Negative Pressure Wound Therapy in Maxillofacial Applications
Abstract
:1. Introduction
1.1. Wound Healing
1.2. Wound Dressings
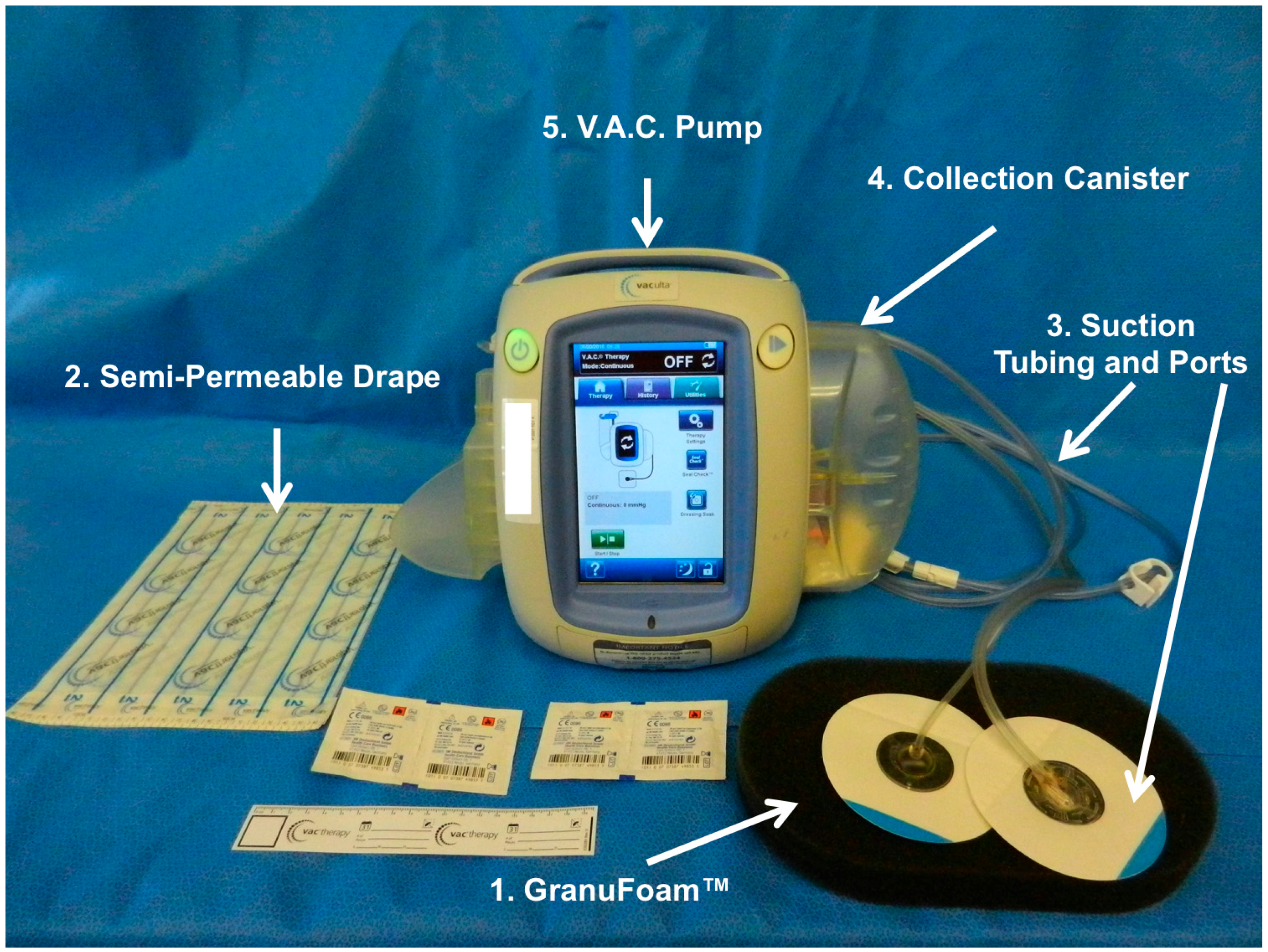
1.3. Vacuum Assisted Closure
2. V.A.C. Mechanisms of Action
2.1. Macrostrain
2.2. Microstrain
2.3. Fluid Removal and Edema Reduction
2.4. Reduction of Infectious Material
2.5. Wound Stabilization and Secondary Events
3. Maxillofacial Considerations
3.1. Upper Third Maxillofacial Reconstruction
3.2. Middle Third Maxillofacial Reconstruction
3.3. Lower Third Maxillofacial Reconstruction
3.4. Neck Reconstruction
3.5. Technical Considerations
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Banwell, P.; Withey, S.; Holten, I. The use of negative pressure to promote healing. Br. J. Plast. Surg. 1998, 51, 79. [Google Scholar] [CrossRef]
- Orgill, D.P.; Bayer, L.R. Update on negative-pressure wound therapy. Plast. Reconstr. Surg. 2011, 127 (Suppl. 1), 105S–115S. [Google Scholar] [CrossRef] [PubMed]
- Asher, S.A.; White, H.N.; Golden, J.B.; Magnuson, J.S.; Carroll, W.R.; Rosenthal, E.L. Negative pressure wound therapy in head and neck surgery. JAMA Facial Plast. Surg. 2014, 16, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.G.; Andros, G. Use of negative pressure wound therapy to help facilitate limb preservation. Int. Wound J. 2012, 9 (Suppl. 1), 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dumville, J.C.; Land, L.; Evans, D.; Peinemann, F. Negative pressure wound therapy for treating leg ulcers. Cochrane Database Syst. Rev. 2015, 7, CD011354. [Google Scholar] [PubMed]
- Evans, D.; Land, L. Topical negative pressure for treating chronic wounds: A systematic review. Br. J. Plast. Surg. 2001, 54, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Stannard, J.P.; Gabriel, A.; Lehner, B. Use of negative pressure wound therapy over clean, closed surgical incisions. Int. Wound J. 2012, 9 (Suppl. 1), 32–39. [Google Scholar] [CrossRef] [PubMed]
- Poglio, G.; Grivetto, F.; Nicolotti, M.; Arcuri, F.; Benech, A. Management of an exposed mandibular plate after fibula free flap with vacuum-assisted closure system. J. Craniofac. Surg. 2011, 22, 905–908. [Google Scholar] [CrossRef] [PubMed]
- Strub, G.M.; Moe, K.S. The use of negative-pressure therapy in the closure of complex head and neck wounds. Facial Plast. Surg. Clin. N. Am. 2013, 21, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.W.; Yi, K.I.; Kang, J.H.; Kim, S.G.; Cha, W. Negative pressure wound therapy for cervical esophageal perforation with abscess. Auris Nasus Larynx 2015, 42, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Agha, R.; Ogawa, R.; Pietramaggiori, G.; Orgill, D.P. A review of the role of mechanical forces in cutaneous wound healing. J. Surg. Res. 2011, 171, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.F.; Bartolo, P.J. Traditional therapies for skin wound healing. Adv. Wound Care (New Rochelle) 2016, 5, 208–229. [Google Scholar] [CrossRef] [PubMed]
- Rittie, L. Cellular mechanisms of skin repair in humans and other mammals. J. Cell Commun. Signal. 2016, 10, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Silver, I.A. The mechanics of wound healing. Equine Vet. J. 1979, 11, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Valero, C.; Javierre, E.; Garcia-Aznar, J.M.; Menzel, A.; Gomez-Benito, M.J. Challenges in the modeling of wound healing mechanisms in soft biological tissues. Ann. Biomed. Eng. 2015, 43, 1654–1665. [Google Scholar] [CrossRef] [PubMed]
- Broughton, G., 2nd; Janis, J.E.; Attinger, C.E. The basic science of wound healing. Plast. Reconstr. Surg. 2006, 117, 12S–34S. [Google Scholar] [CrossRef] [PubMed]
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Borregaard, N.; Wynn, T.A. Phenotypic and functional plasticity of cells of innate immunity: Macrophages, mast cells and neutrophils. Nat. Immunol. 2011, 12, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Jameson, J.; Havran, W.L. Skin gammadelta T-cell functions in homeostasis and wound healing. Immunol. Rev. 2007, 215, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Vannella, K.M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Lech, M.; Anders, H.J. Macrophages and fibrosis: How resident and infiltrating mononuclear phagocytes orchestrate all phases of tissue injury and repair. Biochim. Biophys. Acta 2013, 1832, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Diegelmann, R.F.; Evans, M.C. Wound healing: An overview of acute, fibrotic and delayed healing. Front. Biosci. 2004, 9, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Pietramaggiori, G.; Liu, P.; Scherer, S.S.; Kaipainen, A.; Prsa, M.J.; Mayer, H.; Newalder, J.; Alperovich, M.; Mentzer, S.J.; Konerding, M.A.; et al. Tensile forces stimulate vascular remodeling and epidermal cell proliferation in living skin. Ann. Surg. 2007, 246, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Tomasek, J.J.; Gabbiani, G.; Hinz, B.; Chaponnier, C.; Brown, R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 2002, 3, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.A. Cutaneous tissue repair: Basic biologic considerations. I. J. Am. Acad. Dermatol. 1985, 13, 701–725. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Yoshikawa, K. Cutaneous wound healing: An update. J. Dermatol. 2001, 28, 521–534. [Google Scholar] [PubMed]
- Bochaton-Piallat, M.L.; Gabbiani, G.; Hinz, B. The myofibroblast in wound healing and fibrosis: Answered and unanswered questions. F1000 Res. 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Pennacchi, P.C.; Almeida, M.E.; Gomes, O.L.; Faiao-Flores, F.; Crepaldi, M.C.; Dos Santos, M.F.; Barros, S.B.; Maria-Engler, S.S. Glycated reconstructed human skin as a platform to study the pathogenesis of skin aging. Tissue Eng. Part A 2015, 21, 2417–2425. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Tredget, E.E. The role of chemokines in fibrotic wound healing. Adv. Wound Care (New Rochelle) 2015, 4, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, R.; Akaishi, S.; Kuribayashi, S.; Miyashita, T. Keloids and hypertrophic scars can now be cured completely: Recent progress in our understanding of the pathogenesis of keloids and hypertrophic scars and the most promising current therapeutic strategy. J. Nippon Med. Sch. 2016, 83, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Trace, A.P.; Enos, C.W.; Mantel, A.; Harvey, V.M. Keloids and hypertrophic scars: A spectrum of clinical challenges. Am. J. Clin. Dermatol. 2016, 17, 201–223. [Google Scholar] [CrossRef] [PubMed]
- Winter, G.D. Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature 1962, 193, 293–294. [Google Scholar] [CrossRef] [PubMed]
- Dabiri, G.; Damstetter, E.; Phillips, T. Choosing a wound dressing based on common wound characteristics. Adv. Wound Care (New Rochelle) 2016, 5, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Gould, L.J. Topical collagen-based biomaterials for chronic wounds: Rationale and clinical application. Adv. Wound Care (New Rochelle) 2016, 5, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Maessen-Visch, M.B.; van Montfrans, C. Wound dressings, does it matter and why? Phlebology 2016, 31, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Mayet, N.; Choonara, Y.E.; Kumar, P.; Tomar, L.K.; Tyagi, C.; Du Toit, L.C.; Pillay, V. A comprehensive review of advanced biopolymeric wound healing systems. J. Pharm. Sci. 2014, 103, 2211–2230. [Google Scholar] [CrossRef] [PubMed]
- Ovington, L.G. Advances in wound dressings. Clin. Dermatol. 2007, 25, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Li, W.R.; Xie, X.B.; Shi, Q.S.; Duan, S.S.; Ouyang, Y.S.; Chen, Y.B. Antibacterial effect of silver nanoparticles on staphylococcus aureus. Biometals 2011, 24, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Abrigo, M.; McArthur, S.L.; Kingshott, P. Electrospun nanofibers as dressings for chronic wound care: Advances, challenges, and future prospects. Macromol. Biosci. 2014, 14, 772–792. [Google Scholar] [CrossRef] [PubMed]
- Berkland, C.; Kim, K.; Pack, D.W. Fabrication of PLG microspheres with precisely controlled and monodisperse size distributions. J. Control. Release 2001, 73, 59–74. [Google Scholar] [CrossRef]
- Berkland, C.; Kim, K.; Pack, D.W. PLG microsphere size controls drug release rate through several competing factors. Pharm. Res. 2003, 20, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L.; McCarty, S.M. Silver and alginates: Role in wound healing and biofilm control. Adv. Wound Care (New Rochelle) 2015, 4, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Salamone, J.C.; Salamone, A.B.; Swindle-Reilly, K.; Leung, K.X.; McMahon, R.E. Grand challenge in biomaterials-wound healing. Regen. Biomater. 2016, 3, 127–128. [Google Scholar] [CrossRef] [PubMed]
- Tartarini, D.; Mele, E. Adult stem cell therapies for wound healing: Biomaterials and computational models. Front. Bioeng. Biotechnol. 2015, 3, 206. [Google Scholar] [CrossRef] [PubMed]
- Argenta, L.C.; Morykwas, M.J. Vacuum-assisted closure: A new method for wound control and treatment: Clinical experience. Ann. Plast. Surg. 1997, 38, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Morykwas, M.J.; Argenta, L.C.; Shelton-Brown, E.I.; McGuirt, W. Vacuum-assisted closure: A new method for wound control and treatment: Animal studies and basic foundation. Ann. Plast. Surg. 1997, 38, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S. Optimal use of negative pressure wound therapy for skin grafts. Int. Wound J. 2012, 9 (Suppl. 1), 40–47. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Eastman, S. Negative pressure wound therapy. Plast. Surg. Nurs. 1998, 18, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Orgill, D.P.; Bayer, L.R. Negative pressure wound therapy: Past, present and future. Int. Wound J. 2013, 10 (Suppl. 1), 15–19. [Google Scholar] [CrossRef] [PubMed]
- Wood, B.C.; Molnar, J.A. Subatmospheric pressure therapy: Basic science review. J. Surg. Orthop. Adv. 2011, 20, 168–175. [Google Scholar] [PubMed]
- Huang, C.; Leavitt, T.; Bayer, L.R.; Orgill, D.P. Effect of negative pressure wound therapy on wound healing. Curr. Probl. Surg. 2014, 51, 301–331. [Google Scholar] [CrossRef] [PubMed]
- Scherer, S.S.; Pietramaggiori, G.; Mathews, J.C.; Prsa, M.J.; Huang, S.; Orgill, D.P. The mechanism of action of the vacuum-assisted closure device. Plast. Reconstr. Surg. 2008, 122, 786–797. [Google Scholar] [CrossRef] [PubMed]
- Subotic, U.; Kluwe, W.; Oesch, V. Community-associated methicillin-resistant staphylococcus aureus-infected chronic scalp wound with exposed dura in a 10-year-old boy: Vacuum-assisted closure is a feasible option: Case report. Neurosurgery 2011, 68, E1481–E1483. [Google Scholar] [CrossRef] [PubMed]
- Kairinos, N.; Solomons, M.; Hudson, D.A. Negative-pressure wound therapy I: The paradox of negative-pressure wound therapy. Plast. Reconstr. Surg. 2009, 123, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Kairinos, N.; Solomons, M.; Hudson, D.A. The paradox of negative pressure wound therapy–in vitro studies. J. Plast. Reconstr. Aesthet. Surg. 2010, 63, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Kairinos, N.; Voogd, A.M.; Botha, P.H.; Kotze, T.; Kahn, D.; Hudson, D.A.; Solomons, M. Negative-pressure wound therapy II: Negative-pressure wound therapy and increased perfusion. Just an illusion? Plast. Reconstr. Surg. 2009, 123, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Lancerotto, L.; Bayer, L.R.; Orgill, D.P. Mechanisms of action of microdeformational wound therapy. Semin. Cell Dev. Biol. 2012, 23, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Holfeld, J.; Schaden, W.; Orgill, D.; Ogawa, R. Mechanotherapy: Revisiting physical therapy and recruiting mechanobiology for a new era in medicine. Trends Mol. Med. 2013, 19, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Saxena, V.; Hwang, C.W.; Huang, S.; Eichbaum, Q.; Ingber, D.; Orgill, D.P. Vacuum-assisted closure: Microdeformations of wounds and cell proliferation. Plast. Reconstr. Surg. 2004, 114, 1086–1096. [Google Scholar] [CrossRef] [PubMed]
- Hanson, D.; Langemo, D.; Thompson, P.; Anderson, J.; Hunter, S. Understanding wound fluid and the phases of healing. Adv. Skin Wound Care 2005, 18, 360–362. [Google Scholar] [CrossRef] [PubMed]
- Kadohama, T.; Nishimura, K.; Hoshino, Y.; Sasajima, T.; Sumpio, B.E. Effects of different types of fluid shear stress on endothelial cell proliferation and survival. J. Cell. Physiol. 2007, 212, 244–251. [Google Scholar] [CrossRef] [PubMed]
- DeFranzo, A.J.; Argenta, L.C.; Marks, M.W.; Molnar, J.A.; David, L.R.; Webb, L.X.; Ward, W.G.; Teasdall, R.G. The use of vacuum-assisted closure therapy for the treatment of lower-extremity wounds with exposed bone. Plast. Reconstr. Surg. 2001, 108, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Weed, T.; Ratliff, C.; Drake, D.B. Quantifying bacterial bioburden during negative pressure wound therapy: Does the wound VAC enhance bacterial clearance? Ann. Plast. Surg. 2004, 52, 276–279. [Google Scholar] [CrossRef] [PubMed]
- Scalise, A.; Bianchi, A.; Tartaglione, C.; Bolletta, E.; Pierangeli, M.; Torresetti, M.; Marazzi, M.; di Benedetto, G. Microenvironment and microbiology of skin wounds: The role of bacterial biofilms and related factors. Semin. Vasc. Surg. 2015, 28, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Daeschlein, G. Antimicrobial and antiseptic strategies in wound management. Int. Wound J. 2013, 10 (Suppl. 1), 9–14. [Google Scholar] [CrossRef] [PubMed]
- Reed, B.R.; Clark, R.A. Cutaneous tissue repair: Practical implications of current knowledge. II. J. Am. Acad. Dermatol. 1985, 13, 919–941. [Google Scholar] [CrossRef]
- Mertz, P.M.; Eaglstein, W.H. The effect of a semiocclusive dressing on the microbial population in superficial wounds. Arch. Surg. 1984, 119, 287–289. [Google Scholar] [CrossRef] [PubMed]
- Moues, C.M.; Vos, M.C.; van den Bemd, G.J.; Stijnen, T.; Hovius, S.E. Bacterial load in relation to vacuum-assisted closure wound therapy: A prospective randomized trial. Wound Repair Regen. 2004, 12, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Sachsenmaier, S.; Peschel, A.; Ipach, I.; Kluba, T. Antibacterial potency of V.A.C. Granufoam silver(®) dressing. Injury 2013, 44, 1363–1367. [Google Scholar] [CrossRef] [PubMed]
- Potter, M.J.; Banwell, P.; Baldwin, C.; Clayton, E.; Irvine, L.; Linge, C.; Grobbelaar, A.O.; Sanders, R.; Dye, J.F. In vitro optimisation of topical negative pressure regimens for angiogenesis into synthetic dermal replacements. Burns 2008, 34, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Petzina, R.; Gustafsson, L.; Mokhtari, A.; Ingemansson, R.; Malmsjo, M. Effect of vacuum-assisted closure on blood flow in the peristernal thoracic wall after internal mammary artery harvesting. Eur. J. Cardiothoracic. Surg. 2006, 30, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Nuutila, K.; Siltanen, A.; Peura, M.; Harjula, A.; Nieminen, T.; Vuola, J.; Kankuri, E.; Aarnio, P. Gene expression profiling of negative-pressure-treated skin graft donor site wounds. Burns 2013, 39, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Arti, H.; Khorami, M.; Ebrahimi-Nejad, V. Comparison of negative pressure wound therapy (NPWT) & conventional wound dressings in the open fracture wounds. Pak. J. Med. Sci. 2016, 32, 65–69. [Google Scholar] [PubMed]
- Nolff, M.C.; Fehr, M.; Reese, S.; Meyer-Lindenberg, A.E. Retrospective comparison of negative-pressure wound therapy and silver-coated foam dressings in open-wound treatment in cats. J. Feline Med. Surg. 2016. [Google Scholar] [CrossRef] [PubMed]
- Andrews, B.T.; Smith, R.B.; Goldstein, D.P.; Funk, G.F. Management of complicated head and neck wounds with vacuum-assisted closure system. Head Neck 2006, 28, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Schuster, R.; Moradzadeh, A.; Waxman, K. The use of vacuum-assisted closure therapy for the treatment of a large infected facial wound. Am. Surg. 2006, 72, 129–131. [Google Scholar] [PubMed]
- Palm, H.G.; Hauer, T.; Simon, C.; Willy, C. Vacuum-assisted closure of head and neck wounds. HNO 2011, 59, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Satteson, E.S.; Crantford, J.C.; Wood, J.; David, L.R. Outcomes of vacuum-assisted therapy in the treatment of head and neck wounds. J. Craniofac. Surg. 2015, 26, e599–e602. [Google Scholar] [CrossRef] [PubMed]
- Hsia, J.C.; Moe, K.S. Vacuum-assisted closure therapy for reconstruction of soft-tissue forehead defects. Arch. Facial Plast. Surg. 2011, 13, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Swan, M.C.; Flynn, M.; Tiernan, E.P. Retained vac therapy sponge as a complication of abdominoplasty. J. Plast. Reconstr. Aesthet. Surg. 2010, 63, e497–e499. [Google Scholar] [CrossRef] [PubMed]
- Powers, A.K.; Neal, M.T.; Argenta, L.C.; Wilson, J.A.; DeFranzo, A.J.; Tatter, S.B. Vacuum-assisted closure for complex cranial wounds involving the loss of dura mater. J. Neurosurg. 2013, 118, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Prince, N.; Blackburn, S.; Murad, G.; Mast, B.; Sapountzis, S.; Shaw, C.; Werning, J.; Singhal, D. Vacuum-assisted closure therapy to the brain: A safe method for wound temporization in composite scalp and calvarial defects. Ann. Plast. Surg. 2015, 74 (Suppl. 4), S218–S221. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.M.; Yang, Z.H.; Zhuang, P.L.; Wang, Y.Y.; Chen, W.L.; Zhang, B. Role of negative-pressure wound therapy in the management of submandibular fistula after reconstruction for osteoradionecrosis. J. Oral Maxillofac. Surg. 2016, 74, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Jeng, S.F.; Hsieh, C.H.; Feng, G.M.; Chen, C.C. Vacuum-assisted closure for complicated wounds in head and neck region after reconstruction. J. Plast. Reconstr. Aesthet. Surg. 2013, 66, e209–e216. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Khoo, D.; Tay, A.C.; Soo, K.C.; Tan, N.C.; Tan, H.K.; Iyer, N.G. Management of orocutaneous fistulas using a vacuum-assisted closure system. Head Neck 2014, 36, 873–881. [Google Scholar] [CrossRef] [PubMed]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mellott, A.J.; Zamierowski, D.S.; Andrews, B.T. Negative Pressure Wound Therapy in Maxillofacial Applications. Dent. J. 2016, 4, 30. https://doi.org/10.3390/dj4030030
Mellott AJ, Zamierowski DS, Andrews BT. Negative Pressure Wound Therapy in Maxillofacial Applications. Dentistry Journal. 2016; 4(3):30. https://doi.org/10.3390/dj4030030
Chicago/Turabian StyleMellott, Adam J., David S. Zamierowski, and Brian T. Andrews. 2016. "Negative Pressure Wound Therapy in Maxillofacial Applications" Dentistry Journal 4, no. 3: 30. https://doi.org/10.3390/dj4030030
APA StyleMellott, A. J., Zamierowski, D. S., & Andrews, B. T. (2016). Negative Pressure Wound Therapy in Maxillofacial Applications. Dentistry Journal, 4(3), 30. https://doi.org/10.3390/dj4030030




