 2024, 35, e246134. [Google Scholar] [CrossRef]
- Queiroz, A.; Albuquerque-Souza, E.; Gasparoni, L.M.; de França, B.N.; Pelissari, C.; Trierveiler, M.; Holzhausen, M. Therapeutic potential of periodontal ligament stem cells. World J. Stem Cells 2021, 13, 605–618. [Google Scholar] [CrossRef]
- Petrus-Reure, S.; Romano, M.; Howlett, S.; Jones, J.L.; Lombardi, G.; Saeb-Parsy, K. Immunological considerations and challenges for regenerative cellular therapies. Commun. Biol. 2021, 4, 798. [Google Scholar]
- Howard, D.; Buttery, L.D.; Shakesheff, K.M.; Roberts, S.J. Tissue engineering: Strategies, stem cells and scaffolds. J. Anat. 2008, 213, 66–72. [Google Scholar] [CrossRef]
- Shah, P.; Aghazadeh, M.; Rajasingh, S.; Dixon, D.; Jain, V.; Rajasingh, J. Stem cells in regenerative dentistry: Current understanding and future directions. J. Oral Biosci. 2024, 66, 288–299. [Google Scholar] [CrossRef]
- Panda, S.; Doraiswamy, J.; Malaiappan, S.; Varghese, S.S.; Del Fabbro, M. Additive effect of autologous platelet concentrates in treatment of intrabony defects: A systematic review and meta-analysis. J. Investig. Clin. Dent. 2016, 7, 13–26. [Google Scholar] [CrossRef]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, e37–e44. [Google Scholar] [CrossRef]
- Baca-Gonzalez, L.; Serrano Zamora, R.; Rancan, L.; González Fernández-Tresguerres, F.; Fernández-Tresguerres, I.; López-Pintor, R.M.; López-Quiles, J.; Leco, I.; Torres, J. Plasma rich in growth factors (PRGF) and leukocyte-platelet rich fibrin (L-PRF): Comparative release of growth factors and biological effect on osteoblasts. Int. J. Implant Dent. 2022, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- do Lago, E.S.; Ferreira, S.; Garcia, I.R., Jr.; Okamoto, R.; Mariano, R.C. Improvement of bone repair with l-PRF and bovine bone in calvaria of rats. histometric and immunohistochemical study. Clin. Oral Investig. 2020, 24, 1637–1650. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Cao, L.; Li, H. Effects of platelet-rich fibrin combined with guided bone regeneration in the reconstruction of peri-implantitis bone defect. Am. J. Transl. Res. 2021, 13, 8397–8402. [Google Scholar] [PubMed]
- Marew, T.; Birhanu, G. Three-dimensional printed nanostructure biomaterials for bone tissue engineering. Regen. Ther. 2021, 18, 102–111. [Google Scholar] [CrossRef]
- Lam, E.H.Y.; Yu, F.; Zhu, S.; Wang, Z. 3D Bioprinting for Next-Generation Personalized Medicine. Int. J. Mol. Sci. 2023, 24, 6357. [Google Scholar] [CrossRef]
- Slavin, B.V.; Ehlen, Q.T.; Costello, J.P.; Nayak, V.V.; Bonfante, E.A.; Benalcázar Jalkh, E.B.; Runyan, C.M.; Witek, L.; Coelho, P.G. 3D Printing Applications for Craniomaxillofacial Reconstruction: A Sweeping Review. ACS Biomater. Sci. Eng. 2023, 9, 6586–6609. [Google Scholar] [CrossRef]
- Paludetto, L.V.; Monteiro, N.G.; Breseghello, I.; Batista, F.R.S.; Antoniali, C.; Lisboa-Filho, P.N.; Okamoto, R. Smart Delivery of Biomolecules Interfering with Peri-Implant Repair in Osteoporotic Rats. Int. J. Mol. Sci. 2024, 25, 8963. [Google Scholar] [CrossRef]
- Miron, R.J.; Pikos, M.A.; Estrin, N.E.; Kobayashi-Fujioka, M.; Espinoza, A.R.; Basma, H.; Zhang, Y. Extended platelet-rich fibrin. Periodontol. 2000 2024, 94, 114–130. [Google Scholar] [CrossRef]
- Palioto, D.B.; Finoti, L.S.; Kinane, D.F.; Benakanakere, M. Epigenetic and inflammatory events in experimental periodontitis following systemic microbial challenge. J. Clin. Periodontol. 2019, 46, 819–829. [Google Scholar] [CrossRef]
- Gradinaru, L.M.; Barbalata-Mandru, M.; Enache, A.A.; Rimbu, C.M.; Badea, G.I.; Aflori, M. Chitosan Membranes Containing Plant Extracts: Preparation, Characterization and Antimicrobial Properties. Int. J. Mol. Sci. 2023, 24, 8673. [Google Scholar] [CrossRef]
- Eo, M.Y.; Fan, H.; Cho, Y.J.; Kim, S.M.; Lee, S.K. Cellulose membrane as a biomaterial: From hydrolysis to depolymerization with electron beam. Biomater. Res. 2016, 20, 16. [Google Scholar] [CrossRef] [PubMed]
- Popal, Z.; Nickel, K.F.; Wöltje, M.; Aibibu, D.; Knipfer, C.; Smeets, R.; Renné, T. Polyphosphate-loaded silk fibroin membrane as hemostatic agent in oral surgery: A pilot study. Int. J. Implant Dent. 2023, 9, 44. [Google Scholar] [CrossRef] [PubMed]

| Commercial Name | Manufacturer | Composition | Reference | |
|---|---|---|---|---|
| e-PTFE | Gore-Tex® | W.L. Gore & Associates | Expanded polytetrafluoroethylene | [22] |
| TefGen® | Geistlich Pharma | [22] | ||
| d-PTFE | Cytoplast® | Osteogenics Biomedical | High-density polytetrafluoroethylen | [22] |
| Permamem® | Botiss Biomaterials | [22] | ||
| OsseoGuard® | Zimmer Biomet | [22] | ||
| Ti-PTFE | Cytoplast® Ti-150 | Osteogenics Biomedical | Polytetrafluoroethylene + titanium | [22] |
| Cytoflex® Ti-reinforced | Unicare Biomedical | [22] | ||
| OpenTex®-TR | Purgo Biologics | [24] |
| Commercial Name | Manufacturer | Collagen Type | Collagen Source | Reference | |
|---|---|---|---|---|---|
| Cross-linked | BioGide® | Geistlich Pharma | I and III | Porcine | [46] |
| OSSIX Plus® | Dentsply Sirona | I | Porcine | [46] | |
| OsseoGuard® | Zimmer Biomet | I | Bovine | [47] | |
| Non-cross-linked | CollaTape® | Zimmer Biomet | I | Bovine | [29] |
| Jason® | Straumann | I and III | Porcine | [48] | |
| Mucograft® | Geistlich Pharma | I and III | Porcine | [49] |
| Commercial Name | Manufacturer | Composition | Reference |
|---|---|---|---|
| Plenum® Guide | Plenum | Polydioxanone | [52] |
| Resolut Adapt® | W.L. Gore & Associates | Poly-d,l-lactide and co-glycolide | [53] |
| Guidor® | Sunstar Americas | Poly-d,l-lactide and Poly-l-lactide blended with Acetyl tri-n-butyl Citrate | [53] |
| Epi-Guide® | Curasan Inc. | Poly-d,l-lactic acid | [53] |
| Vivosorb® | Polyganics | Poly(d,l-lactide-ɛ-caprolactone) | [53] |
| Commercial Name | Manufacturer | Deproteinized Bone Origin | Reabsorption Rate | Reference |
|---|---|---|---|---|
| Bio-Oss® | Geistlich Pharma | Bovine | 5–10 years | [84] |
| Cerabone® | Botiss Biomaterials | Bovine | ±10 years | [85] |
| Endobon® | Zimmer Biomet | Bovine | ±10 years | [86] |
| OsteoBiol® Equimatrix | Tecnoss | Equine | 6–12 months | [87] |
| OsteoBiol® Gen-Os | Tecnoss | Porcine | 4–6 months | [87] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duarte, N.D.; Frigério, P.B.; Chica, G.E.A.; Okamoto, R.; Buchaim, R.L.; Buchaim, D.V.; Messora, M.R.; Issa, J.P.M. Biomaterials for Guided Tissue Regeneration and Guided Bone Regeneration: A Review. Dent. J. 2025, 13, 179. https://doi.org/10.3390/dj13040179
Duarte ND, Frigério PB, Chica GEA, Okamoto R, Buchaim RL, Buchaim DV, Messora MR, Issa JPM. Biomaterials for Guided Tissue Regeneration and Guided Bone Regeneration: A Review. Dentistry Journal. 2025; 13(4):179. https://doi.org/10.3390/dj13040179
Chicago/Turabian StyleDuarte, Nathália Dantas, Paula Buzo Frigério, Gloria Estefania Amaya Chica, Roberta Okamoto, Rogério Leone Buchaim, Daniela Vieira Buchaim, Michel Reis Messora, and João Paulo Mardegan Issa. 2025. "Biomaterials for Guided Tissue Regeneration and Guided Bone Regeneration: A Review" Dentistry Journal 13, no. 4: 179. https://doi.org/10.3390/dj13040179
APA StyleDuarte, N. D., Frigério, P. B., Chica, G. E. A., Okamoto, R., Buchaim, R. L., Buchaim, D. V., Messora, M. R., & Issa, J. P. M. (2025). Biomaterials for Guided Tissue Regeneration and Guided Bone Regeneration: A Review. Dentistry Journal, 13(4), 179. https://doi.org/10.3390/dj13040179













