Early Diagnosis on Oral and Potentially Oral Malignant Lesions: A Systematic Review on the VELscope® Fluorescence Method
Abstract
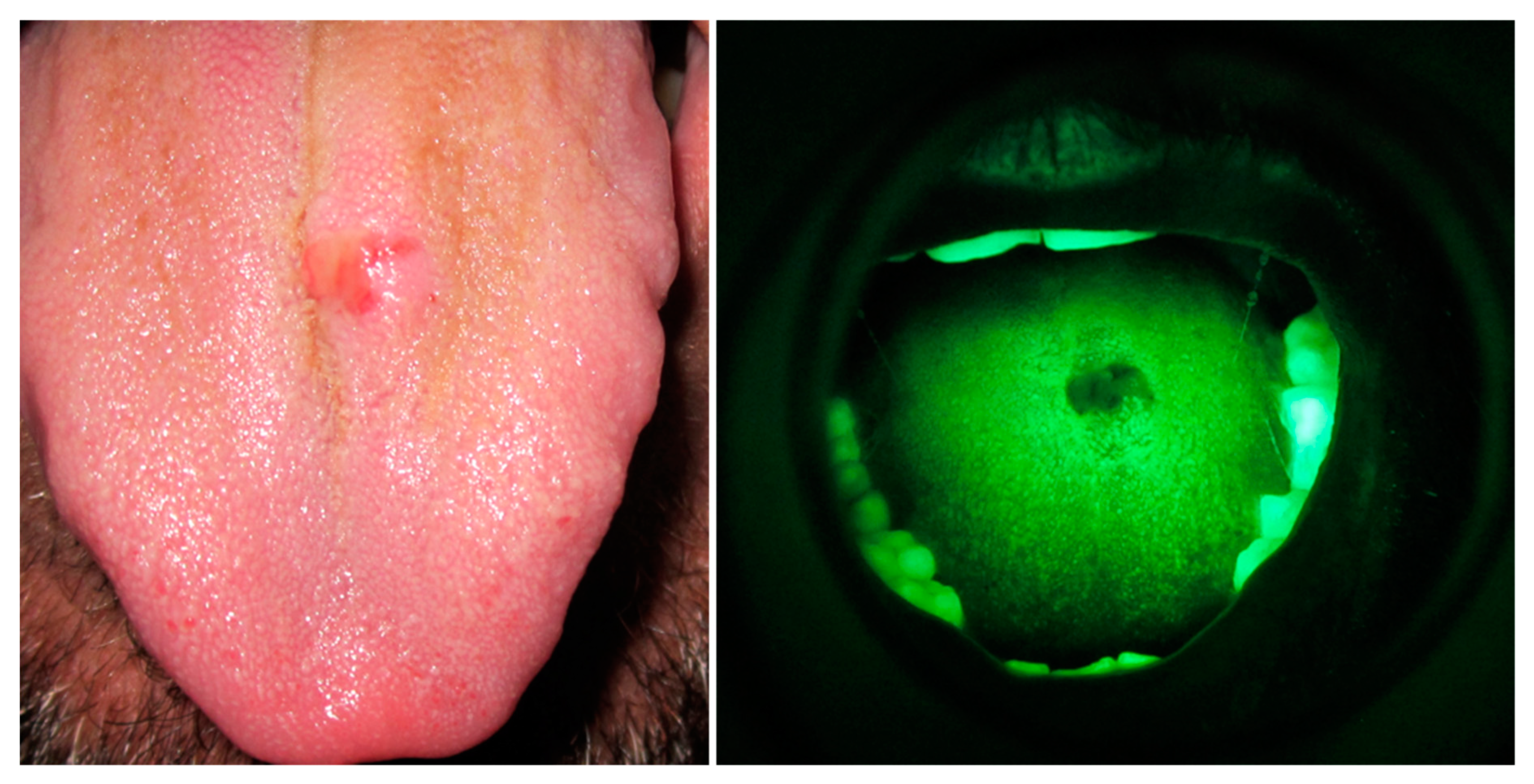
:1. Introduction
1.1. Background
1.2. Objectives
2. Results
2.1. Study Selection
2.2. Study Characteristics
2.3. Risk of Bias within Studies
2.4. Results of Individual Studies
2.5. Synthesis of Results
2.6. Risk of Bias Across Studies
2.7. Additional Analysis
3. Discussion
3.1. Summary of Evidence
3.2. Limitations
4. Materials and Methods
4.1. Protocol and Registration
4.2. Eligibility Criteria
- VELscope® diagnostic tool study
- VELscope® Randomized Controlled Trial (RCT) or Clinical Trial (CT)
- Human studies
- Patients involving systemic or syndromic diseases
- Declared a conflict of interest
- Not enough information about VELscope®
- Animal studies
- Older than 10 years studies
- Not accessible title or abstract
4.3. Information Sources
4.4. Search
4.5. Study Selection
- Last 10 years
- English language studies
- Humans
- Full text
4.6. Data Collection Process
4.7. Items
- Authors (years)—Author name and year of publication
- VELscope® investigation—VELscope® use and compared group
- Oral Pathology—Information about clinical condition and examined sample
- Results—Synthesis of results
- Statistical analysis—Significant or not results
- Sample size—study analyzed sample size
4.8. Risk of Bias in Individual Studies
4.9. Summary Measures
4.10. Additional Analyses
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A

References
- Stornetta, A.; Guidolin, V.; Balbo, S. Alcohol-Derived Acetaldehyde Exposure in the Oral Cavity. Cancers 2018, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Porcheri, C.; Meisel, C.T.; Mitsiadis, T. Multifactorial Contribution of Notch Signaling in Head and Neck Squamous Cell Carcinoma. Int. J. Mol. Sci. 2019, 20, 1520. [Google Scholar] [CrossRef] [PubMed]
- Shashidara, R.; Sreeshyla, H.S.; Sudheendra, U.S. Chemiluminescence: A diagnostic adjunct in oral precancer and cancer: A review. J. Cancer Res. Ther. 2014, 10, 487–491. [Google Scholar] [PubMed]
- Lousada-Fernandez, F.; Rapado-Gonzalez, O.; Lopez-Cedrun, J.-L.; Lopez-Lopez, R.; Muinelo-Romay, L.; Suarez-Cunqueiro, M.M. Liquid Biopsy in Oral Cancer. Int. J. Mol. Sci. 2018, 19, 1704. [Google Scholar] [CrossRef]
- Liyanage, C.; Wathupola, A.; Muraleetharan, S.; Perera, K.; Punyadeera, C.; Udagama, P. Promoter Hypermethylation of Tumor-Suppressor Genes p16INK4a, RASSF1A, TIMP3, and PCQAP/MED15 in Salivary DNA as a Quadruple Biomarker Panel for Early Detection of Oral and Oropharyngeal Cancers. Biomolecules 2019, 9, 148. [Google Scholar] [CrossRef]
- Cervino, G.; Fiorillo, L.; Herford, A.S.; Romeo, U.; Bianchi, A.; Crimi, S.; D’Amico, C.; De Stefano, R.; Troiano, G.; Santoro, R.; et al. Molecular Biomarkers Related to Oral Carcinoma: Clinical Trial Outcome Evaluation in a Literature Review. Dis. Markers 2019, 2019, 8040361. [Google Scholar] [CrossRef]
- Salem, A.; Almahmoudi, R.; Hagström, J.; Stark, H.; Nordström, D.; Salo, T.; Eklund, K.K. Human β-Defensin 2 Expression in Oral Epithelium: Potential Therapeutic Targets in Oral Lichen Planus. Int. J. Mol. Sci. 2019, 20, 1780. [Google Scholar] [CrossRef]
- Kurihara-Shimomura, M.; Sasahira, T.; Shimomura, H.; Nakashima, C.; Kirita, T. The Oncogenic Activity of miR-29b-1-5p Induces the Epithelial-Mesenchymal Transition in Oral Squamous Cell Carcinoma. J. Clin. Med. 2019, 8, 273. [Google Scholar] [CrossRef]
- Hunsaker, M.; Barba, G.; Kingsley, K.; Howard, K.M. Differential MicroRNA Expression of miR-21 and miR-155 within Oral Cancer Extracellular Vesicles in Response to Melatonin. Dent. J. 2019, 7, 48. [Google Scholar] [CrossRef]
- Falzone, L.; Lupo, G.; La Rosa, G.R.M.; Crimi, S.; Anfuso, C.D.; Salemi, R.; Rapisarda, E.; Libra, M.; Candido, S. Identification of Novel MicroRNAs and Their Diagnostic and Prognostic Significance in Oral Cancer. Cancers 2019, 11, 610. [Google Scholar] [CrossRef]
- Chang, Y.-A.; Weng, S.-L.; Yang, S.-F.; Chou, C.-H.; Huang, W.-C.; Tu, S.-J.; Chang, T.-H.; Huang, C.-N.; Jong, Y.-J.; Huang, H.-D. A Three–MicroRNA Signature as a Potential Biomarker for the Early Detection of Oral Cancer. Int. J. Mol. Sci. 2018, 19, 758. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, A.; Burtness, B. Novel Molecular Targets for Chemoprevention in Malignancies of the Head and Neck. Cancers 2017, 9, 113. [Google Scholar] [CrossRef] [PubMed]
- Cervino, G.; Terranova, A.; Briguglio, F.; De Stefano, R.; Famà, F.; D’Amico, C.; Amoroso, G.; Marino, S.; Gorassini, F.; Mastroieni, R.; et al. Diabetes: Oral health related quality of life and oral alterations. BioMed Res. Int. 2019, 2019, 5907195. [Google Scholar] [CrossRef] [PubMed]
- Cervino, G.; Fiorillo, L.; Laino, L.; Herford, A.S.; Lauritano, F.; Giudice, G.L.; Fama, F.; Santoro, R.; Troiano, G.; Iannello, G.; et al. Oral Health Impact Profile in Celiac Patients: Analysis of Recent Findings in a Literature Review. Gastroenterol. Res. Pract. 2018, 2018, 7848735. [Google Scholar] [CrossRef] [PubMed]
- Stacchi, C.; Lombardi, T.; Cusimano, P.; Berton, F.; Lauritano, F.; Cervino, G.; Di Lenarda, R.; Cicciù, M. Bone Scrapers Versus Piezoelectric Surgery in the Lateral Antrostomy for Sinus Floor Elevation. J. Craniofac. Surg. 2017, 28, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Fama, F.; Cicciu, M.; Sindoni, A.; Nastro-Siniscalchi, E.; Falzea, R.; Cervino, G.; Polito, F.; De Ponte, F.; Gioffre-Florio, M. Maxillofacial and concomitant serious injuries: An eight-year single center experience. Chin. J. Traumatol. 2017, 20, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Giudice, G.; Cicciù, M.; Cervino, G.; Lizio, A.; Visco, A. Flowable resin and marginal gap on tooth third medial cavity involving enamel and radicular cementum: A SEM evaluation of two restoration techniques. Indian J. Dental Res. 2012, 23, 763–769. [Google Scholar]
- Giudice, G.; Lipari, F.; Lizio, A.; Cervino, G.; Cicciù, M. Tooth fragment reattachment technique on a pluri traumatized tooth. J. Conserv. Dent. 2012, 15, 80–83. [Google Scholar] [PubMed]
- Farah, C.S.; Kordbacheh, F.; John, K.; Bennett, N.; Fox, S.A. Molecular classification of autofluorescence excision margins in oral potentially malignant disorders. Oral Dis. 2018, 24, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Canjau, S.; Todea, D.C.M.; Sinescu, C.; Pricop, M.O.; Duma, V.F. Fluorescence influence on screening decisions for oral malignant lesions. Rom. J. Morphol. Embryol. 2018, 59, 203–209. [Google Scholar]
- Amirchaghmaghi, M.; Mohtasham, N.; Delavarian, Z.; Shakeri, M.T.; Hatami, M.; Mosannen Mozafari, P. The diagnostic value of the native fluorescence visualization device for early detection of premalignant/malignant lesions of the oral cavity. Photodiagnosis Photodyn. Ther. 2018, 21, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Kawaguchi, K.; Fujihara, H.; Hasebe, M.; Kishi, Y.; Yasukawa, M.; Kumagai, K.; Hamada, Y. Detection accuracy for epithelial dysplasia using an objective autofluorescence visualization method based on the luminance ratio. Int. J. Oral Sci. 2017, 9, e2. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.T.; Huang, J.S.; Wang, Y.Y.; Chen, K.C.; Wong, T.Y.; Chen, Y.C.; Wu, C.W.; Chan, L.P.; Lin, Y.C.; Kao, Y.H.; et al. Novel quantitative analysis of autofluorescence images for oral cancer screening. Oral Oncol. 2017, 68, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Ganga, R.S.; Gundre, D.; Bansal, S.; Shirsat, P.M.; Prasad, P.; Desai, R.S. Evaluation of the diagnostic efficacy and spectrum of autofluorescence of benign, dysplastic and malignant lesions of the oral cavity using VELscope. Oral Oncol. 2017, 75, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Cicciu, M.; Herford, A.S.; Cervino, G.; Troiano, G.; Lauritano, F.; Laino, L. Tissue Fluorescence Imaging (VELscope) for Quick Non-Invasive Diagnosis in Oral Pathology. J. Craniofac. Surg. 2017, 28, e112–e115. [Google Scholar] [CrossRef] [PubMed]
- Burian, E.; Schulz, C.; Probst, F.; Palla, B.; Troltzsch, M.; Maglitto, F.; Califano, L.; Ehrenfeld, M.; Otto, S. Fluorescence based characterization of early oral squamous cell carcinoma using the Visually Enhanced Light Scope technique. J. Cranio Maxillo Facial Surg. 2017, 45, 1526–1530. [Google Scholar] [CrossRef] [PubMed]
- Scheer, M.; Fuss, J.; Derman, M.A.; Kreppel, M.; Neugebauer, J.; Rothamel, D.; Drebber, U.; Zoeller, J.E. Autofluorescence imaging in recurrent oral squamous cell carcinoma. Oral Maxillofac. Surg. 2016, 20, 27–33. [Google Scholar] [CrossRef]
- Ohnishi, Y.; Fujii, T.; Ugaki, Y.; Yasui, H.; Watanabe, M.; Dateoka, S.; Kakudo, K. Usefulness of a fluorescence visualization system for the detection of oral precancerous and early cancerous lesions. Oncol. Rep. 2016, 36, 514–520. [Google Scholar] [CrossRef] [Green Version]
- Nagi, R.; Reddy-Kantharaj, Y.B.; Rakesh, N.; Janardhan-Reddy, S.; Sahu, S. Efficacy of light based detection systems for early detection of oral cancer and oral potentially malignant disorders: Systematic review. Med. Oral Patol. Oral Cir. Bucal 2016, 21, e447–e455. [Google Scholar] [CrossRef]
- Kordbacheh, F.; Bhatia, N.; Farah, C.S. Patterns of differentially expressed genes in oral mucosal lesions visualised under autofluorescence (VELscope). Oral Dis. 2016, 22, 285–296. [Google Scholar] [CrossRef]
- Rashid, A.; Warnakulasuriya, S. The use of light-based (optical) detection systems as adjuncts in the detection of oral cancer and oral potentially malignant disorders: A systematic review. J. Oral Pathol. Med. 2015, 44, 307–328. [Google Scholar] [CrossRef] [PubMed]
- Jane-Salas, E.; Blanco-Carrion, A.; Jover-Armengol, L.; Lopez-Lopez, J. Autofluorescence and Diagnostic Accuracy of Lesions of Oral Mucosa: A Pilot Study. Braz. Dent. J. 2015, 26, 580–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elvers, D.; Braunschweig, T.; Hilgers, R.D.; Ghassemi, A.; Mohlhenrich, S.C.; Holzle, F.; Gerressen, M.; Modabber, A. Margins of oral leukoplakia: Autofluorescence and histopathology. Br. J. Oral Maxillofac. Surg. 2015, 53, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Hanken, H.; Kraatz, J.; Smeets, R.; Heiland, M.; Assaf, A.T.; Blessmann, M.; Eichhorn, W.; Clauditz, T.S.; Grobe, A.; Kolk, A.; et al. The detection of oral pre- malignant lesions with an autofluorescence based imaging system (VELscope)—A single blinded clinical evaluation. Head Face Med. 2013, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.; Zapf, A.; Kuehle, M.; Gellrich, N.C.; Eckardt, A.M. Clinical evaluation of an autofluorescence diagnostic device for oral cancer detection: A prospective randomized diagnostic study. Eur. J. Cancer Prev. 2012, 21, 460–466. [Google Scholar] [CrossRef] [PubMed]
- McNamara, K.K.; Martin, B.D.; Evans, E.W.; Kalmar, J.R. The role of direct visual fluorescent examination (VELscope) in routine screening for potentially malignant oral mucosal lesions. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Farah, C.S.; McIntosh, L.; Georgiou, A.; McCullough, M.J. Efficacy of tissue autofluorescence imaging (VELScope) in the visualization of oral mucosal lesions. Head Neck 2012, 34, 856–862. [Google Scholar] [CrossRef]
- Scheer, M.; Neugebauer, J.; Derman, A.; Fuss, J.; Drebber, U.; Zoeller, J.E. Autofluorescence imaging of potentially malignant mucosa lesions. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 111, 568–577. [Google Scholar] [CrossRef]
- Matsumoto, K. Detection of potentially malignant and malignant lesions of oral cavity using autofluorescence visualization device. J. Stomatol. Soc. Jpn. 2011, 78, 73–80. [Google Scholar]
- Lopez-Jornet, P.; De la Mano-Espinosa, T. The efficacy of direct tissue fluorescence visualization in screening for oral premalignant lesions in general practice: An update. Int. J. Dent. Hyg. 2011, 9, 97–100. [Google Scholar] [CrossRef]
- Fricain, J.C. Autofluorescence for the detection of potentially malignant and malignant lesions of the oral cavity lining. Rev. Stomatol. Chir. Maxillofac. 2011, 112, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Awan, K.H.; Morgan, P.R.; Warnakulasuriya, S. Evaluation of an autofluorescence based imaging system (VELscope) in the detection of oral potentially malignant disorders and benign keratoses. Oral Oncol. 2011, 47, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, R.; Singh, M.; Thomas, S.; Nair, P.; Pandya, S.; Nigam, N.S.; Shukla, P. A cross-sectional study evaluating chemiluminescence and autofluorescence in the detection of clinically innocuous precancerous and cancerous oral lesions. J. Am. Dent. Assoc. 2010, 141, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Lingen, M.W.; Tampi, M.P.; Urquhart, O.; Abt, E.; Agrawal, N.; Chaturvedi, A.K.; Cohen, E.; D’Souza, G.; Gurenlian, J.; Kalmar, J.R.; et al. Adjuncts for the evaluation of potentially malignant disorders in the oral cavity: Diagnostic test accuracy systematic review and meta-analysis-a report of the American Dental Association. J. Am. Dent. Assoc. 2017, 148, 797–813. [Google Scholar] [CrossRef] [PubMed]
- Poli, P.P.; Beretta, M.; Cicciù, M.; Maiorana, C. Alveolar ridge augmentation with titanium mesh. A retrospective clinical study. Open Dent. J. 2014, 8, 148–158. [Google Scholar] [CrossRef]
- Beretta, M.; Cicciù, M.; Bramanti, E.; Maiorana, C. Schneider membrane elevation in presence of sinus septa: Anatomic features and surgical management. Int. J. Dent. 2012, 2012, 261905. [Google Scholar] [CrossRef]
- Rancitelli, D.; Borgonovo, A.E.; Cicciù, M.; Re, D.; Rizza, F.; Frigo, A.C.; Maiorana, C. Maxillary sinus septa and anatomic correlation with the Schneiderian membrane. J. Cranio Fac. Surg. 2015, 26, 1394–1398. [Google Scholar] [CrossRef]
- Cicciu, M.; Cervino, G.; Herford, A.S.; Fama, F.; Bramanti, E.; Fiorillo, L.; Lauritano, F.; Sambataro, S.; Troiano, G.; Laino, L. Facial Bone Reconstruction Using both Marine or Non-Marine Bone Substitutes: Evaluation of Current Outcomes in a Systematic Literature Review. Mar. Drugs 2018, 16, 27. [Google Scholar] [CrossRef]
- Stacchi, C.; Berton, F.; Fiorillo, L.; Nicolin, V.; Lombardi, T.; Cicciù, M.; Di Lenarda, R. Fresh frozen allogeneic bone block in maxillary sinus floor elevation: Histomorphometric analysis of a bone specimen retrieved 15 years after grafting procedure. Appl. Sci. 2019, 9, 1119. [Google Scholar] [CrossRef]
- Rullo, R.; Scalzone, P.; Laino, L.; Russo, A.; Festa, V.M.; Fiorillo, L.; Cicciu, M. Solitary Plasmacytoma of the Mandible: Early Diagnosis and Surgical Management. J. Cranio Fac. Surg. 2019, 30, e411–e413. [Google Scholar] [CrossRef]
- Lombardi, T.; Bernardello, F.; Berton, F.; Porrelli, D.; Rapani, A.; Camurri Piloni, A.; Fiorillo, L.; Di Lenarda, R.; Stacchi, C. Efficacy of Alveolar Ridge Preservation after Maxillary Molar Extraction in Reducing Crestal Bone Resorption and Sinus Pneumatization: A Multicenter Prospective Case-Control Study. BioMed Res. Int. 2018, 2018, 9352130. [Google Scholar] [CrossRef] [PubMed]
- Cervino, G.; Fiorillo, L.; Arzukanyan, A.V.; Spagnuolo, G.; Cicciu, M. Dental Restorative Digital Workflow: Digital Smile Design from Aesthetic to Function. Dent. J. 2019, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Herford, A.S.; Cicciù, M.; Eftimie, L.F.; Miller, M.; Signorino, F.; Famà, F.; Cervino, G.; Lo Giudice, G.; Bramanti, E.; Lauritano, F.; et al. rhBMP-2 applied as support of distraction osteogenesis: A split-mouth histological study over nonhuman primates mandibles. Int. J. Clin. Exp. Med. 2016, 9, 17187–17194. [Google Scholar]
- Bramanti, E.; Matacena, G.; Cecchetti, F.; Arcuri, C.; Cicciù, M. Oral health-related quality of life in partially edentulous patients before and after implant therapy: A 2-year longitudinal study. ORAL Implantol. 2013, 6, 37–42. [Google Scholar] [CrossRef]
- Cicciù, M.; Beretta, M.; Risitano, G.; Maiorana, C. Cemented-retained vs. screw-retained implant restorations: An investigation on 1939 dental implants. Minerva Stomatol. 2008, 57, 167–179. [Google Scholar] [PubMed]
- Oteri, G.; Procopio, R.M.; Cicciù, M. Giant salivary gland calculi (GSGC): Report of two cases. Open Dent. J. 2011, 5, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Menditti, D.; Laino, L.; Cicciù, M.; Mezzogiorno, A.; Perillo, L.; Menditti, M.; Cervino, G.; Muzio, L.L.; Baldi, A. Kissing molars: Report of three cases and new prospective on aetiopathogenetic theories. Int. J. Clin. Exp. Pathol. 2015, 8, 15708–15718. [Google Scholar]
- Lo Giudice, G.; Cutroneo, G.; Centofanti, A.; Artemisia, A.; Bramanti, E.; Militi, A.; Rizzo, G.; Favaloro, A.; Irrera, A.; Lo Giudice, R.; et al. Dentin morphology of root canal surface: A quantitative evaluation based on a scanning electronic microscopy study. BioMed Res. Int. 2015, 2015, 7. [Google Scholar] [CrossRef]
- Germano, F.; Bramanti, E.; Arcuri, C.; Cecchetti, F.; Cicciù, M. Atomic force microscopy of bacteria from periodontal subgingival biofilm: Preliminary study results. Eur. J. Dent. 2013, 7, 152–158. [Google Scholar] [Green Version]
- Cicciù, M.; Herford, A.S.; Stoffella, E.; Cervino, G.; Cicciù, D. Protein-signaled guided bone regeneration using titanium mesh and Rh-BMP2 in oral surgery: A case report involving left mandibular reconstruction after tumor resection. Open Dent. J. 2012, 6, 51–55. [Google Scholar] [CrossRef]
- Maiorana, C.; Beretta, M.; Grossi, G.B.; Santoro, F.; Herford, A.S.; Nagursky, H.; Cicciù, M. Histomorphometric evaluation of anorganic bovine bone coverage to reduce autogenous grafts resorption: Preliminary results. Open Dent. J. 2011, 5, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Cervino, G.; Cicciù, M.; Biondi, A.; Bocchieri, S.; Herford, A.S.; Laino, L.; Fiorillo, L. Antibiotic Prophylaxis on Third Molar Extraction: Systematic Review of Recent Data. Antibiotics 2019, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Troiano, G.; Laino, L.; Cicciu, M.; Cervino, G.; Fiorillo, L.; D’Amico, C.; Zhurakivska, K.; Lo Muzio, L. Comparison of Two Routes of Administration of Dexamethasone to Reduce the Postoperative Sequelae After Third Molar Surgery: A Systematic Review and Meta-Analysis. Open Dent. J. 2018, 12, 181–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiorillo, L. Chlorhexidine gel use in the oral district: A systematic review. Gels 2019, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Crimi, S.; Fiorillo, L.; Bianchi, A.; D’Amico, C.; Amoroso, G.; Gorassini, F.; Mastroieni, R.; Marino, S.; Scoglio, C.; Catalano, F.; et al. Herpes Virus, Oral Clinical Signs and QoL: Systematic Review of Recent Data. Viruses 2019, 11, 463. [Google Scholar] [CrossRef] [PubMed]
- Fiorillo, L.; Cervino, G.; Herford, A.S.; Lauritano, F.; D’Amico, C.; Lo Giudice, R.; Laino, L.; Troiano, G.; Crimi, S.; Cicciu, M. Interferon Crevicular Fluid Profile and Correlation with Periodontal Disease and Wound Healing: A Systemic Review of Recent Data. Int. J. Mol. Sci. 2018, 19, 1908. [Google Scholar] [CrossRef] [PubMed]
- Laino, L.; Cicciù, M.; Fiorillo, L.; Crimi, S.; Bianchi, A.; Amoroso, G.; Monte, I.P.; Herford, A.S.; Cervino, G. Surgical Risk on Patients with Coagulopathies: Guidelines on Hemophiliac Patients for Oro-Maxillofacial Surgery. Int. J. Environ. Res. Public Health 2019, 16, 1386. [Google Scholar] [CrossRef] [PubMed]
- Fiorillo, L.; De Stefano, R.; Cervino, G.; Crimi, S.; Bianchi, A.; Campagna, P.; Herford, A.S.; Laino, L.; Cicciù, M. Oral and Psychological Alterations in Haemophiliac Patients. Biomedicines 2019, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Cervino, G.; Fiorillo, L.; Monte, I.P.; De Stefano, R.; Laino, L.; Crimi, S.; Bianchi, A.; Herford, A.S.; Biondi, A.; Cicciù, M. Advances in Antiplatelet Therapy for Dentofacial Surgery Patients: Focus on Past and Present Strategies. Materials 2019, 12, 1524. [Google Scholar] [CrossRef]
- Bachelet, V.C.; Pardo-Hernandez, H. Quality of reporting and risk of bias of randomized clinical trials published in Spanish and Latin American journals. Medwave 2019, 19, e7573. [Google Scholar] [CrossRef]
- Whiting, P.; Savovic, J.; Higgins, J.P.T.; Caldwell, D.M.; Reeves, B.C.; Shea, B.; Davies, P.; Kleijnen, J.; Churchill, R. ROBIS: A new tool to assess risk of bias in systematic reviews was developed. Recenti Prog. Med. 2018, 109, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Savovic, J.; Turner, R.M.; Mawdsley, D.; Jones, H.E.; Beynon, R.; Higgins, J.P.T.; Sterne, J.A.C. Association Between Risk-of-Bias Assessments and Results of Randomized Trials in Cochrane Reviews: The ROBES Meta-Epidemiologic Study. Am. J. Epidemiol. 2018, 187, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Mansournia, M.A.; Higgins, J.P.; Sterne, J.A.; Hernan, M.A. Biases in Randomized Trials: A Conversation Between Trialists and Epidemiologists. Epidemiology 2017, 28, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Coburn, K.M.; Vevea, J.L. Publication bias as a function of study characteristics. Psychol. Methods 2015, 20, 310–330. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Br. Med. J. 2011, 343, d5928. [Google Scholar] [CrossRef]
- Bagri-Manjrekar, K.; Chaudhary, M.; Sridharan, G.; Tekade, S.R.; Gadbail, A.R.; Khot, K. In vivo autofluorescence of oral squamous cell carcinoma correlated to cell proliferation rate. J. Cancer Res. Ther. 2018, 14, 553–558. [Google Scholar] [CrossRef]
- Quang, T.; Tran, E.Q.; Schwarz, R.A.; Williams, M.D.; Vigneswaran, N.; Gillenwater, A.M.; Richards-Kortum, R. Prospective Evaluation of Multimodal Optical Imaging with Automated Image Analysis to Detect Oral Neoplasia In Vivo. Cancer Prev. Res. 2017, 10, 563–570. [Google Scholar] [CrossRef]
- Uthoff, R.D.; Song, B.; Sunny, S.; Patrick, S.; Suresh, A.; Kolur, T.; Keerthi, G.; Spires, O.; Anbarani, A.; Wilder-Smith, P.; et al. Point-of-care, smartphone-based, dual-modality, dual-view, oral cancer screening device with neural network classification for low-resource communities. PLoS ONE 2018, 13, e0207493. [Google Scholar] [CrossRef]
- Fei, B.; Lu, G.; Wang, X.; Zhang, H.; Little, J.V.; Patel, M.R.; Griffith, C.C.; El-Diery, M.W.; Chen, A.Y. Label-free reflectance hyperspectral imaging for tumor margin assessment: A pilot study on surgical specimens of cancer patients. J. Biomed. Opt. 2017, 22, 1–7. [Google Scholar] [CrossRef]
- Amaechi, B.T.; Owosho, A.A.; Fried, D. Fluorescence and Near-Infrared Light Transillumination. Dent. Clin. N. Am. 2018, 62, 435–452. [Google Scholar] [CrossRef]


| Authors (Year) | VELscope Investigation | Oral Pathology | Results | Statistical Analysis | Sample Size |
|---|---|---|---|---|---|
| Farah et al. (2018) [19] | Differences between white light and autofluorescence on oral potentially malignant disorders (OPMD) detected margins. | Oral epithelial dysplasia, oral lichen planus, oral lichenoid dysplasia | Autofluorescence determined margins are safer | 11 | |
| Canjau et al. (2018) [20] | Conventional oral examination vs. visual fluorescence evaluation with VELscope® | Malignant lesions and premalignant lesions | Fluorescence examination cannot replace histopathology, but it may add sensitivity to the conventional examination | 18 | |
| Amirchagmaghi et al. (2018) [21] | Fluorescence evaluation in patients with oral lesions | Dysplasia and oral carcinoma | This method is not capable to distinguish malignant from benign lesions. | 45 | |
| Yamamoto et al. (2017) [22] | Detection accuracy of VELscope on epithelial dysplasia of the tongue | Leukoplakia with and without dysplasia | Autofluorescence visualization could be an auxiliary method for diagnosis | Luminance ration on malignant lesion: p < 0.0001 | 79 |
| Huang et al. (2017) [23] | White light vs. VELscope® method | Oral cancer, precancerous lesions | Fluorescence examination could be used to differentiate oral cancer and precancerous lesions from normal mucosa. | 140 | |
| Ganga et al. (2017) [24] | Conventional Oral examination vs. VELscope method | Fluorescence examination could not provide a definitive diagnosis | 76% of specificity and sensitivity | 200 | |
| Cicciù et al. (2017) [25] | Tissue fluorescence imaging vs. conventional oral examination or biopsy | Oral squamous cell carcinoma | The main criticism of autofluorescence on performing a diagnosis of cancer was the impossibility of discriminating high-risk from low-risk lesions, but it could be a quick and noninvasive method. | 1 | |
| Burian et al. (2017) [26] | Tissue fluorescence examination VELscope® | Oral soft tissue lesions or carcinoma in situ | VELscope® could help clinicians and help to define biopsy margins | Sensitivity of VELscope p = 0.007 | 90 |
| Scheer et al. (2016) [27] | Tissue fluorescence examination VELscope® | Oral squamous cell carcinomas | VELscope® reveals no additional information to analysis | Sensitivity and specificity were 33.3% and 88.6% | 41 |
| Ohnishi et al. (2016) [28] | Tissue fluorescence examination VELscope® | Carcinoma in situ | Fluorescence examination could represent a simple, cost-effective screening. | Sensitivity 95% and specificity 100% | 17 |
| Nagi et al. (2016) [29] | Chemoluminescence and tissue autofluorescence in detection of Oral Squamous Cell Carcinoma (OSCC) and OPMD | OSCC and OPMD | Chemoluminescence and autofluorescence are simple and not invasive tests. | Sensitivity range 22% to 100% and specificity 16% to 100% | Review |
| Kordbacheh et al. (2016) [30] | Molecular pathways associated with fluorescence properties of OPMD | OSCC, Oral epithelial dysplasia (OED), Oral lichen planus (OLP), Oral epithelial hyperplasia (OEH) | Uncovering these molecular mechanisms could provide a reduction in false positive or negative findings with VELscope® | 42 | |
| Rashid et Warnakulasuriya (2015) [31] | Chemoluminescence vs. tissue autofluorescence in detection of OPMD | OPMD | VELscope® may detect erythematous lesions or benign inflammations as a false positive | Review | |
| Jane-Salas et al. (2015) [32] | Conventional oral examination vs. Autofluorescence technique VELscope® | Oral lesions | No clinical benefits were obtained using the VELscope® system | Not significant results | 60 |
| Elvers et al. (2015) [33] | Photo examination vs. autofluorescence examination VELscope® | OPMD | This technique enables clinicians to measure the extent of lesions beyond visible margins | 20 | |
| Hanken et al. (2013) [34] | Early detection with VELscope® | OPMD | VELscope® is a simple noninvasive way to find OPMD | Sensitivity 22% specificity 8.4% | 120 |
| Rana et al. (2012) [35] | Autofluorescence examination vs. white light examination | OPMD | VELscope® is a useful new diagnostic device for the detection of oral cancer diseases. | Sensitivity 100% specificity 74% | 289 |
| McNamara et al. [36] | COE vs. Fluorescence examination VELscope® | OPMD | Biopsy does not confirm VELscope diagnosis | Scalpel biopsy vs. VELscope® p = 0.0001 | 42 |
| Farah et al. (2012) [37] | Tissue autofluorescence VELscope® in oral mucosa lesions detection | OPMD | VELscope® cannot provide a definitive diagnosis alone | Sensitivity 30% Specificity 63% | 112 |
| Scheer et al. (2011) [38] | Autofluorescence evaluation VELscope® | OPMD, OSCC, squamous intraepithelial neoplasia | VELscope® can assist the clinician during the identification of OPMD, but it does not help in discriminating benign or malignant conditions | Sensitivity 100%, specificity 80% | 64 |
| Matsumoto (2011) [39] | Autofluorescence evaluation VELscope® | OSCC, moderate and severe epithelial dysplasia lesions, mild dysplasia lesions and lichen planus. | VELscope® could be a valuable tool in an early detection of potentially malignant and malignant lesions in oral mucosa. | 74 | |
| Lopez-Jornet et al. (2011) [40] | Autofluorescence evaluation VELscope® | Oral cancer | This device needed a conventional oral examination too | Sensitivity 98% to 100% Specificity 94% to 100% | Review |
| Fricain (2011) [41] | Autofluorescence evaluation VELscope® vs. spectroscopy | OPMD | Histological examination remains the gold standard of OPMD diagnosis, VELscope® could only support clinicians | Sensibility 78% to 100% Specificity 75% to 100% | Review |
| Awan et al. (2011) [42] | Autofluorescence vs. COE and oral biopsy | Oral leukoplakia, oral erythoplakia, oral lichen planus, hyperplastic candidiasis, rest frictional keratosis, oral sub-mucous fibrosis | VELscope® is not able to discriminate high or low risk lesions, but it can diagnose mucosal disorders | Sensibility 84.1% Specificity 15.3% | 126 |
| Mehrotra et al. (2010) [43] | Autofluorescence techniques VELscope® vs. chemoluminescence ViziLite | OPMD | VELscope could provide a false negative | Sensitivity 50% Specificity 38.9% | 102 |
| 1342 Patient’s Data | Sensitivity or Sensibility | Specificity |
|---|---|---|
| VELscope® range | 22% to 100% | 8.4% to 100% |
| VELscope® weighted average | 70.19% | 65.95% |
| VELscope® | Oral leukoplakia, oral erythoplakia, oral lichen planus, hyperplastic candidiasis, rest frictional keratosis, oral sub-mucous fibrosis, oral squamous cell carcinoma, moderate and severe epithelial dysplasia lesions, mild dysplasia lesion, squamous intraepithelial neoplasia, oral carcinoma in situ |
| Authors (Year) | Risk of Bias | |||
|---|---|---|---|---|
| Unclear | Low | Moderate | High | |
| Farah et al. (2018) [19] | x | |||
| Canjau et al. (2018) [20] | x | |||
| Amirchagmaghi et al. (2018) [21] | x | |||
| Yamamoto et al. (2017) [22] | x | |||
| Huang et al. (2017) [23] | x | |||
| Ganga et al. (2017) [24] | x | |||
| Cicciù et al. (2017) [25] | x | |||
| Burian et al. (2017) [26] | x | |||
| Scheer et al. (2016) [27] | x | |||
| Ohnishi et al. (2016) [28] | x | |||
| Nagi et al. (2016) [29] | x | |||
| Kordbacheh et al. (2016) [30] | x | |||
| Rashid et Warnakulasuriya (2015) [31] | x | |||
| Jane-Salas et al. (2015) [32] | x | |||
| Elvers et al. (2015) [33] | x | |||
| Hanken et al. (2013) [34] | x | |||
| Rana et al. (2012) [35] | x | |||
| McNamara et al. [36] | x | |||
| Farah et al. (2012) [37] | x | |||
| Scheer et al. (2011) [38] | x | |||
| Matsumoto (2011) [39] | x | |||
| Lopez-Jornet et al. (2011) [40] | x | |||
| Fricain (2011) [41] | x | |||
| Awan et al. (2011) [42] | x | |||
| Mehrotra et al. (2010) [43] | x | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cicciù, M.; Cervino, G.; Fiorillo, L.; D’Amico, C.; Oteri, G.; Troiano, G.; Zhurakivska, K.; Lo Muzio, L.; Herford, A.S.; Crimi, S.; et al. Early Diagnosis on Oral and Potentially Oral Malignant Lesions: A Systematic Review on the VELscope® Fluorescence Method. Dent. J. 2019, 7, 93. https://doi.org/10.3390/dj7030093
Cicciù M, Cervino G, Fiorillo L, D’Amico C, Oteri G, Troiano G, Zhurakivska K, Lo Muzio L, Herford AS, Crimi S, et al. Early Diagnosis on Oral and Potentially Oral Malignant Lesions: A Systematic Review on the VELscope® Fluorescence Method. Dentistry Journal. 2019; 7(3):93. https://doi.org/10.3390/dj7030093
Chicago/Turabian StyleCicciù, Marco, Gabriele Cervino, Luca Fiorillo, Cesare D’Amico, Giacomo Oteri, Giuseppe Troiano, Khrystyna Zhurakivska, Lorenzo Lo Muzio, Alan Scott Herford, Salvatore Crimi, and et al. 2019. "Early Diagnosis on Oral and Potentially Oral Malignant Lesions: A Systematic Review on the VELscope® Fluorescence Method" Dentistry Journal 7, no. 3: 93. https://doi.org/10.3390/dj7030093
APA StyleCicciù, M., Cervino, G., Fiorillo, L., D’Amico, C., Oteri, G., Troiano, G., Zhurakivska, K., Lo Muzio, L., Herford, A. S., Crimi, S., Bianchi, A., Di Stasio, D., Rullo, R., Laino, G., & Laino, L. (2019). Early Diagnosis on Oral and Potentially Oral Malignant Lesions: A Systematic Review on the VELscope® Fluorescence Method. Dentistry Journal, 7(3), 93. https://doi.org/10.3390/dj7030093















