Monolithic Zirconia: An Update to Current Knowledge. Optical Properties, Wear, and Clinical Performance
Abstract
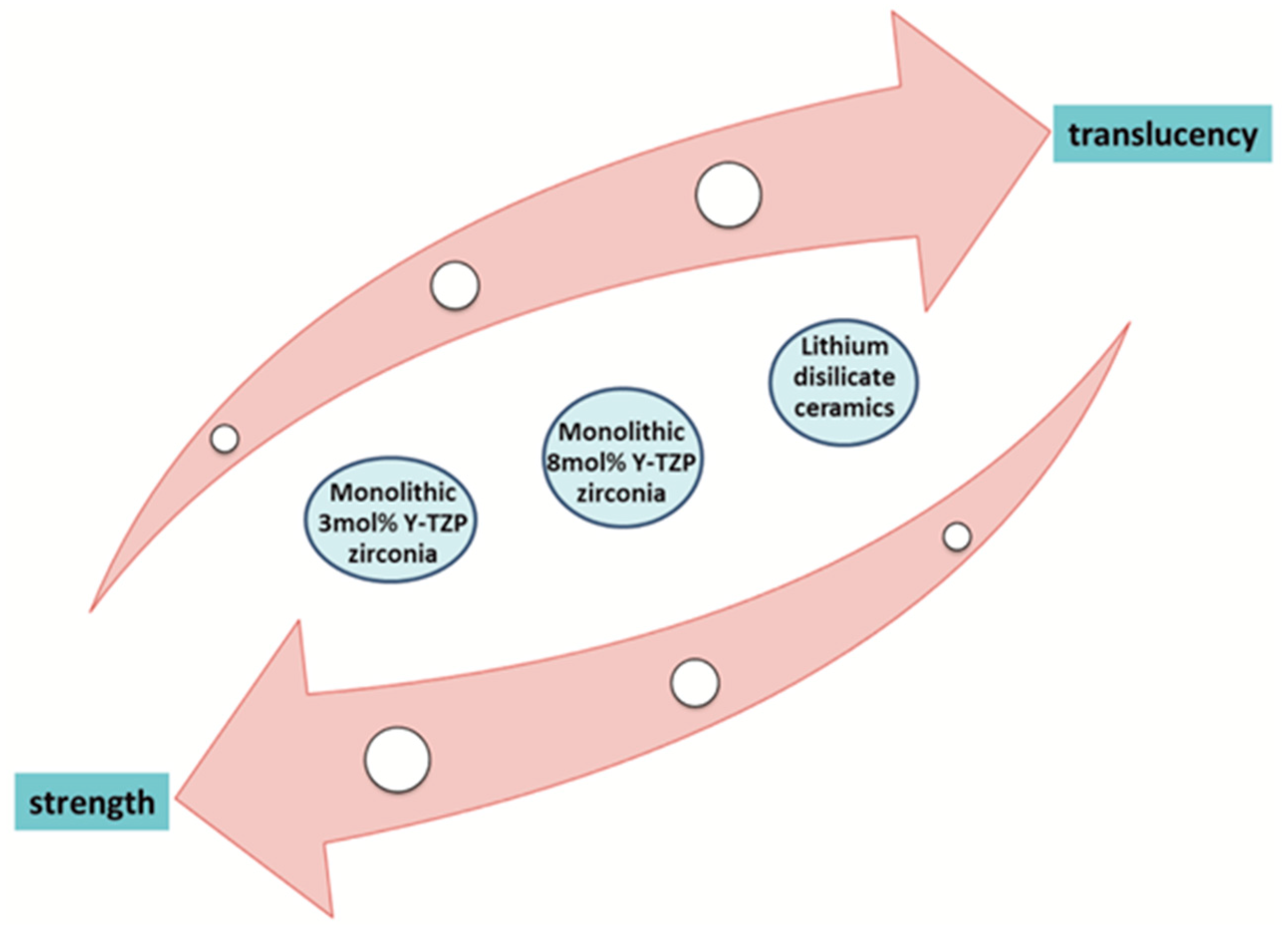
:1. Introduction
2. Optical Properties
2.1. Basic Theory
2.2. Factors Affecting Light Scattering
2.3. Studies Evaluating Optical Properties of Monolithic Zirconia
3. Wear Properties of Monolithic Zirconia
3.1. Laboratory Studies
3.2. Clinical Studies
4. Survival-Clinical Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Albashaireh, Z.S.M.; Ghazal, M.; Kern, M. Two-body wear of different ceramic materials opposed to zirconia ceramic. J. Prosthet. Dent. 2010, 104, 105–113. [Google Scholar] [CrossRef]
- Griffin, J.D. Combining monolithic zirconia crowns, digital impressioning, and regenerative cement for a predictable restorative alternative to PFM. Compend. Contin. Educ. Dent. 2013, 34, 212–222. [Google Scholar] [PubMed]
- Christensen, G.; BruxZir, J. Milled e.maxCAD: Superior clinical performance at 3+ years. Clin. Rep. 2014, 7, 1–3. [Google Scholar]
- Tong, H.; Tanaka, C.B.; Kaizer, M.R.; Zhang, Y. Characterization of three commercial Y-TZP ceramics produced for their High-Translucency, High-Strength and High-Surface Area. Ceram. Int. 2016, 42, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. Making yttria-stabilized tetragonal zirconia translucent. Dent. Mater. 2014, 30, 1195–1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinke, S.; Fischer, C. Range of indications for translucent zirconia modifications: Clinical and technical aspects. Quintessence Int. 2013, 44, 557–566. [Google Scholar] [PubMed]
- Elsaka, S.E. Optical and Mechanical Properties of Newly Developed Monolithic Multilayer Zirconia. J. Prosthodont. 2019, 28, e279–e284. [Google Scholar] [CrossRef]
- Muñoz, E.M.; Longhini, D.; Antonio, S.G.; Adabo, G.L. The effects of mechanical and hydrothermal aging on microstructure and biaxial flexural strength of an anterior and a posterior monolithic zirconia. J. Dent. 2017, 63, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Griffin, J.D. Tooth in a bag: Same-day monolithic zirconia crown. Dent. Today 2013, 32, 126–131. [Google Scholar]
- Lameira, D.P.; Silva, W.A.B.E.; Silva, F.A.E.; De Souza, G.M. Fracture Strength of Aged Monolithic and Bilayer Zirconia-Based Crowns. Biomed. Res. Int. 2015, 2015, 7. [Google Scholar] [CrossRef]
- Sun, T.; Zhou, S.; Lai, R.; Liu, R.; Ma, S.; Zhou, Z.; Longquan, S. Load-bearing capacity and the recommended thickness of dental monolithic zirconia single crowns. J. Mech. Behav. Biomed. Mater. 2014, 35, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Dresselhaus, M.S. Absorpion of light in silids. In Solid State Physics Part II: Optical Properties of Solids; Springer: Berlin/Heidelberg, Germany, 2001; p. 36. [Google Scholar]
- Miyagawa, Y.; Powers, J.M.; O’brien, W.J. Optical Properties of Direct Restorative Materials. J. Dent. Res. 1981, 60, 890–894. [Google Scholar] [CrossRef]
- Johnston, W.M.; Ma, T.; Kienle, B.H. Translucency parameter of colorants for maxillofacial prostheses. Int. J. Prosthodont. 1995, 8, 79–86. [Google Scholar] [PubMed]
- Sakka, Y.; Suzuki, T.S.; Morita, K.; Nakano, K.; Hiraga, K. Colloidal processing and superplastic properties of zirconia- and alumina-based nanocomposites. Scr. Mater. 2001, 44, 2075–2078. [Google Scholar] [CrossRef]
- Vasylkiv, O.; Sakka, Y.; Skorokhod, V.V. Hardness and Fracture Toughness of Alumina-Doped Tetragonal Zirconia with Different Yttria Contents. Mater. Trans. 2003, 44, 2235–2238. [Google Scholar] [CrossRef] [Green Version]
- Liebermann, A.; Rafael, F.C.; Kauling, A.E.; Edelhoff, D.; Ueda, K.; Seiffert, A.; Volpato, M.C.A. Transmittance of visible and blue light through zirconia. Dent. Mater. J. 2018, 37, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, L.; Readey, M.J. Effect of heat treatment on grain size, phase assemblage, and mechanical properties of 3 mol% Y-TZP. J. Am. Ceram. Soc. 1996, 79, 2331–2340. [Google Scholar] [CrossRef]
- Denry, I.; Kelly, J.R. State of the art of zirconia for dental applications. Dent. Mater. 2008, 24, 299–307. [Google Scholar] [CrossRef]
- Zhang, F.; Vanmeensel, K.; Batuk, M.; Hadermann, J.; Inokoshi, M.; Van Meerbeek, B.; Naert, I.; Vleugels, J. Highly-translucent, strong and aging-resistant 3Y-TZP ceramics for dental restoration by grain boundary segregation. Acta Biomater. 2015, 16, 215–222. [Google Scholar] [CrossRef]
- Zhang, F.; Chevalier, J.; Olagnon, C.; Batuk, M.; Hadermann, J.; Van Meerbeek, B.; Vleugels, J. Grain-Boundary Engineering for Aging and Slow-Crack-Growth Resistant Zirconia. J. Dent. Res. 2017, 96, 774–779. [Google Scholar] [CrossRef]
- Zhang, Y.; Lawn, B.R. Novel Zirconia Materials in Dentistry. J. Dent. Res. 2018, 97, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Liao, Y.; Wan, Q.; Li, W. Effects of sintering temperature and particle size on the translucency of zirconium dioxide dental ceramic. J. Mater. Sci. Mater. Med. 2011, 22, 2429–2435. [Google Scholar] [CrossRef] [PubMed]
- Sen, N.; Sermet, I.B.; Cinar, S. Effect of coloring and sintering on the translucency and biaxial strength of monolithic zirconia. J. Prosthet. Dent. 2017, 119, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ebeid, K.; Wille, S.; Hamdy, A.; Salah, T.; El-Etreby, A.; Kern, M. Effect of changes in sintering parameters on monolithic translucent zirconia. Dent. Mater. 2014, 30, e419–e424. [Google Scholar] [CrossRef] [PubMed]
- Stawarczyk, B.; Özcan, M.; Hallmann, L.; Ender, A.; Mehl, A.; Hämmerlet, C.H.F. The effect of zirconia sintering temperature on flexural strength, grain size, and contrast ratio. Clin. Oral Investig. 2013, 17, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Ahn, J.S.; Kim, J.H.; Kim, H.Y.; Kim, W.C. Effects of the sintering conditions of dental zirconia ceramics on the grain size and translucency. J. Adv. Prosthodont. 2013, 5, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Kim, S.H. Comparison of the optical properties of pre-colored dental monolithic zirconia ceramics sintered in a conventional furnace versus a microwave oven. J. Adv. Prosthodont. 2017, 9, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Tuncel, İ.; Turp, I.; Üşümez, A. Evaluation of translucency of monolithic zirconia and framework zirconia materials. J. Adv. Prosthodont. 2016, 8, 181–186. [Google Scholar] [CrossRef] [Green Version]
- Klimke, J.; Trunec, M.; Krell, A. Transparent tetragonal yttria-stabilized zirconia ceramics: Influence of scattering caused by birefringence. J. Am. Ceram. Soc. 2011, 94, 1850–1858. [Google Scholar] [CrossRef]
- Ban, S. Reliability and properties of core materials for all-ceramic dental restorations. Jpn. Dent. Sci. Rev. 2008, 44, 3–21. [Google Scholar] [CrossRef] [Green Version]
- Carrabba, M.; Keeling, A.J.; Aziz, A.; Vichi, A.; Fonzar, F.R.; Wood, D.; Ferrari, M. Translucent zirconia in the ceramic scenario for monolithic restorations: A flexural strength and translucency comparison test. J. Dent. 2017, 60, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez, S.; Suárez, G.; Rendtorff, N.M.; Aglietti, E.F. Relation between mechanical and textural properties of dense materials of tetragonal and cubic zirconia. Sci. Sinter. 2016, 48, 119–130. [Google Scholar] [CrossRef]
- Sabet, H.; Wahsh, M.; Sherif, A.; Salah, T. Effect of different immersion times and sintering temperatures on translucency of monolithic nanocrystalline zirconia. Futur. Dent. J. 2018, 4, 84–89. [Google Scholar] [CrossRef]
- Hao, C.C.; Muchtar, A.; Azhari, C.H.; Razali, M.; Aboras, M. Influence of sintering temperature on translucency of yttria-stabilized zirconia for dental crown applications. J. Teknol. 2016, 78, 13–18. [Google Scholar] [CrossRef]
- Stawarczyk, B.; Emslander, A.; Roos, M.; Sener, B.; Noack, F.; Keul, C. Zirconia ceramics, their contrast ratio and grain size depending on sintering parameters. Dent. Mater. J. 2014, 33, 591–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alaniz, J.E.; Perez-Gutierrez, F.G.; Aguilar, G.; Garay, J.E. Optical properties of transparent nanocrystalline yttria stabilized zirconia. Opt. Mater. (Amst) 2009, 32, 62–68. [Google Scholar] [CrossRef]
- Heffernan, M.J.; Aquilino, S.A.; Diaz-Arnold, A.M.; Haselton, D.R.; Stanford, C.M.; Vargas, M.A. Relative translucency of six all-ceramic systems. Part II: Core and veneer materials. J. Prosthet. Dent. 2002, 88, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Vagkopoulou, T.; Koutayas, S.O.; Koidis, P.; Strub, J.R. Zirconia in dentistry: Part 1. Discovering the nature of an upcoming bioceramic. Eur. J. Esthet. Dent. 2009, 4, 130–151. [Google Scholar] [PubMed]
- Anselmi-Tamburini, U.; Woolman, J.N.; Munir, Z.A. Transparent nanometric cubic and tetragonal zirconia obtained by high-pressure pulsed electric current sintering. Adv. Funct. Mater. 2007, 17, 3267–3273. [Google Scholar] [CrossRef]
- Tsukuma, K.; Yamashita, I.; Kusunose, T. Transparent 8 mol% Y2O3-ZrO2 (8Y) ceramics. J. Am. Ceram. Soc. 2008, 91, 813–818. [Google Scholar] [CrossRef]
- Casolco, S.R.; Xu, J.; Garay, J.E. Transparent/translucent polycrystalline nanostructured yttria stabilized zirconia with varying colors. Scr. Mater. 2008, 58, 516–519. [Google Scholar] [CrossRef]
- Zhang, H.; Kim, B.N.; Morita, K.; Yoshida, H.; Lim, J.H.; Hiraga, K. Optical properties and microstructure of nanocrystalline cubic zirconia prepared by high-pressure spark plasma sintering. J. Am. Ceram. Soc. 2011, 94, 2981–2986. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Z.; Kim, B.N.; Morita, K.; Yoshida, H.; Hiraga, K.; Sakka, Y. Effect of alumina dopant on transparency of tetragonal zirconia. J. Nanomater. 2012, 2012, 5. [Google Scholar] [CrossRef]
- Vichi, A.; Sedda, M.; Fabian Fonzar, R.; Carrabba, M.; Ferrari, M. Comparison of contrast ratio, translucency parameter, and flexural strength of traditional and “augmented translucency” zirconia for CEREC CAD/CAM system. J. Esthet. Restor. Dent. 2016, 28, S32–S39. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, F.; Sekine, H.; Honma, S.; Takanashi, T.; Furuya, K.; Yajima, Y.; Yoshinari, M. Translucency and flexural strength of monolithic translucent zirconia and porcelain-layered zirconia. Dent. Mater. J. 2015, 34, 910–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldissara, P.; Wandscher, V.F.; Marchionatti, A.M.E.; Parisi, C.; Monaco, C.; Ciocca, L. Translucency of IPS e.max and cubic zirconia monolithic crowns. J. Prosthet. Dent. 2018, 120, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Malkondu, O.; Tinastepe, N.; Kazazoglu, E. Influence of type of cement on the color and translucency of monolithic zirconia. J. Prosthet. Dent. 2016, 116, 902–908. [Google Scholar] [CrossRef]
- Putra, A.; Chung, K.H.; Flinn, B.D.; Kuykendall, T.; Zheng, C.; Harada, K.; Raigrodski, A.J. Effect of hydrothermal treatment on light transmission of translucent zirconias. J. Prosthet. Dent. 2017, 118, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Fathy, S.M.; El-Fallal, A.A.; El-Negoly, S.A.; El Bedawy, A.B. Translucency of monolithic and core zirconia after hydrothermal aging. Acta Biomater. Odontol. Scand. 2015, 1, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Abdelbary, O.; Wahsh, M.; Sherif, A.; Salah, T. Effect of accelerated aging on translucency of monolithic zirconia. Futur. Dent. J. 2016, 2, 65–69. [Google Scholar] [CrossRef]
- Walczak, K.; Meißner, H.; Range, U.; Sakkas, A.; Boening, K.; Wieckiewicz, M.; Konstantinidis, I. Translucency of Zirconia Ceramics before and after Artificial Aging. J. Prosthodont. 2019, 28, e319–e324. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, T.A.; Abdulmajeed, A.A.; Shahramian, K.; Hupa, L.; Donovan, T.E.; Vallittu, P.; Närhi, T.O. Impact of gastric acidic challenge on surface topography and optical properties of monolithic zirconia. Dent. Mater. 2015, 31, 1445–1452. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.; Rothrock, J.; Thompson, J. Impact of Gastric Acid Induced Surface Changes on Mechanical Behavior and Optical Characteristics of Dental Ceramics. J. Prosthodont. 2018. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, S.H.; Han, J.S.; Yeo, I.S.L.; Yoon, H.I. Optical and Surface Properties of Monolithic Zirconia after Simulated Toothbrushing. Materials 2019, 12, 1158. [Google Scholar] [CrossRef] [PubMed]
- Shamseddine, L.; Majzoub, Z. Relative Translucency of a Multilayered Ultratranslucent Zirconia Material. J. Contemp. Dent. Pract. 2017, 18, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Raigrodski, A.J.; Chung, K.H.; Flinn, B.D.; Dogan, S.; Mancl, L.A. A comparative evaluation of the translucency of zirconias and lithium disilicate for monolithic restorations. J. Prosthet. Dent. 2016, 116, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Camposilvan, E.; Leone, R.; Gremillard, L.; Sorrentino, R.; Zarone, F.; Ferrari, M.; Chevalier, J. Aging resistance, mechanical properties and translucency of different yttria-stabilized zirconia ceramics for monolithic dental crown applications. Dent. Mater. 2018, 34, 879–890. [Google Scholar] [CrossRef]
- Huh, Y.H.; Yang, E.C.; Park, C.J.; Cho, L.R. In vitro evaluation of the polishing effect and optical properties of monolithic zirconia. J. Prosthet. Dent. 2018, 119, 994–999. [Google Scholar] [CrossRef]
- Kim, H.K.; Kim, S.H.; Lee, J.B.; Han, J.S.; Yeo, I.S.; Ha, S.R. Effect of the amount of thickness reduction on color and translucency of dental monolithic zirconia ceramics. J. Adv. Prosthodont. 2016, 8, 37–42. [Google Scholar] [CrossRef]
- Kim, H.K.; Kim, S.H. Optical properties of pre-colored dental monolithic zirconia ceramics. J. Dent. 2016, 55, 75–81. [Google Scholar] [CrossRef]
- Kwon, S.J.; Lawson, N.C.; McLaren, E.E.; Nejat, A.H.; Burgess, J.O. Comparison of the mechanical properties of translucent zirconia and lithium disilicate. J. Prosthet. Dent. 2018, 120, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, T.A.; Abdulmajeed, A.A.; Donovan, T.E.; Ritter, A.V.; Vallittu, P.K.; Närhi, T.O.; Lassila, L.V. Optical properties and light irradiance of monolithic zirconia at variable thicknesses. Dent. Mater. 2015, 31, 1180–1187. [Google Scholar] [CrossRef] [PubMed]
- Inokoshi, M.; Shimizu, H.; Nozaki, K.; Takagaki, T.; Yoshihara, K.; Nagaoka, N.; Zhang, F.; Vleugels, J.; Van Meerbeek, B.; Minakuchi, S. Crystallographic and morphological analysis of sandblasted highly translucent dental zirconia. Dent. Mater. 2018, 34, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Church, T.D.; Jessup, J.P.; Guillory, V.L.; Vandewalle, K.S. Translucency and strength of high-translucency monolithic zirconium oxide materials. Gen. Dent. 2017, 65, 48–52. [Google Scholar] [PubMed]
- Kanchanavasita, W.; Triwatana, P.; Suputtamongkol, K.; Thanapitak, A.; Chatchaiganan, M. Contrast Ratio of Six Zirconia-Based Dental Ceramics. J. Prosthodont. 2014, 23, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Subaşı, M.G.; Alp, G.; Johnston, W.M.; Yilmaz, B. Effect of thickness on optical properties of monolithic CAD-CAM ceramics. J. Dent. 2018, 71, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Zadeh, N.P.; Lümkemann, N.; Sener, B.; Eichberger, M.; Stawarczyk, B. Flexural strength, fracture toughness, and translucency of cubic/tetragonal zirconia materials. J. Prosthet. Dent. 2018, 120, 948–954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakai, T.; Sato, T.; Hisanaga, R.; Shinya, A.; Takemoto, S.; Yoshinari, M. Optical properties and flexural strength of translucent zirconia layered with high-translucent zirconia. Dent. Mater. J. 2019, 38, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Kim, S.H. Effect of hydrothermal aging on the optical properties of precolored dental monolithic zirconia ceramics. J. Prosthet. Dent. 2019, 121, 676–682. [Google Scholar] [CrossRef]
- Turssi, C.P.; De Purquerio, M.B.; Serra, M.C. Wear of dental resin composites: Insights into underlying processes and assessment methods--a review. J. Biomed. Mater. Res. B Appl. Biomater. 2003, 65, 280–285. [Google Scholar] [CrossRef]
- Oh, W.S.; De Long, R.; Anusavice, K.J. Factors affecting enamel and ceramic wear: A literature review. J. Prosthet. Dent. 2002, 87, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Alghazzawi, T.F.; Lemons, J.; Liu, P.R.; Essig, M.E.; Bartolucci, A.A.; Janowski, G.M. Influence of Low-Temperature Environmental Exposure on the Mechanical Properties and Structural Stability of Dental Zirconia. J. Prosthodont. 2012, 21, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Mörmann, W.H.; Stawarczyk, B.; Ender, A.; Sener, B.; Attin, T.; Mehl, A. Wear characteristics of current aesthetic dental restorative CAD/CAM materials: Two-body wear, gloss retention, roughness and Martens hardness. J. Mech. Behav. Biomed. Mater. 2013, 20, 113–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hmaidouch, R.; Müller, W.D.; Lauer, H.C.; Weigl, P. Surface roughness of zirconia for full-contour crowns after clinically simulated grinding and polishing. Int. J. Oral Sci. 2014, 6, 241–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preis, V.; Schmalzbauer, M.; Bougeard, D.; Schneider-Feyrer, S.; Rosentritt, M. Surface properties of monolithic zirconia after dental adjustment treatments and in vitro wear simulation. J. Dent. 2015, 43, 133–139. [Google Scholar] [CrossRef]
- Mitov, G.; Heintze, S.D.; Walz, S.; Woll, K.; Muecklich, F.; Pospiech, P. Wear behavior of dental Y-TZP ceramic against natural enamel after different finishing procedures. Dent. Mater. 2012, 28, 909–918. [Google Scholar] [CrossRef]
- Janyavula, S.; Lawson, N.; Cakir, D.; Beck, P.; Ramp, L.C.; Burgess, J.O. The wear of polished and glazed zirconia against enamel. J. Prosthet. Dent. 2013, 109, 22–29. [Google Scholar] [CrossRef]
- Preis, V.; Weiser, F.; Handel, G.; Rosentritt, M. Wear performance of monolithic dental ceramics with different surface treatments. Quintessence Int. 2013, 44, 393–405. [Google Scholar]
- Luangruangrong, P.; Cook, N.B.; Sabrah, A.H.; Hara, A.T.; Bottino, M.C. Influence of full-contour zirconia surface roughness on wear of glass-ceramics. J. Prosthodont. 2014, 23, 198–205. [Google Scholar] [CrossRef]
- Sabrah, A.H.A.; Cook, N.B.; Luangruangrong, P.; Hara, A.T.; Bottino, M.C. Full-contour Y-TZP ceramic surface roughness effect on synthetic hydroxyapatite wear. Dent. Mater. 2013, 29, 666–673. [Google Scholar] [CrossRef]
- Rosentritt, M.; Preis, V.; Behr, M.; Hahnel, S.; Handel, G.; Kolbeck, C. Two-body wear of dental porcelain and substructure oxide ceramics. Clin. Oral Investig. 2012, 16, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Preis, V.; Behr, M.; Hahnel, S.; Handel, G.; Rosentritt, M. In vitro failure and fracture resistance of veneered and full-contour zirconia restorations. J. Dent. 2012, 40, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Kaizer, M.R.; Bano, S.; Borba, M.; Garg, V.; dos Santos, M.B.F.; Zhang, Y. Wear Behavior of Graded Glass/Zirconia Crowns and Their Antagonists. J. Dent. Res. 2019, 98, 437–442. [Google Scholar] [CrossRef] [PubMed]
- D’Arcangelo, C.; Vanini, L.; Rondoni, G.D.; Vadini, M.D.A.F. Wear Evaluation of Prosthetic Materials Opposing Themselves. Oper. Dent. 2018, 43, 38–50. [Google Scholar] [CrossRef] [Green Version]
- Sripetchdanond, J.; Leevailoj, C. Wear of human enamel opposing monolithic zirconia, glass ceramic, and composite resin: An in vitro study. J. Prosthet. Dent. 2014, 112, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Habib, S.R.; Alotaibi, A.; Al Hazza, N.; Allam, Y.; AlGhazi, M. Two-body wear behavior of human enamel versus monolithic zirconia, lithium disilicate, ceramometal and composite resin. J. Adv. Prosthodont. 2019, 11, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Bolaca, A.E.Y. In Vitro evaluation of the wear of primary tooth enamel against different ceramic and composite resin materials. Niger. J. Clin. Pr. 2019, 4, 11–13. [Google Scholar]
- Pereira, G.K.R.; Dutra, D.M.; Werner, A.; Prochnow, C.; Valandro, L.F.; Kleverlaan, C.J. Effect of zirconia polycrystal and stainless steel on the wear of resin composites, dentin and enamel. J. Mech. Behav. Biomed. Mater. 2019, 91, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Ludovichetti, F.S.; Trindade, F.Z.; Werner, A.; Kleverlaan, C.J.; Fonseca, R.G. Wear resistance and abrasiveness of CAD-CAM monolithic materials. J. Prosthet. Dent. 2018, 120, 318. [Google Scholar] [CrossRef] [PubMed]
- Sarıkaya, I.H.Y. Effects of dynamic aging on the wear and fracture strength of monolithic zirconia restorations. BMC Oral Health 2018, 18, 146. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.K.; Satterthwaite, J.D. The effect of chewing simulation on surface roughness of resin composite when opposed by zirconia ceramic and lithium disilicate ceramic. Dent. Mater. 2018, 34, e15–e24. [Google Scholar] [CrossRef] [PubMed]
- Amer, R.; Kürklü, D.; Kateeb, E.; Seghi, R.R. Three-body wear potential of dental yttrium-stabilized zirconia ceramic after grinding, polishing, and glazing treatments. J. Prosthet. Dent. 2014, 112, 1151–1155. [Google Scholar] [CrossRef] [PubMed]
- Beuer, F.; Stimmelmayr, M.; Gueth, J.F.; Edelhoff, D.; Naumann, M. In vitro performance of full-contour zirconia single crowns. Dent. Mater. 2012, 28, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Stawarczyk, B.; Özcan, M.; Schmutz, F.; Trottmann, A.; Roos, M.; Hämmerle, C.H.F. Two-body wear of monolithic, veneered and glazed zirconia and their corresponding enamel antagonists. Acta Odontol. Scand. 2013, 71, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Kontos, L.; Schille, C.; Schweizer, E.; Geis-Gerstorfer, J. Influence of surface treatment on the wear of solid zirconia. Acta Odontol. Scand. 2013, 71, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Park, S.; Lee, K.; Yun, K.D.; Lim, H.P. Antagonist wear of three CAD/CAM anatomic contour zirconia ceramics. J. Prosthet. Dent. 2014, 111, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Gundugollu, Y.; Yalavarthy, R.S.; Krishna, M.H.; Kalluri, S.; Pydi, S.K. Comparison of the effect of monolithic and layered zirconia on natural teeth wear: An in vitro study. J. Indian Prosthodont. Soc. 2018, 18, 336. [Google Scholar]
- Ghazal, M.; Kern, M. The influence of antagonistic surface roughness on the wear of human enamel and nanofilled composite resin artificial teeth. J. Prosthet. Dent. 2009, 101, 342–349. [Google Scholar] [CrossRef]
- Lawson, N.C.; Janyavula, S.; Syklawer, S.; McLaren, E.A.; Burgess, J.O. Wear of enamel opposing zirconia and lithium disilicate after adjustment, polishing and glazing. J. Dent. 2014, 42, 1586–1591. [Google Scholar] [CrossRef]
- Kaizer, M.R.; Moraes, R.R.; Cava, S.S.; Zhang, Y. The progressive wear and abrasiveness of novel graded glass/zirconia materials relative to their dental ceramic counterparts. Dent. Mater. 2019, 35, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.S.; Lee, J.W.; Choi, Y.J.; Ahn, J.S.; Shin, S.W.; Huh, J.B. A study on the in-vitro wear of the natural tooth structure by opposing zirconia or dental porcelain. J. Adv. Prosthodont. 2010, 2, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Oh, S.H.; Kim, J.H.; Ju, S.W.; Seo, D.G.; Jun, S.H.; Ahn, J.S.; Ryu, J.J. Wear evaluation of the human enamel opposing different Y-TZP dental ceramics and other porcelains. J. Dent. 2012, 40, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Preis, V.; Behr, M.; Handel, G.; Schneider-Feyrer, S.; Hahnel, S.; Rosentritt, M. Wear performance of dental ceramics after grinding and polishing treatments. J. Mech. Behav. Biomed. Mater. 2012, 10, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Bae, I.; Noh, T.; Ju, S.; Lee, T.; Ahn, J. Wear of primary teeth caused by opposed all- ceramic or stainless steel crowns. J. Adv. Prosthodont. 2016, 438, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Rupawala, A.; Musani, S.I.; Madanshetty, P.; Dugal, R.; Shah, U.D.; Sheth, E.J. A study on the wear of enamel caused by monolithic zirconia and the subsequent phase transformation compared to two other ceramic systems. J. Indian Prosthodont. Soc. 2017, 17, 8–14. [Google Scholar] [PubMed]
- Stawarczyk, B.; Frevert, K.; Ender, A.; Roos, M.; Sener, B.; Wimmer, T. Comparison of four monolithic zirconia materials with conventional ones: Contrast ratio, grain size, four-point flexural strength and two-body wear. J. Mech. Behav. Biomed. Mater. 2016, 59, 128–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaizer, M.R.; Gierthmuehlen, P.C.; dos Santos, M.B.; Cava, S.S.; Zhang, Y. Speed sintering translucent zirconia for chairside one-visit dental restorations: Optical, mechanical, and wear characteristics. Ceram. Int. 2017, 43, 10999–11005. [Google Scholar] [CrossRef] [PubMed]
- Preis, V.; Behr, M.; Kolbeck, C.; Hahnel, S.; Handel, G.; Rosentritt, M. Wear performance of substructure ceramics and veneering porcelains. Dent. Mater. 2011, 27, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Mundhe, K.; Jain, V.; Pruthi, G.; Shah, N. Clinical study to evaluate the wear of natural enamel antagonist to zirconia and metal ceramic crowns. J. Prosthet. Dent. 2015, 114, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Esquivel-upshaw, J.F.; Kim, M.J.; Hsu, S.M.; Abdulhameed, N.; Jenkins, R.; Neal, D.; Ren, F.; Clark, A.E. Randomized clinical study of wear of enamel antagonists against polished monolithic zirconia crowns. J. Dent. 2018, 68, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Stober, T.; Bermejo, J.L.; Schwindling, F.S.; Schmitter, M. Clinical assessment of enamel wear caused by monolithic zirconia crowns. J. Oral Rehabil. 2016, 43, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Lohbauer, U.; Reich, S. Antagonist wear of monolithic zirconia crowns after 2 years. Clin. Oral Investig. 2017, 21, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Dogan, S.; Raigrodski, A.J.; Zhang, H.; Mancl, L.A. Prospective cohort clinical study assessing the 5-year survival and success of anterior maxillary zirconia-based crowns with customized zirconia copings. J. Prosthet. Dent. 2017, 117, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Örtorp, A.; Kihl, M.L.; Carlsson, G.E. A 5-year retrospective study of survival of zirconia single crowns fitted in a private clinical setting. J. Dent. 2012, 40, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Sailer, I.; Fehér, A.; Filser, F.; Gauckler, L.J.; Lüthy, H.; Hämmerle, C.H.F. Five-year clinical results of zirconia frameworks for posterior fixed partial dentures. Int. J. Prosthodont 2007, 20, 383–388. [Google Scholar] [PubMed]
- Burke, F.J.T.; Crisp, R.J.; Cowan, A.J.; Lamb, J.; Thompson, O.; Tulloch, N. Five-year clinical evaluation of zirconia-based bridges in patients in UK general dental practices. J. Dent. 2013, 41, 992–999. [Google Scholar] [CrossRef] [PubMed]
- Molin, M.K.; Karlsson, S.L. Five-year clinical prospective evaluation of zirconia-based Denzir 3-unit FPDs. Int. J. Prosthodont. 2008, 21, 223–227. [Google Scholar]
- Sax, C.; Hammerle, C.H.F.; Sailer, I. 10-Year Clinical Outcomes of Fixed Dental Prostheses with Zirconia Frameworks. Int. J. Comput. Dent. 2011, 14, 183–202. [Google Scholar]
- Sorrentino, R.; De Simone, G.; Tetè, S.; Russo, S.; Zarone, F. Five-year prospective clinical study of posterior three-unit zirconia-based fixed dental prostheses. Clin. Oral Investig. 2012, 16, 977–985. [Google Scholar] [CrossRef]
- Pjetursson, B.E.; Sailer, I.; Makarov, N.A.; Zwahlen, M.; Thoma, D.S. Corrigendum to “All-ceramic or metal-ceramic tooth-supported fixed dental prostheses (FDPs)? A systematic review of the survival and complication rates. Part II: Multiple-unit FDPs”. Dent. Mater. 2017, 33, e48–e51. [Google Scholar] [CrossRef]
- Thoma, D.S.; Sailer, I.; Ioannidis, A.; Zwahlen, M.; Makarov, N.; Pjetursson, B.E. A systematic review of the survival and complication rates of resin-bonded fixed dental prostheses after a mean observation period of at least 5 years. Clin. Oral Implants Res. 2017, 28, 1421–1432. [Google Scholar] [CrossRef]
- Sad Chaar, M.; Kern, M. Five-year clinical outcome of posterior zirconia ceramic inlay-retained FDPs with a modified design. J. Dent. 2015, 43, 1411–1415. [Google Scholar] [CrossRef] [PubMed]
- Sasse, M.; Kern, M. Survival of anterior cantilevered all-ceramic resin-bonded fixed dental prostheses made from zirconia ceramic. J. Dent. 2014, 42, 660–663. [Google Scholar] [CrossRef] [PubMed]
- Sasse, M.; Kern, M. CAD/CAM single retainer zirconia-ceramic resin-bonded fixed dental prostheses: Clinical outcome after 5 years. Int. J. Comput. Dent. 2013, 16, 109–118. [Google Scholar] [PubMed]
- Bömicke, W.; Rammelsberg, P.; Stober, T.; Schmitter, M. Short-Term Prospective Clinical Evaluation of Monolithic and Partially Veneered Zirconia Single Crowns. J. Esthet. Restor. Dent. 2017, 29, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Worni, A.; Katsoulis, J.; Kolgeci, L.; Worni, M.; Mericske-Stern, R. Monolithic zirconia reconstructions supported by teeth and implants: 1- to 3-year results of a case series. Quintessence Int. 2017, 48, 459–467. [Google Scholar] [PubMed]
- Gunge, H.; Ogino, Y.; Kihara, M.; Tsukiyama, Y.; Koyano, K. Retrospective clinical evaluation of posterior monolithic zirconia restorations after 1 to 3.5 years of clinical service. J. Oral Sci. 2017, 60, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Güngör, M.B.; Nemli, S.K.; Çağlar, A.; Aydın, C.; Yılmaz, H. Clinical study on the success of posterior monolithic zirconia crowns and fixed dental prostheses: Preliminary report. Acta Odontol. Turc. 2017, 34, 104–108. [Google Scholar]
- Pihlaja, J.; Ãnkangas, R.; Raustia, A. Outcome of zirconia partial fixed dental prostheses made by predoctoral dental students: A clinical retrospective study after 3 to 7 years of clinical service. J. Prosthet. Dent. 2016, 116, 40–46. [Google Scholar] [CrossRef]
- Sulaiman, T.A.; Abdulmajeed, A.A.; Donovan, T.E.; Cooper, L.F. Fracture rate of monolithic zirconia restorations up to 5 years: A dental laboratory survey. J. Prosthet. Dent. 2016, 10, 436–439. [Google Scholar] [CrossRef]
- Levartovsky, S.; Pilo, R.; Shadur, A.; Matalon, S.; Winocur, E. Complete rehabilitation of patients with bruxism by veneered and non-veneered zirconia restorations with an increased vertical dimension of occlusion: An observational case-series study. J. Prosthodont. Res. 2019. [Google Scholar] [CrossRef]
- Hansen, T.L.; Schriwer, C.; Øilo, M.; Gjengedal, H. Monolithic zirconia crowns in the aesthetic zone in heavy grinders with severe tooth wear an observational case-series. J. Dent. 2018, 72, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Pathan, M.S.; Kheur, M.G.; Patankar, A.H.; Kheur, S.M. Assessment of Antagonist Enamel Wear and Clinical Performance of Full-Contour Monolithic Zirconia Crowns: One-Year Results of a Prospective Study. J. Prosthodont. 2019, 28, e411–e416. [Google Scholar] [CrossRef] [PubMed]
- Shahdad, S.; Cattell, M.J.; Cano-Ruiz, J.; Gamble, E. Clinical Evaluation of All Ceramic Zirconia Framework Resin Bonded Bridges. Eur. J. Prosthodont. Restor. Dent. 2018, 26, 203–211. [Google Scholar] [PubMed]
- Cheng, C.W.; Chien, C.H.; Chen, C.J.; Papaspyridakos, P. Clinical Results and Technical Complications of Posterior Implant-Supported Modified Monolithic Zirconia Single Crowns and Short-Span Fixed Dental Prostheses: A 2-Year Pilot Study. J. Prosthodont. 2018, 27, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.W.; Chien, C.H.; Chen, C.J.; Papaspyridakos, P. Randomized Controlled Clinical Trial to Compare Posterior Implant-Supported Modified Monolithic Zirconia and Metal-Ceramic Single Crowns: One-Year Results. J. Prosthodont. 2019, 28, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Degidi, M.; Nardi, D.; Gianluca, S.; Piattelli, A. The Conometric Concept: A 5-Year Follow-up of Fixed Partial Monolithic Zirconia Restorations Supported by Cone-in-Cone Abutments. Int. J. Periodontics Restorative Dent. 2018, 38, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Vizcaya, R.F. Retrospective 2 to 7Year Follow-Up Study of 20 Double Full-Arch Implant-Supported Monolithic Zirconia Fixed Prostheses: Measurements and Recommendations for Optimal Design. J. Prosthodont. 2018, 27, 501–508. [Google Scholar] [CrossRef]
- Bidra, A.S.; Tischler, M.; Patch, C. Survival of 2039 complete arch fixed implant-supported zirconia prostheses: A retrospective study. J. Prosthet. Dent. 2018, 119, 220–224. [Google Scholar] [CrossRef]
- Mangano, F.; Margiani, B.; Admakin, O. A Novel Full-Digital Protocol (SCAN-PLAN-MAKE-DONE®) for the Design and Fabrication of Implant-Supported Monolithic Translucent Zirconia Crowns Cemented on Customized Hybrid Abutments: A Retrospective Clinical Study on 25 Patients. Int. J. Environ. Res. Public Health 2019, 16, 317. [Google Scholar] [CrossRef]
- Limmer, B.; Sanders, A.E.; Reside, G.; Cooper, L.F. Complications and patient-centered outcomes with an implant-supported monolithic zirconia fixed dental prosthesis: 1 year results. J. Prosthodont. 2014, 23, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Pjetursson, B.E.; Valente, N.A.; Strasding, M.; Zwahlen, M.; Liu, S.; Sailer, I. A systematic review of the survival and complication rates of zirconia-ceramic and metal-ceramic single crowns. Clin. Oral Implants Res. 2018, 29, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Sailer, I.; Strasding, M.; Valente, N.A.; Zwahlen, M.; Liu, S.; Pjetursson, B.E. A systematic review of the survival and complication rates of zirconia-ceramic and metal-ceramic multiple-unit fixed dental prostheses. Clin. Oral Implants Res. 2018, 29, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Schriwer, C.; Skjold, A.; Gjerdet, N.R.; Øilo, M. Monolithic zirconia dental crowns. Internal fit, margin quality, fracture mode and load at fracture. Dent. Mater. 2017, 33, 1012–1020. [Google Scholar] [CrossRef] [PubMed]
- Schatz, C.; Strickstrock, M.; Roos, M.; Edelhoff, D.; Eichberger, M.; Zylla, I.M.; Stawarczyk, B. Influence of specimen preparation and test methods on the flexural strength results of monolithic zirconia materials. Materials 2016, 9, 144. [Google Scholar] [CrossRef] [PubMed]
- Yener, S.E.; Ozcan, M.; Kazazoglu, E. A comparative study of biaxial flexural strength and Vickers microhardness of different zirconia materials: Effect of glazing and thermal cycling. Brazilian Dent. Sci. 2015, 18, 19–30. [Google Scholar] [CrossRef]
- Livaditis, J.M.; Livaditis, G.J. The use of custom-milled zirconia teeth to address tooth abrasion in complete dentures: A clinical report. J. Prosthodont. 2013, 22, 208–213. [Google Scholar] [CrossRef]
- Feitosa, S.; Patel, D.; Borges, A.; Alshehri, E.; Bottino, M.; Özcan, M.; Valandro, L.; Bottino, M. Effect of Cleansing Methods on Saliva-Contaminated Zirconia—An Evaluation of Resin Bond Durability. Oper. Dent. 2015, 40, 163–171. [Google Scholar] [CrossRef]
- Sulaiman, T.A.; Abdulmajeed, A.A.; Donovan, T.A.; Vallittu, P.K.; Narhi, T.O.; Lassila, L.V. The effect of staining and vacuum sintering on optical and mechanical properties of partially and fully stabilized monolithic zirconia. Dent. Mater. J. 2015, 34, 605–610. [Google Scholar] [CrossRef] [Green Version]
- Mitov, G.; Anastassova-Yoshida, Y.; Nothdurft, F.P.; von See, C.; Pospiech, P. Influence of the preparation design and artificial aging on the fracture resistance of monolithic zirconia crowns. J. Adv. Prosthodont. 2016, 8, 30–36. [Google Scholar] [CrossRef] [Green Version]


| Authors | Zirconia System | Test Method | Sample Thickness | Results | ||||
|---|---|---|---|---|---|---|---|---|
| Fathy et al., 2015 [50] | Zirkonzahn | TP measured with a spectrophotometer Steam autoclave: 134 °C, 2 bars, 15 h | 1 mm | TP values: | ||||
| Before aging = 16.4 ± 0.316 | ||||||||
| After aging = 13.35 ± 0.158 | ||||||||
| Sulaiman et al., 2015 [53] | -Pretau (PRT) | TP measured with a spectrophotometer | 1.2 mm | Arithmetic values cannot be extrapolated from the data provided in the article. Acid immersion had no effect on the TP and surface gloss of KAT and BRX. TP values increased significantly for PRT, ZEN and IPS e.max | ||||
| -Pretau anterior (PRTA) | ||||||||
| -Katana HT (KAT) | ||||||||
| -Zenostar (ZEN) | Simulating gastric acid, 96 h, 37 °C | |||||||
| -Bruxzir (BRX) | ||||||||
| Abdelbary et al., 2016 [51] | inCoris TZI | TP measured with a spectrophotometer | 0.5 mm, 0.8 mm, 1 mm and 1.2 mm | TP | Before aging | After aging | ||
| 0.5 | 16.12 | 12.56 | ||||||
| Steam autoclave: 134 °C, 0.2 MPa for 5 h | 0.8 | 13.67 | 13.24 | |||||
| 1 | 11.49 | 11.08 | ||||||
| 1.2 | 9.25 | 9.74 | ||||||
| Putra et al., 2017 [49] | -BruxZir Anterior (BA) | Tt% measured with a spectrophotometer | 1 mm | Tt% | ||||
| -Lava Plus High Translucency (LPHT) | ||||||||
| 0 h | 5 h | 50 h | 100 h | |||||
| -Katana Zirconia Super Translucent (KST) | ||||||||
| DLT | 28.3 | 27.6 | 26.8 | 28.0 | ||||
| UT | 23.4 | 22.9 | 22.5 | 22.6 | ||||
| Steam autoclave: 134 °C, 0.2 Mpa for 0, 5, 50 and 100 h | ||||||||
| ST | 22.6 | 22.8 | 22.1 | 21.9 | ||||
| -Katana Zirconia Ultra Translucent (KUT) | ||||||||
| PHT | 6.5 | 7.0 | 7.8 | 8.9 | ||||
| BA | 7.2 | 6.6 | 7.8 | 7.4 | ||||
| Subaşı et al., 2018 [67] | -İnCoris TZI C (MonZr) | Color difference and relative TP (RTP) was calculated using a spectroradiometer Specimens were subjected to 5000 coffee thermocycling | 0.5, 0.7 and 1 mm | Arithmetic values cannot be extrapolated from the graphs provided in the article. However, significant interactions between material and different thickness was recorded for both TP and color difference. Pre-shaded monolithic zirconia presented the lowest translucency and the smallest color change, and its color change was not perceptible at any thickness, while coffee thermocycling did not have any effect on the translucency. | ||||
| Kim et al., 2019 [70] | -Katana ML A Light -IPS e.max CAD lithium dis- ilicate glass-ceramic | L*, a*, b* values were measured with a spectrophotometer and ΔE00 values were calculated | 1.5 mm | Katana (no aging) | 4.81 ± 0.22 | ΔΕ00 | ||
| Katana (aging for 1 h) | 4.93 ± 0.27 | Katana | e.max | |||||
| Katana (aging for 3 h) | 4.95 ± 0.08 | Aging for 1 h | 2.52 | 0.22 | ||||
| Katana (aging for 5 h) | 5.07 ± 0.16 | |||||||
| Katana (aging for 10 h) | 4.88 ± 0.09 | Aging for 3 h | 2.49 | 0.09 | ||||
| Specimens were stored in an autoclave at 134 °C under 0.2 MPa for 0, 1, 3, 5 or 10 h. | e.max (no aging) | 7.95 ± 0.28 | ||||||
| e.max (aging for 1 h) | 8.14 ± 0.25 | Aging for 5 h | 2.03 | 0.23 | ||||
| e.max (aging for 3 h) | 8.24 ± 0.13 | |||||||
| e.max (aging for 5 h) | 8.22 ± 0.18 | Aging for 10 h | 2.1 | 0.07 | ||||
| e.max (aging for 10 h) | 8.42 ± 0.06 | |||||||
| Walczak et al., 2019 [52] | Cercon ht white | L*, a*, b* values and Y tristimulus values against a white and a black background were measured using a spectrophotometer. CR and TP we calculated. | 0.5 mm | CR values | TP values | |||
| Before aging | After aging | Before aging | After aging | |||||
| BruxZir Solid Zirconia ZenostarT0 | Cercon ht white | 0.76 ± 0.03 | 0.78 ± 0.04 | 11.72 ± 1.61 | 11.12 ± 2.03 | |||
| BruxZir Solid Zirconia | 0.76 ± 0.01 | 0.80 ± 0.02 | 11.66 ± 0.73 | 10.08 ± 0.67 | ||||
| ZenostarT0 | 0.74 ± 0.18 | 0.78 ± 0.15 | 12.96 ± 0.89 | 10.49 ± 0.75 | ||||
| Artificial aging with storage in steam autoclave at 134 °C and 0.2 MPa pressure for 5 h | Lava Plus | 0.79 ± 0.14 | 0.80 ± 0.21 | 10.59 ± 0.72 | 10.13 ± 0.84 | |||
| Lava Plus | ||||||||
| Authors | Zirconia System | Number/Teeth | Mean Follow-Up | Survival Rate | Complications | |
|---|---|---|---|---|---|---|
| Limmer et al., 2014 [142] | ZirkonZahn | Full-arch fixed prosthesis (MZ-FDP) | 1 year | 1-year: 88% | Chipped denture tooth | 6 |
| Fractured abutment | 2 | |||||
| Loose abutment | 1 | |||||
| Fractured MZ-FDP | 1 | |||||
| Debonded component | 1 | |||||
| Implant failure | 1 | |||||
| Bömicke et al., 2016 [126] | Cercon ht | Single tooth crowns: | 35.16 ± 6.3 months | 3-year: | Monolithic: | |
| 82 monolithic | ||||||
| 66 monolithic partially veneered | loss of retention | 2 | ||||
| Cementation: | 100% for monolithic | endodontic problems | 4 | |||
| 98.5% for partially veneered | secondary caries | 1 | ||||
| vertical root fracture | 1 | |||||
| Glass Ionomer, self-etch or self-adhesive resin | Partially veneered: | |||||
| loss of retention | 1 | |||||
| minor chipping | 1 | |||||
| periodontits | 2 | |||||
| Pihlaja et al., 2016 [130] | Pretau | 3–12 units; mean, 4.5 units FPDs | 3–7 years | 100% | No complication at al | |
| Güngör et al., 2017 [129] | InCoris TZI | Single tooth crown: | 18.6 ± 3.9 months | 2-year: | Crown fracture | 1 |
| 30 (18 molar, 12 premolar) | ||||||
| Fixed dental prosthesis: 13 | 86.7% for crowns | Connector fracture | 1 | |||
| 92.3% for FDPs | Decementation | 1 | ||||
| Cementation: adhesive resin cement | ||||||
| Endodontic treatment requirement | 1 | |||||
| Unesthetic appearance | 2 | |||||
| Gunge et al., 2017 [128] | Cercon ht | Single tooth crowns: | 25.0 ± 9.9 months | 3.5 years: 91.5% | Severe hyperesthesia | 1 |
| 148 monolithic premolar or molar | ||||||
| Cementation: | Root fracture | 1 | ||||
| self-etch, dual-cure, composite cement system | Restoration fracture | 1 | ||||
| Pulpitis | 2 | |||||
| Abutment tooth for fixed partial denture | 1 | |||||
| Worni et al., 2017 [127] | Ceramill Zolid | Single tooth crowns: 56 | 12–36 months | 3 year: 100%, | No technical or biological complications | |
| Fixed dental prostheses: 15 on teeth | ||||||
| Shahdad et al., 2018 [135] | Zerion | 58 single unit resin-bonded bridges | 36.2 months | 3 year: 82.7% | Debonding | 9 |
| Framework fracture | 1 | |||||
| Hansen et al., 2018 [133] | Bruxzir | Single tooth crowns: 84 | 20 months | 20 months: 93.5% | Fractured crown | 1 |
| Chipping | 4 | |||||
| Levartovsky et al., 2019 [132] | Prettau (veneered and non-veneered) | Single tooth crowns | 28.2 (± 16.8) months | Overall mean survival 99.6% | Horizontal tooth fracture | 1 |
| 108 veneered | Chipping of the veneering ceramic | 15 | ||||
| 142 non-veneered | ||||||
| Pathan et al., 2019 [134] | DGStar | Single tooth crowns: 60 | 12 months | 12 months: 100% | No complications | |
| Authors | Zirconia System | Number/Teeth | Mean Follow-Up | Survival Rate | Complications | |
|---|---|---|---|---|---|---|
| Cheng et al., 2017 [136] | Ceramil zi or Ceramill Zolid | Posterior single crowns: 44 | 2 years | 2-year: | Porcelain fracture | 1 |
| 91.7% for FDPs | ||||||
| 100% for single crowns | ||||||
| 3-unit FDPs: 12 | Loss of retention | 1 | ||||
| Screw loosening | 2 | |||||
| Framework fracture | 1 | |||||
| Opposing tooth fracture | 1 | |||||
| Cheng et al., 2018 [137] | Ceramil zi or Ceramill Zolid | Posterior single crowns (MZ): 36 | 2 years | 2-year: | MZ: | |
| 97.2% for MZ | Screw loosening | 1 | ||||
| 100% for MC | Loss of retention | 0 | ||||
| Complication free: | Ceramic fracture | 0 | ||||
| Posterior metal-ceramic (MC) crowns: 34 | ||||||
| 97.1% for MZ | MC: | |||||
| 79.4% for MC | Screw loosening | 5 | ||||
| Loss of retention | 2 | |||||
| Ceramic fracture | 1 | |||||
| Rojas Vizcaya et al., 2018 [139] | Prettau | Double full arch fixed prosthesis: 20 | 2–7 years | 2–7 years: 100% | Chipping of pink ceramic | 1 |
| Screw loosening | 2 | |||||
| Bidra et al., 2018 [140] | Pretau | Full arch fixed prosthesis: 2039 | 5 years | 5 years: 99.3% | Prosthesis fracture | 6 |
| Debonding of Ti cylinder | 6 | |||||
| Fracture of Ti cylinder | 3 | |||||
| Degidi et al., 2018 [138] | Pretau | 3-unit FDPs: 76 | 5 years | 5 years: 97.4% | Prosthesis fracture | 1 |
| Antagonist fracture | 3 | |||||
| Detachment of resin veneer of antagonist | 1 | |||||
| Minor chipping of antagonist | 6 | |||||
| Detachment of resin on screw hole | 1 | |||||
| Worni et al., 2017 [127] | Ceramill Zolid | Single crowns: 18 | 12–36 months | 3 year: 98.4% | Implant loss with single crown | 1 |
| Fixed dental prostheses: 20 | ||||||
| Levartovsky et al., 2019 [132] | Prettau (veneered and non-veneered) | Single crowns: 63 | 28.2 (± 16.8) months | 100% | Open proximal contacts | 5 |
| Mangano et al., 2019 [141] | Not specified | Single crowns: 40 | 1 year | 1 year: 97.5% | Implant loss | 1 |
| hybrid abutment loss of connection | 1 | |||||
| zirconia abutment decementation | 1 | |||||
| zirconia crown decementation | 1 | |||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kontonasaki, E.; Rigos, A.E.; Ilia, C.; Istantsos, T. Monolithic Zirconia: An Update to Current Knowledge. Optical Properties, Wear, and Clinical Performance. Dent. J. 2019, 7, 90. https://doi.org/10.3390/dj7030090
Kontonasaki E, Rigos AE, Ilia C, Istantsos T. Monolithic Zirconia: An Update to Current Knowledge. Optical Properties, Wear, and Clinical Performance. Dentistry Journal. 2019; 7(3):90. https://doi.org/10.3390/dj7030090
Chicago/Turabian StyleKontonasaki, Eleana, Athanasios E. Rigos, Charithea Ilia, and Thomas Istantsos. 2019. "Monolithic Zirconia: An Update to Current Knowledge. Optical Properties, Wear, and Clinical Performance" Dentistry Journal 7, no. 3: 90. https://doi.org/10.3390/dj7030090
APA StyleKontonasaki, E., Rigos, A. E., Ilia, C., & Istantsos, T. (2019). Monolithic Zirconia: An Update to Current Knowledge. Optical Properties, Wear, and Clinical Performance. Dentistry Journal, 7(3), 90. https://doi.org/10.3390/dj7030090





