Effect of Collagen Cross-Link Deficiency on Incorporation of Grafted Bone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collagen and Animal Models
2.2. Animal Surgery
2.3. Histology
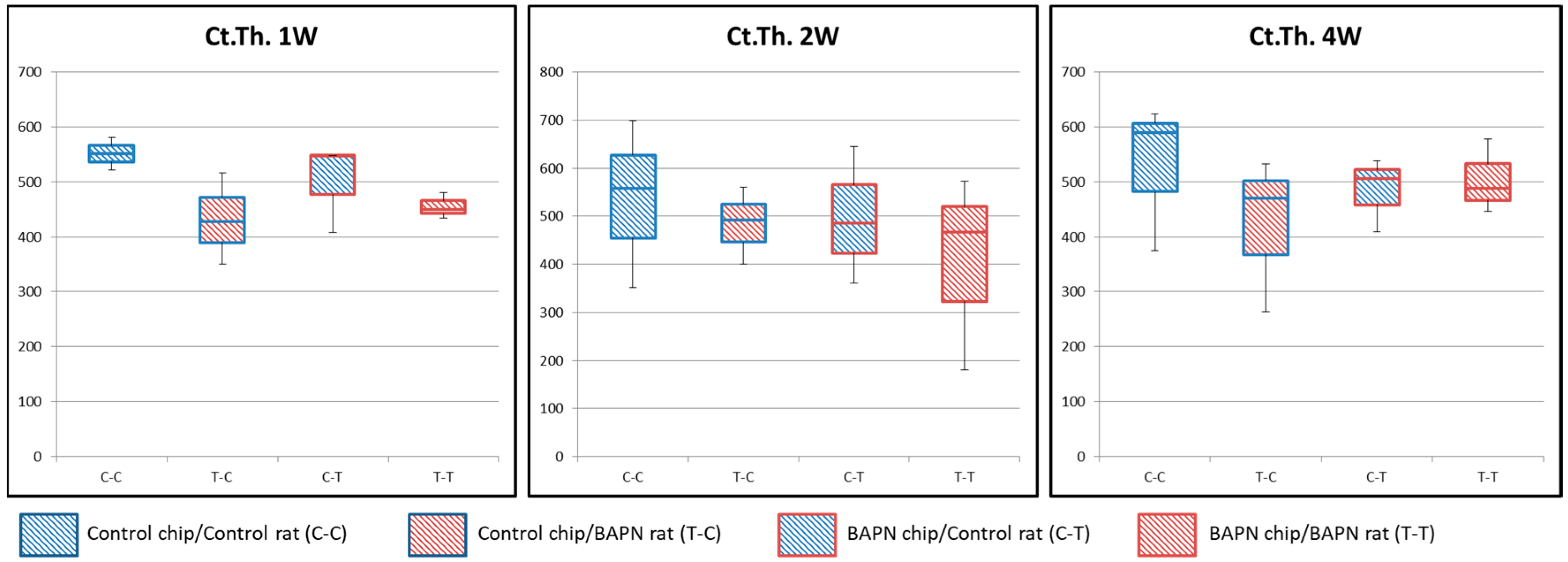
2.4. Histomorphometric Analysis
2.5. Immunohistochemical Analyses
2.6. Statistical Analysis
3. Results
3.1. Characterization of Collagen Structure
3.2. Histological and Immunohistochemical Analyses of Transferred Bone Chips
3.3. Periosteum Surrounding Transferred Bone
3.4. Bone Formation and Osteoclastic Activity Associated with the Host
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brugnami, F.; Caiazzo, A.; Leone, C. Local intraoral autologous bone harvesting for dental implant treatment: Alternative sources and criteria of choice. Keio J. Med. 2009, 58, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Sittitavornwong, S.; Gutta, R. Bone graft harvesting from regional sites. Oral Maxillofac. Surg. Clin. N. Am. 2010, 22, 317–330. [Google Scholar] [CrossRef]
- Zouhary, K.J. Bone graft harvesting from distant sites: Concepts and techniques. Oral Maxillofac. Surg. Clin. N. Am. 2010, 22, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Cypher, T.J.; Grossman, J.P. Biological principles of bone graft healing. J. Foot Ankle Surg. 1996, 35, 413–417. [Google Scholar] [CrossRef]
- Knott, L.; Bailey, A.J. Collagen cross-links in mineralizing tissues: A review of their chemistry, function, and clinical relevance. Bone 1998, 22, 181–187. [Google Scholar] [CrossRef]
- McNerny, E.M.B.; Gong, B.; Morris, M.D.; Kohn, D.H. Bone fracture toughness and strength correlate with collagen cross-link maturity in a dose-controlled lathyrism mouse model. J. Bone Miner. Res. 2015, 30, 455–464. [Google Scholar] [CrossRef]
- Oxlund, H.; Barckman, M.; Ørtoft, G.; Andreassen, T.T. Reduced concentrations of collagen cross-links are Associated with reduced strength of bone. Bone 1995, 17, 365–371. [Google Scholar] [CrossRef]
- Yamauchi, M.; Mechanic, G.L.; Katz, E.P. Intermolecular cross-linking and stereospecific molecular packing in type I collagen fibrils of the periodontal ligament. Biochemistry 1986, 25, 4907–4913. [Google Scholar] [CrossRef]
- Kuroshima, S.; Kaku, M.; Ishimoto, T.; Sasaki, M.; Nakano, T.; Sawase, T. A paradigm shift for bone quality in dentistry: A literature Review. J. Prosthodont. Res. 2017, 61, 353–362. [Google Scholar] [CrossRef]
- Paschalis, E.P.; Tatakis, D.N.; Robins, S.; Fratzl, P.; Manjubala, I.; Zoehrer, R.; Gamsjaeger, S.; Buchinger, B.; Roschger, A.; Phipps, R.; et al. Lathyrism-induced alterations in collagen cross-links influence the mechanical properties of bone material without affecting the mineral. Bone 2011, 49, 1232–1241. [Google Scholar] [CrossRef]
- Cengiz, M.I.; Kirtiloğlu, T.; Acikgoz, G.; Trisi, P.; Wang, H.-L. Effect of defective collagen synthesis on epithelial implant interface: Lathyritic model in dogs. An experimental preliminary study. J. Oral Implantol. 2012, 38, 105–114. [Google Scholar] [CrossRef]
- Canelón, S.P.; Wallace, J.M. β-Aminopropionitrile-induced reduction in enzymatic crosslinking causes in vitro changes in collagen morphology and molecular composition. PLoS ONE 2016, 11, 1–13. [Google Scholar] [CrossRef]
- Sharma-Bhandari, A.; Park, S.H.; Kim, J.Y.; Oh, J.; Kim, Y. Lysyl oxidase modulates the osteoblast differentiation of primary mouse calvaria cells. Int. J. Mol. Med. 2015, 36, 1664–1670. [Google Scholar] [CrossRef]
- Tang, S.S.; Trackman, P.C.; Kagan, H.M. Reaction of aortic lysyl oxidase with beta-aminopropionitrile. J. Biol. Chem. 1983, 258, 4331–4338. [Google Scholar]
- Ida, T.; Masaru, K.; Megumi, K.; Masahiko, T.; Juan, M.R.R.; Yosuke, A.; Masako, N.; Mitsuo, Y.; Katsumi, U. Extracellular matrix with defective collagen cross-linking affects the differentiation of bone cells. PLoS ONE 2018. [Google Scholar] [CrossRef]
- Ida, T.; Id, M.K.; Kitami, M.; Terajima, M.; Rocabado, M.R.; Akiba, Y.; Nagasawa, M.; Yamauchi, M.; Uoshima, K. Extracellular matrix with defective collagen cross-linking affects the differentiation of bone cells. 2018, 13, 1–18. [Google Scholar] [CrossRef]
- Junqueira, L.C.U.; Bignolas, G.; Brentani, R.R. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem. J. 1979, 11, 447–455. [Google Scholar] [CrossRef]
- Dempster, D.W.; Compston, J.E.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R.; Parfitt, A.M. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. JBMR 2014, 28, 2–17. [Google Scholar] [CrossRef]
- Sohn, J.Y.; Park, J.C.; Um, Y.J.; Jung, U.W.; Kim, C.S.; Cho, K.S.; Choi, S.H. Spontaneous healing capacity of rabbit cranial defects of various sizes. J. Periodontal Implant Sci. 2010, 40, 180–187. [Google Scholar] [CrossRef]
- Stevenson, S.; Emery, S.E.; Goldberg, V.M. Factors affecting bone graft incorporation. Clin. Orthop. Relat. Res. 1996, 324, 66–74. [Google Scholar] [CrossRef]
- Saito, M.; Marumo, K. Collagen cross-links as a determinant of bone quality: A possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporos. Int. 2010, 21, 195–214. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Li, J.; Córdova, L.A.; Liu, B.; Mouraret, S.; Sun, Q.; Salmon, B.; Helms, J. A WNT protein therapeutic improves the bone-forming capacity of autografts from aged animals. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Leucht, P.; Jiang, J.; Cheng, D.; Liu, B.; Dhamdhere, G.; Fang, M.Y.; Monica, S.D.; Urena, J.J.; Cole, W.; Smith, L.R.; et al. Wnt3a reestablishes osteogenic capacity to bone grafts from aged animals. J. Bone Jt. Surg. Am. Vol. 2013, 95, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- Unno, H.; Suzuki, H.; Nakakura-Ohshima, K.; Jung, H.S.; Ohshima, H. Pulpal regeneration following allogenic tooth transplantation into mouse maxilla. Anat. Rec. 2009, 292, 570–579. [Google Scholar] [CrossRef]
- Delgado-Calle, J.; Sato, A.Y.; Bellido, T. Role and mechanism of action of sclerostin in bone. Bone 2017, 96, 29–37. [Google Scholar] [CrossRef]
- Fan, W. Physiological Investigation of Periosteum Structure and Its Application in Periosteum Tissue Engineering. Ph.D. Thesis, Queensland University of Technology, Brisbane, Australia, 2010. [Google Scholar]
- Kubota, S.; Yuguchi, M.; Yamazaki, Y.; Kanazawa, H.; Isokawa, K. Highly reproducible skeletal deformities induced by administration of β-aminopropionitrile to developing Chick embryos. J. Oral Sci. 2016, 58, 255–263. [Google Scholar] [CrossRef]
- Fernandes, H.; Dechering, K.; Van Someren, E.; Steeghs, I.; Apotheker, M.; Leusink, A.; Bank, R.; Janeczek, K.; Van Blitterswijk, C.; de Boer, J. The role of collagen crosslinking in differentiation of human mesenchymal stem cells and MC3T3-E1 cells. Tissue Eng. Part A 2009, 15, 3857–3867. [Google Scholar] [CrossRef]
- Turecek, C.; Fratzl-Zelman, N.; Rumpler, M.; Buchinger, B.; Spitzer, S.; Zoehrer, R.; Durchschlag, E.; Klaushofer, K.; Paschalis, E.P.; Varga, F. Collagen cross-linking influences osteoblastic differentiation. Calcif. Tissue Int. 2008, 82, 392–400. [Google Scholar] [CrossRef]
- Goldberg, V.M. Natural history of autografts and allografts the function of bone grafts stages of bone graft. Bone Implant Grafting 1992, 3, 2–5. [Google Scholar]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10, S96–S101. [Google Scholar] [CrossRef]
- Eyre, D.R.; Dickson, I.R.; Ness, K.V.A.N. Collagen cross-linking in human bone and articular cartilage age-related changes in the content of mature hydroxypyridinium residues. Biochem. J. 1988, 252, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Fujii, K.; Kuboki, Y.; Sasaki, S. Aging of human bone and articular cartilage collagen. Gerontology 1976, 22, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Boskey, A.L. Bone composition: Relationship to bone fragility and antiosteoporotic drug effects. BoneKEy Rep. 2013, 2, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Seeman, E.; Delmas, P.D. Bone quality—the material and structural basis of bone strength and fragility. N. Engl. J. Med. 2006, 354, 2250–2261. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Shou, P.; Zheng, C.; Jiang, M.; Cao, G.; Yang, Q.; Cao, J.; Xie, N.; Velletri, T.; Zhang, X.; et al. Fate decision of mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death Differ. 2016, 23, 1128–1139. [Google Scholar] [CrossRef] [PubMed]







| Host Animal | Transferred Chip | Week 4 |
|---|---|---|
| Control | Control chip | 75% |
| BAPN chip | 75% | |
| BAPN | Control chip | 100% |
| BAPN chip | 100% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mubarak, S.; Masako, N.; Al-Omari, F.A.; Keisuke, H.; Katsumi, U. Effect of Collagen Cross-Link Deficiency on Incorporation of Grafted Bone. Dent. J. 2019, 7, 45. https://doi.org/10.3390/dj7020045
Mubarak S, Masako N, Al-Omari FA, Keisuke H, Katsumi U. Effect of Collagen Cross-Link Deficiency on Incorporation of Grafted Bone. Dentistry Journal. 2019; 7(2):45. https://doi.org/10.3390/dj7020045
Chicago/Turabian StyleMubarak, Suliman, Nagasawa Masako, Farah A. Al-Omari, Hamaya Keisuke, and Uoshima Katsumi. 2019. "Effect of Collagen Cross-Link Deficiency on Incorporation of Grafted Bone" Dentistry Journal 7, no. 2: 45. https://doi.org/10.3390/dj7020045
APA StyleMubarak, S., Masako, N., Al-Omari, F. A., Keisuke, H., & Katsumi, U. (2019). Effect of Collagen Cross-Link Deficiency on Incorporation of Grafted Bone. Dentistry Journal, 7(2), 45. https://doi.org/10.3390/dj7020045




