Effect of Methods of Biosilicate Microparticle Application on Dentin Adhesion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Outline
Ethical Aspects
2.2. Selection and Preparation of Teeth
2.3. Dentin Surface Treatments
2.4. Restorative Procedure
2.5. Microtensile Bond Strength Test
2.6. Statistical Analysis
2.7. Failure Mode Analysis
2.8. SEM Analysis of the Adhesive/Dentine Interfaces
3. Results
3.1. Microtensile Bond Strength Test
3.2. Failure Mode Analysis
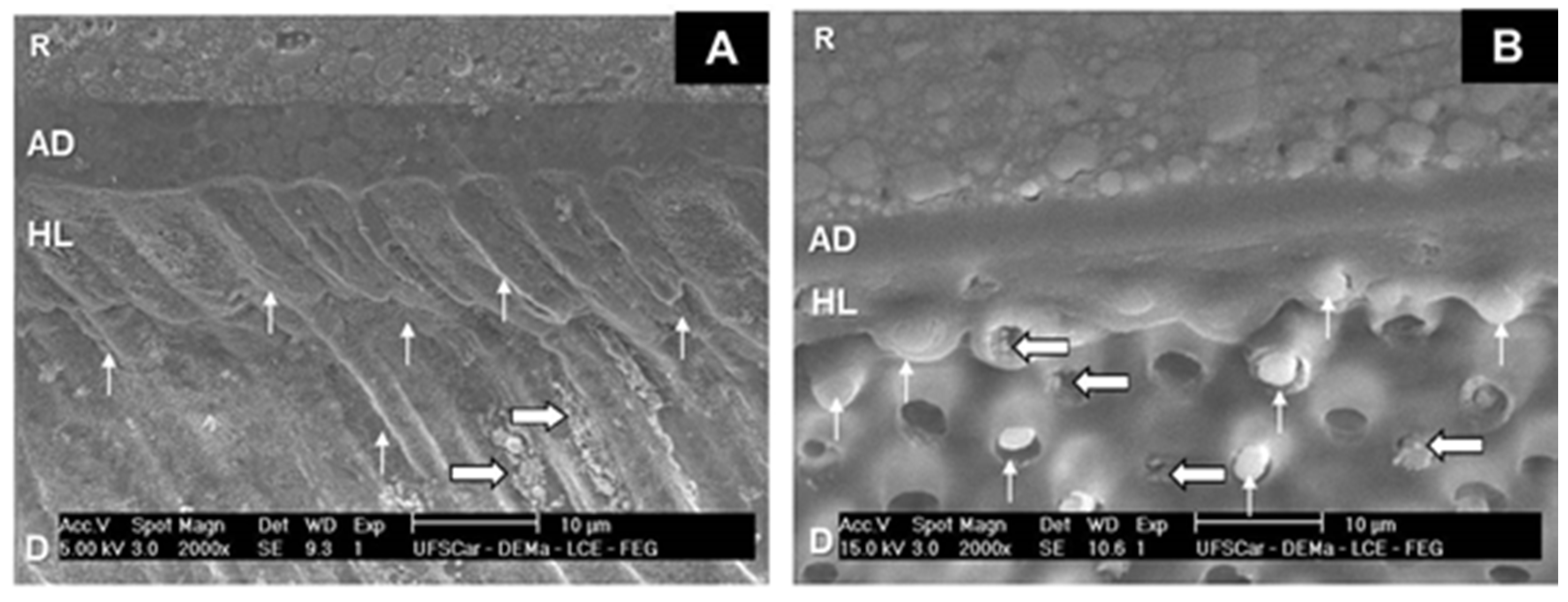
3.3. SEM Analysis of Adhesive Interfaces
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Mackenzie, L.; Banerjee, A. The minimally invasive management of early occlusal caries: A practical guide. Prim. Dent. J. 2014, 3, 34–41. [Google Scholar] [CrossRef]
- Peters, M.C.; McLean, M.E. Minimally invasive operative care. II. Contemporary techniques and materials: An overview. J. Adhes. Dent. 2001, 3, 17–31. [Google Scholar] [PubMed]
- Peters, M.C.; Bresciani, E.; Barata, T.J.; Fagundes, T.C.; Navarro, R.L.; Navarro, M.F.; Dickens, S.H. In vivo dentin remineralization by calcium-phosphate cement. J. Dent. Res. 2010, 89, 286–291. [Google Scholar] [CrossRef]
- Banerjee, A.; Paolinelis, G.; Socker, M.; McDonald, F.; Watson, T.F. An in vitro investigation of the effectiveness of bioactive glass air-abrasion in the ‘selective’ removal of orthodontic resin adhesive. Eur. J. Oral Sci. 2008, 116, 488–492. [Google Scholar] [CrossRef]
- Bresciani, E.; Wagner, W.C.; Navarro, M.F.; Dickens, S.H.; Peters, M.C. In vivo dentin microhardness beneath a calcium-phosphate cement. J. Dent. Res. 2010, 89, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Gillam, D.G.; Tang, J.Y.; Mordan, N.J.; Newman, H.N. The effects of a novel Bioglass dentifrice on dentine sensitivity: A scanning electron microscopy investigation. J. Oral Rehabil. 2002, 29, 305–313. [Google Scholar] [CrossRef]
- Hench, L.L. The story of Bioglass. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef]
- Profeta, A.C.; Mannocci, F.; Foxton, R.M.; Thompson, I.; Watson, T.F.; Sauro, S. Bioactive effects of a calcium/sodium phosphosilicate on the resin-dentine interface: A microtensile bond strength, scanning electron microscopy, and confocal microscopy study. Eur. J. Oral Sci. 2012, 120, 353–362. [Google Scholar] [CrossRef]
- Pires-de-Souza, F.C.P.; Marco, F.F.; Casemiro, L.A.; Panzeri, H. Desensitizing bioactive agents improves bond strength of indirect resin-cemented restorations: Preliminary results. J. Appl. Oral Sci. 2007, 15, 120–126. [Google Scholar] [CrossRef]
- Renno, A.C.; Bossini, P.S.; Crovace, M.C.; Rodrigues, A.C.; Zanotto, E.D.; Parizotto, N.A. Characterization and in vivo biological performance of biosilicate. Biomed. Res. Int. 2013, 2013, 141427. [Google Scholar] [CrossRef]
- Crovace, M.C.; Souza, M.T.; Chinaglia, C.R.; Peitl, O.; Zanotto, E.D. Biosilicate®—A multipurpose, highly bioactive glass-ceramic. In vitro, in vivo and clinical trials. J. Non-Cryst. Solids 2016, 432, 90–110. [Google Scholar] [CrossRef]
- Tirapelli, C.; Panzeri, H.; Lara, E.H.; Soares, R.G.; Peitl, O.; Zanotto, E.D. The effect of a novel crystallized bioactive glass-ceramic powder on dentine hypersensitivity: A long-term clinical study. J. Oral Rehabil. 2011, 38, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Tirapelli, C.; Panzeri, H.; Soares, R.G.; Peitl, O.; Zanotto, E.D. A novel bioactive glass-ceramic for treating dentin hypersensitivity. Braz. Oral Res. 2010, 24, 381–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chinelatti, M.A.; Tirapelli, C.; Corona, S.A.M.; Jasinevicius, R.G.; Peitl, O.; Zanotto, E.D.; Pires-de-Souza, F.C.P. Effect of a Bioactive Glass Ceramic on the Control of Enamel and Dentin Erosion Lesions. Braz. Dent. J. 2017, 28, 489–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Morais, R.C.; Silveira, R.E.; Chinelatti, M.; Geraldeli, S.; Pires-de, F.D. Bond strength of adhesive systems to sound and demineralized dentin treated with bioactive glass ceramic suspension. Clin. Oral Investig. 2018, 22, 1923–1931. [Google Scholar] [CrossRef]
- De Morais, R.C.; Silveira, R.E.; Chinelatti, M.A.; Pires-de-Souza, F.C.P. Biosilicate as a dentin pretreatment for total-etch and self-etch adhesives: In vitro study. Int. J. Adhes. Adhes. 2016, 70, 271–276. [Google Scholar] [CrossRef]
- Sauro, S.; Watson, T.F.; Thompson, I. Dentine desensitization induced by prophylactic and air-polishing procedures: An in vitro dentine permeability and confocal microscopy study. J. Dent. 2010, 38, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Sauro, S.; Watson, T.F.; Thompson, I.; Banerjee, A. One-bottle self-etching adhesives applied to dentine air-abraded using Bioactive glasses containing polyacrylic acid: An in vitro μTBS and confocal microscopy study. J. Dent. 2012, 40, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Sauro, S.; Watson, T.F.; Thompson, I.; Toledano, M.; Nucci, C.; Banerjee, A. Influence of air-abrasion executed with polyacrylic acid-Bioglass 45S5 on the bonding performance of a resin-modified glass ionomer cement. Eur. J. Oral Sci. 2012, 120, 168–177. [Google Scholar] [CrossRef]
- Beltrami, R.; Chiesa, M.; Scribante, A.; Allegretti, J.; Poggio, C. Comparison of shear bond strength of universal adhesives on etched and nonetched enamel. J. Appl. Biomater. Funct. Mater. 2016, 14, e78–e83. [Google Scholar] [CrossRef]
- Ozer, F.; Blatz, M.B. Self-etch and etch-and-rinse adhesive systems in clinical dentistry. Compend. Contin. Educ. Dent. 2013, 34, 12–14. [Google Scholar] [PubMed]
- Sezinando, A.; Serrano, M.L.; Pérez, V.M.; Muñoz, R.A.; Ceballos, L.; Perdigão, J. Chemical Adhesion of Polyalkenoate-based Adhesives to Hydroxyapatite. J. Adhes. Dent. 2016, 18, 257–265. [Google Scholar] [PubMed]
- Tay, F.R.; Pashley, D.H. Biomimetic remineralization of resin-bonded acid-etched dentin. J. Dent. Res. 2009, 88, 719–724. [Google Scholar] [CrossRef] [PubMed]


| Material | Composition | Manufacturer |
|---|---|---|
| Biosilicate microparticles | Fully crystallized glass-ceramic of the Na2O-CaO-SiO2-P2O5 system, with additions of Li2O and K2O; 1–10 µm. | Vitrovita, São Carlos, SP, Brazil |
| Filtek™ Z350—composite resin | Inorganic Fillers: 78.5% by weight. Non-agglomerated/non-aggregated 20 nm silica filler, non-agglomerated/non-aggregated 4–11 nm zirconia filler, and aggregated zirconia/silica, cluster filler (20 nm silica and 4–11 nm zirconia). Resins: Bis-GMA *, UDMA **, Bis-EMA ***, TEGDMA ##. | 3M ESPE Dental Products, St. Paul, CA, USA |
| Adper™ Easy Bond Self-Etch Adhesive | 2-Hydroxyethyl methacryate (HEMA), Bis-GMA, methacrylated phosphoric esters, 1,6 hexanediol dimethacrylate, methacrylate functionalized, polyalkenoic acid (Vitrebond™ Copolymer), 7nm silica filler, ethanol, water, camphorquinone, stabilizers. | 3M ESPE Dental Products, St. Paul, CA, USA |
| Surface Treatment (Group) | MPa (±SD) |
|---|---|
| Biosilicate microparticles blasting (Group 1) | 28.4 (±2.3) a |
| Biosilicate microparticles paste (Group 2) | 27.7 (±2.1) a |
| Control—No treatment (Group 3) | 22.3 (±1.4) b |
| Group | Adhesive | Cohesive | Mixed |
|---|---|---|---|
| 1 | 21 | 8 | 71 |
| 2 | 20 | 11 | 69 |
| 3 | 18 | 17 | 65 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chinelatti, M.A.; Santos, E.L.; Tirapelli, C.; Pires-de-Souza, F.C.P. Effect of Methods of Biosilicate Microparticle Application on Dentin Adhesion. Dent. J. 2019, 7, 35. https://doi.org/10.3390/dj7020035
Chinelatti MA, Santos EL, Tirapelli C, Pires-de-Souza FCP. Effect of Methods of Biosilicate Microparticle Application on Dentin Adhesion. Dentistry Journal. 2019; 7(2):35. https://doi.org/10.3390/dj7020035
Chicago/Turabian StyleChinelatti, Michelle Alexandra, Egle Leitão Santos, Camila Tirapelli, and Fernanda Carvalho Panzeri Pires-de-Souza. 2019. "Effect of Methods of Biosilicate Microparticle Application on Dentin Adhesion" Dentistry Journal 7, no. 2: 35. https://doi.org/10.3390/dj7020035
APA StyleChinelatti, M. A., Santos, E. L., Tirapelli, C., & Pires-de-Souza, F. C. P. (2019). Effect of Methods of Biosilicate Microparticle Application on Dentin Adhesion. Dentistry Journal, 7(2), 35. https://doi.org/10.3390/dj7020035






