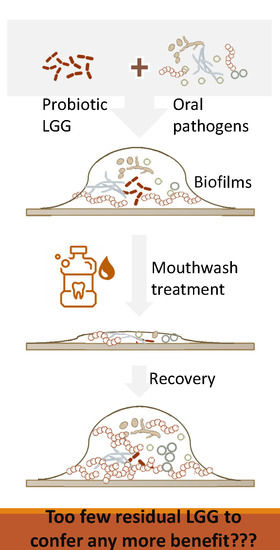
Mouthwash Effects on LGG-Integrated Experimental Oral Biofilms
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains, Growth Conditions, and Inoculum Preparation
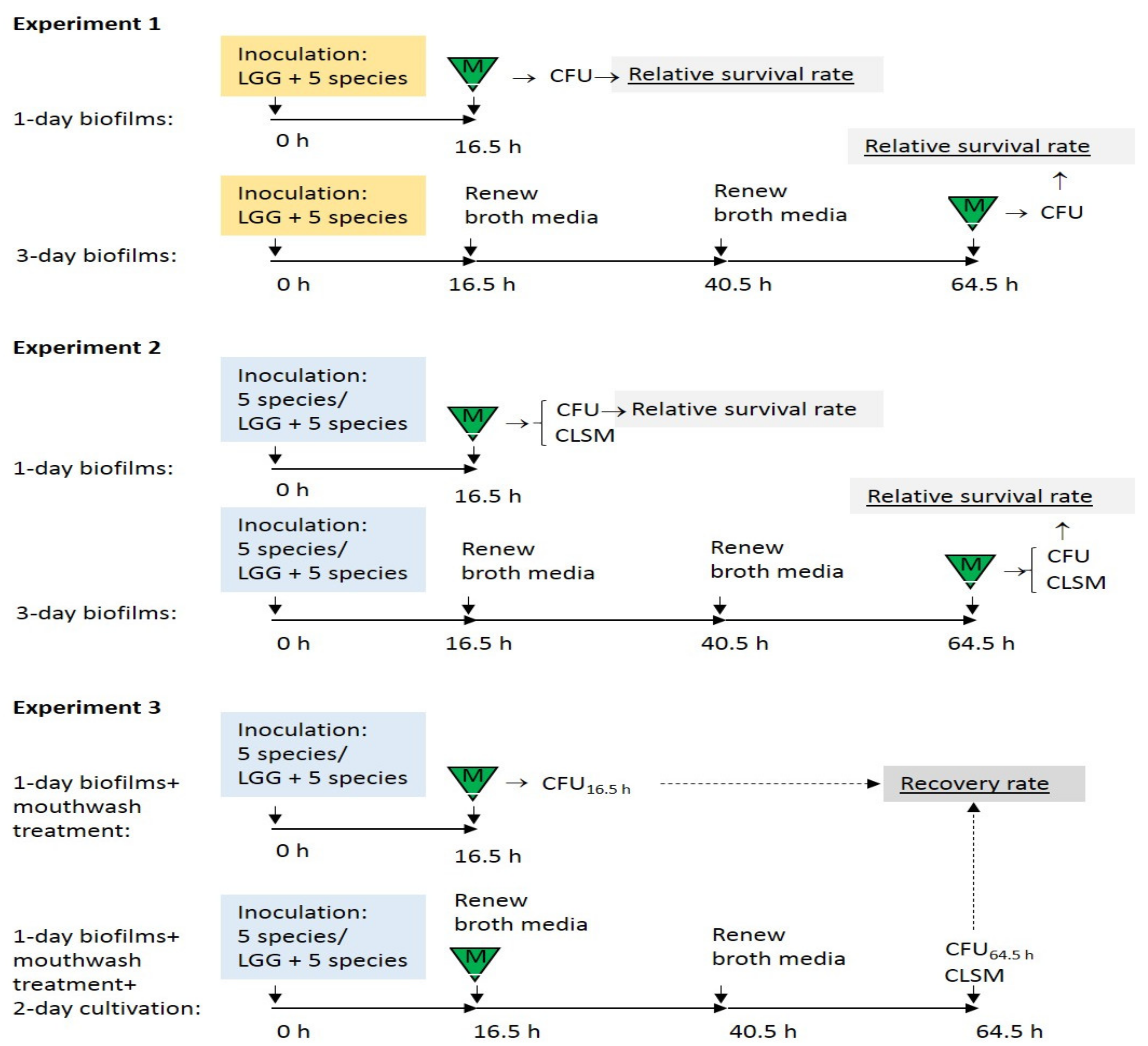
2.2. Preparation of Biofilms
2.3. Susceptibility of Probiotic LGG to Mouthwashes Compared with Streptococci and Candida in the Biofilms
2.3.1. Enumeration of Live Cells
2.3.2. Calculation of the Relative Survival Rates
2.4. Influence of LGG on the Efficacy of Mouthwashes on Biofilm Streptococci and Candida
2.5. Recovery of Streptococci and Candida in LGG-Free and LGG-Integrated Biofilms after Mouthwash Rinsing
2.6. Statistical Analysis
3. Results
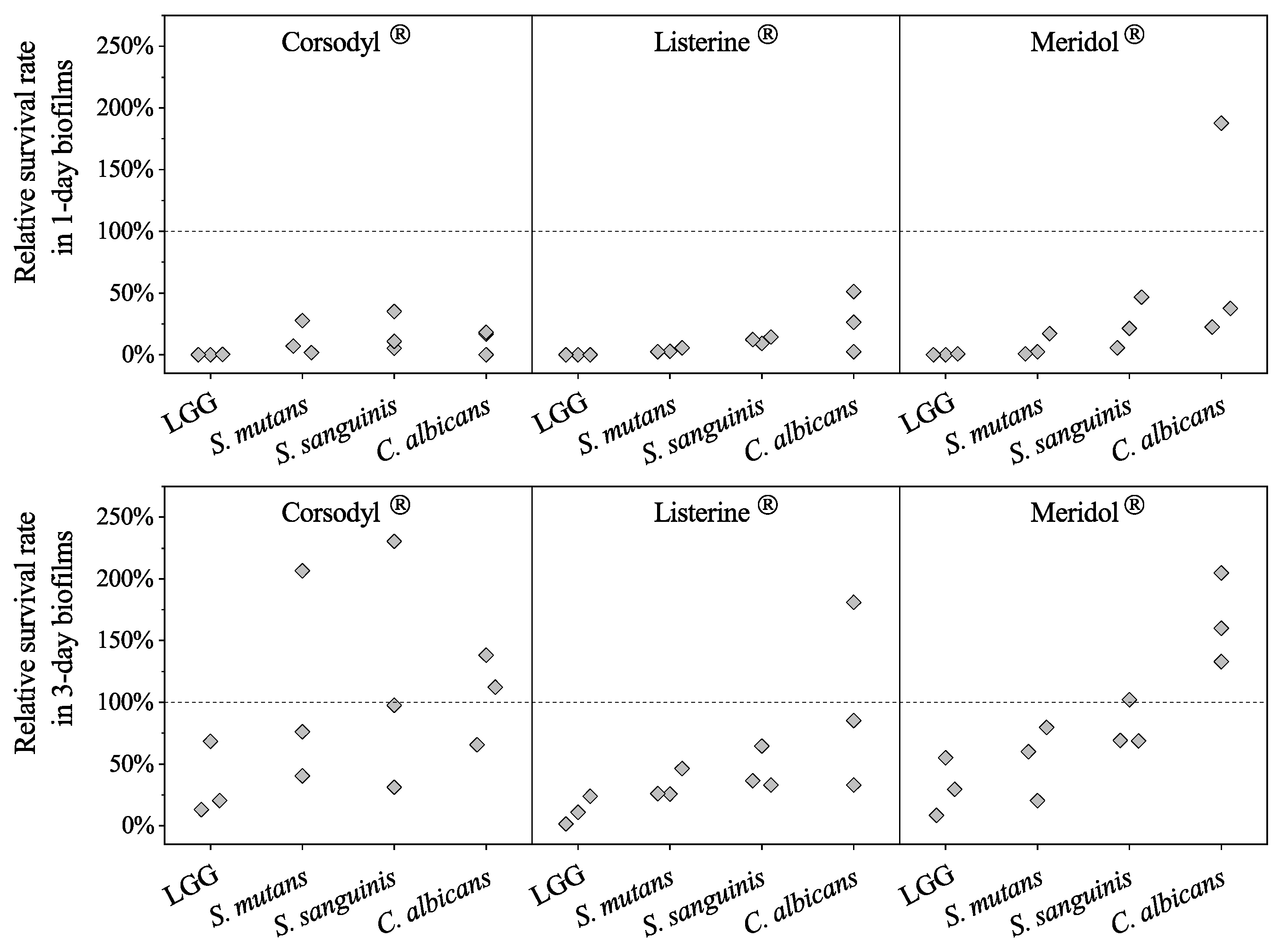
3.1. Susceptibility to Mouthwashes of Probiotic LGG Compared with Susceptibility of Streptococci and of Candida in the Biofilms
Relative Survival Rates of LGG, Streptococci, and Candida in LGG-Integrated Biofilms
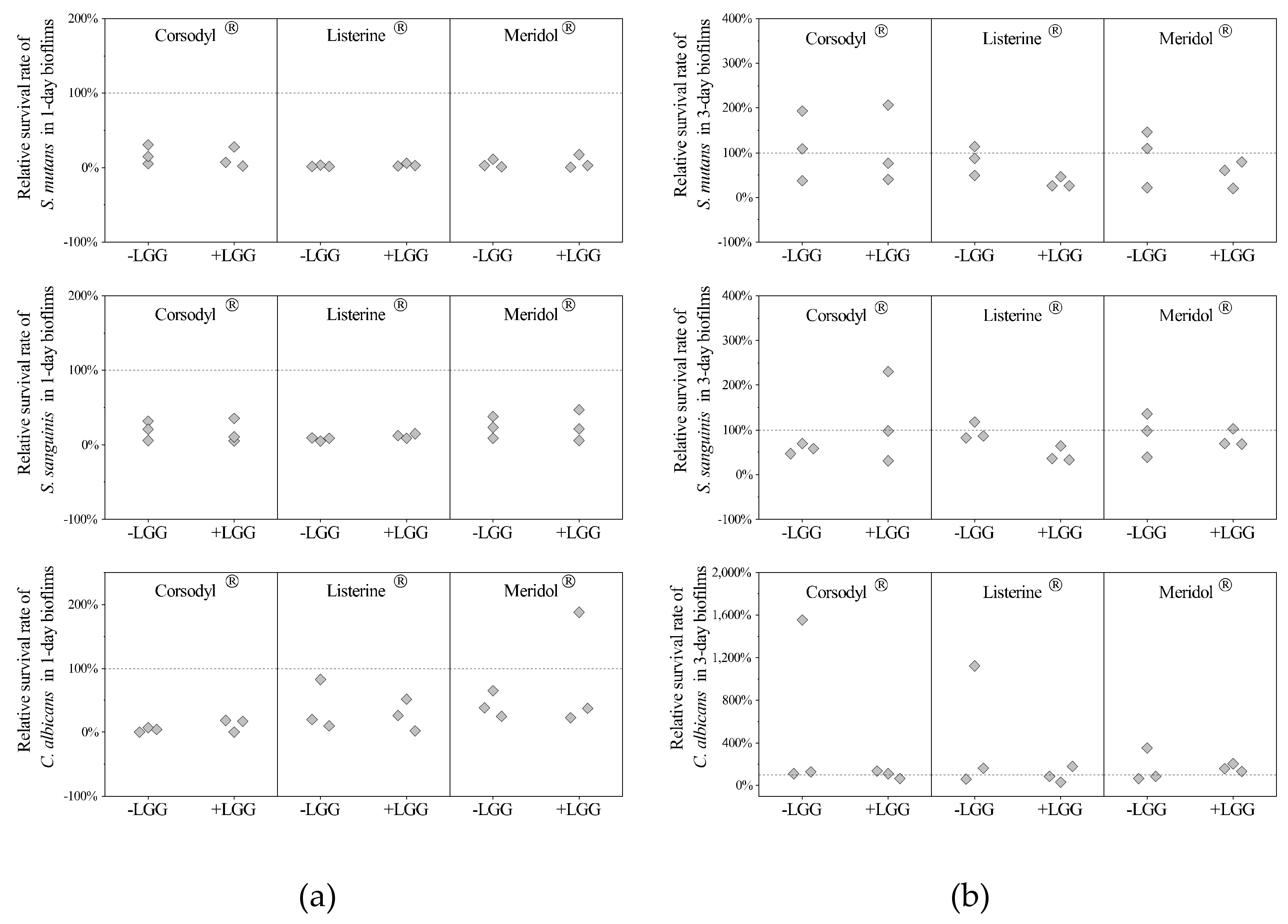
3.2. Influence of LGG on Mouthwash Efficacy against Streptococci and Candida in the Biofilms
3.2.1. Relative Survival Rates of Streptococci and Candida in LGG-Free and LGG-Integrated Biofilms
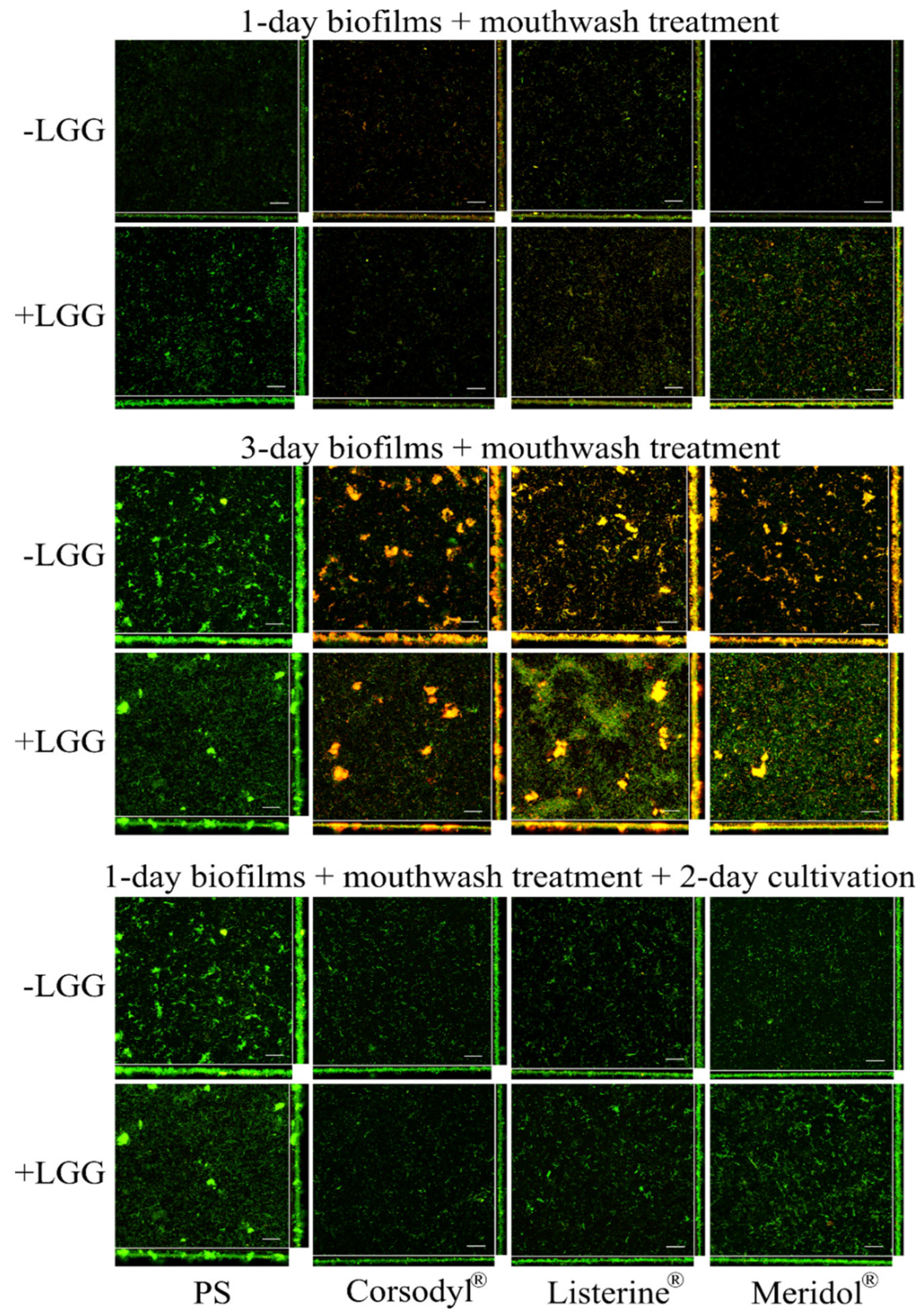
3.2.2. Structural Analysis of Biofilms
3.3. Recovery of Streptococci and Candida in LGG-Free and LGG-Integrated Biofilms after the Mouthwash Rinsing
3.3.1. Recovery Rates of Streptococci and Candida in LGG-Free and LGG-Integrated Biofilms
3.3.2. Structural Analysis of Biofilms
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hollmann, B.; Perkins, M.; Walsh, D. Biofilms and Their Role in Pathogenesis. British Society for Immunology. Available online: https://www.immunology.org/public-information/bitesized-immunology/pathogens-and-disease/biofilms-and-their-role-in (accessed on 7 August 2020).
- Kumar, S.; Tadakamadla, J.; Johnson, N.W. Effect of toothbrushing frequency on incidence and increment of dental caries. J. Dent. Res. 2016, 95, 1230–1236. [Google Scholar] [CrossRef] [PubMed]
- Sicca, C.; Bobbio, E.; Quartuccio, N.; Nicolò, G.; Cistaro, A. Prevention of dental caries: A review of effective treatments. J. Clin. Exp. Dent. 2016, 8, e604–e610. [Google Scholar] [CrossRef] [PubMed]
- Lertpimonchai, A.; Rattanasiri, S.; Vallibhakara, S.A.-O.; Attia, J.; Thakkinstian, A. The association between oral hygiene and periodontitis: A systematic review and meta-analysis. Int. Dent. J. 2017, 67, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Yaacob, M.; Worthington, H.; Deacon, S.A.; Deery, C.; Walmsley, A.D.; Robinson, P.G.; Glenny, A.M. Powered versus manual toothbrushing for oral health. Cochrane Database Syst. Rev. 2014, 2014, CD002281. [Google Scholar] [CrossRef]
- Jongsma, M.A.; Van De Lagemaat, M.; Busscher, H.J.; Geertsema-Doornbusch, G.I.; Atema-Smit, J.; Van Der Mei, H.C.; Ren, Y. Synergy of brushing mode and antibacterial use on in vivo biofilm formation. J. Dent. 2015, 43, 1580–1586. [Google Scholar] [CrossRef]
- Sheiham, A.; Sabbah, W. Using universal patterns of caries for planning and evaluating dental care. Caries Res. 2010, 44, 141–150. [Google Scholar] [CrossRef]
- Tartaglia, G.M.; Kumar, S.; Fornari, C.D.; Corti, E.; Connelly, S.T. Mouthwashes in the 21st century: A narrative review about active molecules and effectiveness on the periodontal outcomes. Expert Opin. Drug Deliv. 2016, 14, 973–982. [Google Scholar] [CrossRef]
- Jones, C.G. Chlorhexidine: Is it still the gold standard? Periodontol. 2000 1997, 15, 55–62. [Google Scholar] [CrossRef]
- Supranoto, S.; Slot, D.E.; Addy, M.; Van Der Weijden, G. The effect of chlorhexidine dentifrice or gel versus chlorhexidine mouthwash on plaque, gingivitis, bleeding and tooth discoloration:a systematic review. Int. J. Dent. Hyg. 2014, 13, 83–92. [Google Scholar] [CrossRef]
- Slot, D.E.; Berchier, C.; Addy, M.; Van Der Velden, U.; Van Der Weijden, G. The efficacy of chlorhexidine dentifrice or gel on plaque, clinical parameters of gingival inflammation and tooth discoloration: A systematic review. Int. J. Dent. Hyg. 2013, 12, 25–35. [Google Scholar] [CrossRef]
- Van Strydonck, D.A.C.; Slot, D.E.; Van Der Velden, U.; Van Der Weijden, F. Effect of a chlorhexidine mouthrinse on plaque, gingival inflammation and staining in gingivitis patients: A systematic review. J. Clin. Periodontol. 2012, 39, 1042–1055. [Google Scholar] [CrossRef]
- Zimmer, S.; Korte, P.; Verde, P.; Ohmann, C.; Naumova, E.; Jordan, R. Randomized controlled trial on the efficacy of new alcohol-free chlorhexidine mouthrinses after 8 weeks. Int. J. Dent. Hyg. 2014, 13, 110–116. [Google Scholar] [CrossRef]
- Turkoglu, O.; Becerik, S.; Emingil, G.; Kütükçüler, N.; Baylas, H.; Atilla, G. The effect of adjunctive chlorhexidine mouthrinse on clinical parameters and gingival crevicular fluid cytokine levels in untreated plaque-associated gingivitis. Inflamm. Res. 2009, 58, 277–283. [Google Scholar] [CrossRef]
- Pizzo, G.; La Cara, M.; Licata, M.E.; Pizzo, I.; D’Angelo, M. The effects of an essential oil and an amine fluoride/stannous fluoride mouthrinse on supragingival plaque regrowth. J. Periodontol. 2008, 79, 1177–1183. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Rodríguez, G.; Ruiz, B.; Faleiros, S.; Vistoso, A.; Marró, M.; Sánchez, J.; Urzúa, I.; Cabello, R. Probiotic compared with standard milk for high-caries children. J. Dent. Res. 2016, 95, 402–407. [Google Scholar] [CrossRef]
- Çaglar, E.; Topcuoglu, N.; Özbey, H.; Sandalli, N.; Külekçi, G. Early colonization of lactobacillus reuteri after exposure to probiotics. J. Clin. Pediatr. Dent. 2015, 39, 326–330. [Google Scholar] [CrossRef]
- Ravn, I.; Dige, I.; Meyer, R.L.; Nyvad, B. Colonization of the oral cavity by probiotic bacteria. Caries Res. 2012, 46, 107–112. [Google Scholar] [CrossRef]
- Çaglar, E.; Topcuoglu, N.; Cildir, S.K.; Sandalli, N.; Kulekci, G. Oral colonization byLactobacillus reuteriATCC 55730 after exposure to probiotics. Int. J. Paediatr. Dent. 2009, 19, 377–381. [Google Scholar] [CrossRef]
- Yli-Knuuttila, H.; Snäll, J.; Kari, K.; Meurman, J.H. Colonization of Lactobacillus rhamnosus GG in the oral cavity. Oral Microbiol. Immunol. 2006, 21, 129–131. [Google Scholar] [CrossRef]
- Meurman, J.H.; Antila, H.; Salminen, S. Recovery of LactobacillusStrain GG (ATCC 53103) from saliva of healthy volunteers after consumption of yoghurt prepared with the bacterium. Microb. Ecol. Health Dis. 1994, 7, 295–298. [Google Scholar] [CrossRef][Green Version]
- Aminabadi, N.A.; Erfanparast, L.; Ebrahimi, A.; Oskouei, S. Effect of Chlorhexidine pretreatment on the stability of Salivary Lactobacilli Probiotic in six- to twelve-year-old children: A randomized controlled trial. Caries Res. 2011, 45, 148–154. [Google Scholar] [CrossRef]
- Emilson, C.G. Susceptibility of various microorganisms to chlorhexidine. Eur. J. Oral Sci. 1977, 85, 255–265. [Google Scholar] [CrossRef]
- Evans, A.; Leishman, S.; Walsh, L.; Seow, W.K.; Leishman, S.; Walsh, L.J. Inhibitory effects of antiseptic mouthrinses on Streptococcus mutans, Streptococcus sanguinis and Lactobacillus acidophilus. Aust. Dent. J. 2015, 60, 247–254. [Google Scholar] [CrossRef]
- Maltz, M.; Emilson, C. Susceptibility of oral bacteria to various fluoride salts. J. Dent. Res. 1982, 61, 786–790. [Google Scholar] [CrossRef]
- Jiang, Q.; Stamatova, I.; Kainulainen, V.A.; Korpela, R.; Meurman, J.H. Interactions between Lactobacillus rhamnosus GG and oral micro-organisms in an in vitro biofilm model. BMC Microbiol. 2016, 16, 149. [Google Scholar] [CrossRef]
- Deshmukh, M.A.; Dodamani, A.S.; Karibasappa, G.; Khairnar, M.R.; Naik, R.G.; Jadhav, H.C. Comparative evaluation of the efficacy of probiotic, herbal and chlorhexidine mouthwash on gingival health: A randomized clinical trial. J. Clin. Diagn. Res. 2017, 11, ZC13–ZC16. [Google Scholar] [CrossRef]
- Stewart, P.S. Mechanisms of antibiotic resistance in bacterial biofilms. Int. J. Med. Microbiol. 2002, 292, 107–113. [Google Scholar] [CrossRef]
- Hawser, S.P.; Douglas, L.J. Biofilm formation by Candida species on the surface of catheter materials in vitro. Infect. Immun. 1994, 62, 915–921. [Google Scholar] [CrossRef]
- Singh, S.; Singh, S.K.; Chowdhury, I.; Singh, R. Understanding the mechanism of bacterial biofilms resistance to antimicrobial agents. Open Microbiol. J. 2017, 11, 53–62. [Google Scholar] [CrossRef]
- Marsh, P.D. Plaque as a biofilm: Pharmacological principles of drug delivery and action in the sub- and supragingival environment. Oral Dis. 2003, 9, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.B.; Tyler, K.Z.; Low, S.B.; King, C.J. Penicillin-degrading enzymes in sites associated with adult periodontitis. Oral Microbiol. Immunol. 1987, 2, 129–131. [Google Scholar] [CrossRef] [PubMed]
- Wood, T.K.; Knabel, S.J.; Kwan, B.W. Bacterial persister cell formation and dormancy. Appl. Environ. Microbiol. 2013, 79, 7116–7121. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Stamatova, I.; Kari, K.; Meurman, J.H. Inhibitory activity in vitro of probiotic lactobacilli against oral Candida under different fermentation conditions. Benef. Microbes 2015, 6, 361–368. [Google Scholar] [CrossRef]
- Jiang, Q.; Kainulainen, V.; Stamatova, I.A.; Korpela, R.; Meurman, J.H. Lactobacillus rhamnosus GG in experimental oral biofilms exposed to different carbohydrate sources. Caries Res. 2018, 52, 220–229. [Google Scholar] [CrossRef]
- Zaura, E.; Van Marle, J.; Cate, J.T. Confocal microscopy study of undisturbed and Chlorhexidine-treated dental biofilm. J. Dent. Res. 2001, 80, 1436–1440. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Orgaz, B.; Puga, C.; Martínez-Suárez, J.; Sanjosé, C. Biofilm recovery from chitosan action: A possible clue to understand Listeria monocytogenes persistence in food plants. Food Control 2013, 32, 484–489. [Google Scholar] [CrossRef]
- Yousefimanesh, H.; Amin, M.; Robati, M.; Goodarzi, H.; Otoufi, M. Comparison of the antibacterial properties of three mouthwashes containing Chlorhexidine against oral microbial plaques: An in vitro study. Jundishapur J. Microbiol. 2015, 8. [Google Scholar] [CrossRef]
- Zheng, C.Y.; Wang, Z.H. Effects of chlorhexidine, listerine and fluoride listerine mouthrinses on four putative root-caries pathogens in the biofilm. Chin. J. Dent. Res. 2011, 14, 135–140. [Google Scholar]
- McBain, A.J.; Bartolo, R.G.; Catrenich, C.E.; Charbonneau, D.; Ledder, R.G.; Gilbert, P. Effects of a Chlorhexidine gluconate-containing mouthwash on the vitality and antimicrobial susceptibility of in vitro oral bacterial Ecosystems. Appl. Environ. Microbiol. 2003, 69, 4770–4776. [Google Scholar] [CrossRef] [PubMed]
- McDermid, A.; McKee, A.; Marsh, P. A mixed-culture chemostat system to predict the effect of anti-microbial agents on the oral flora: Preliminary studies using Chlorhexidine. J. Dent. Res. 1987, 66, 1315–1320. [Google Scholar] [CrossRef] [PubMed]
- Sari, E.; Birinci, I. Microbiological evaluation of 0.2% Chlorhexidine gluconate mouth rinse in orthodontic patients. Angle Orthod. 2007, 77, 881–884. [Google Scholar] [CrossRef] [PubMed]
- Milnes, A.; Bowden, G.; Hamilton, I. Effect of NaF and pH on the growth and glycolytic rate of recently isolated strains of oral Lactobacillus species. J. Dent. Res. 1985, 64, 401–404. [Google Scholar] [CrossRef]
- Ostengo, M.D.C.A.; Wiese, B.; Nader-Macías, M.E.F. Inhibitory effect of sodium fluoride and chlorhexidine on the growth of oral lactobacilli. Can. J. Microbiol. 2005, 51, 133–140. [Google Scholar] [CrossRef]
- Wicht, M.J.; Haak, R.; Schütt-Gerowitt, H.; Kneist, S.; Noack, M.J. Suppression of caries-related microorganisms in dentine lesions after short-term Chlorhexidine or antibiotic treatment. Caries Res. 2004, 38, 436–441. [Google Scholar] [CrossRef]
- Stojicic, S.; Shen, Y.; Haapasalo, M. Effect of the source of biofilm bacteria, level of biofilm maturation, and type of disinfecting agent on the susceptibility of biofilm bacteria to antibacterial agents. J. Endod. 2013, 39, 473–477. [Google Scholar] [CrossRef]
- Zanatta, F.B.; Antoniazzi, R.P.; Rösing, C.K. The effect of 0.12% Chlorhexidine gluconate rinsing on previously plaque-free and plaque-covered surfaces: A randomized, controlled clinical trial. J. Periodontol. 2007, 78, 2127–2134. [Google Scholar] [CrossRef]
- Dalleau, S.; Cateau, E.; Bergès, T.; Berjeaud, J.-M.; Imbert, C. In vitro activity of terpenes against Candida biofilms. Int. J. Antimicrob. Agents 2008, 31, 572–576. [Google Scholar] [CrossRef]
- Exterkate, R.; Crielaard, W.; Cate, J.T. Different response to amine fluoride by streptococcus mutans and polymicrobial biofilms in a novel high-throughput active attachment model. Caries Res. 2010, 44, 372–379. [Google Scholar] [CrossRef]
- Shen, Y.; Zhao, J.; De La Fuente-Núñez, C.; Wang, Z.; Hancock, R.E.W.; Roberts, C.R.; Ma, J.; Li, J.; Haapasalo, M.; Wang, Q. Experimental and theoretical investigation of multispecies oral biofilm resistance to chlorhexidine treatment. Sci. Rep. 2016, 6, 27537. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.; Townsend, K.M.; Fenwick, S.G.; Maker, G.; Trengove, R.; O’Handley, R.M. Comparative susceptibility of SalmonellaTyphimurium biofilms of different ages to disinfectants. Biofouling 2010, 26, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Balbuena, L.; Stambaugh, K.I.; Ramirez, S.G.; Yeager, C. Effects of topical oral antiseptic rinses on bacterial counts of saliva in healthy human subjects. Otolaryngol. Neck Surg. 1998, 118, 625–629. [Google Scholar] [CrossRef]
- NaikTari, R.S.; Dharmadhikari, C.; Gurav, A.N.; Kakade, S. Determining the antibacterial substantivity of Triphala mouthwash and comparing it with 0.2% chlorhexidine gluconate after a single oral rinse: A crossover clinical trial. J. Indian Soc. Periodontol. 2018, 22, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Elworthy, A.; Greenman, J.; Doherty, F.; Newcombe, R.; Addy, M. The Substantivity of a number of oral hygiene products determined by the duration of effects on salivary bacteria. J. Periodontol. 1996, 67, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Tomás, I.; Cousido, M.; García-Caballero, L.; Rubido, S.; Limeres, J.; Diz, P. Substantivity of a single chlorhexidine mouthwash on salivary flora: Influence of intrinsic and extrinsic factors. J. Dent. 2010, 38, 541–546. [Google Scholar] [CrossRef]
- Hall, M.W.; Singh, N.; Ng, K.F.; Lam, D.K.; Goldberg, M.B.; Tenenbaum, H.C.; Neufeld, J.D.; Beiko, R.G.; Senadheera, D.B. Inter-personal diversity and temporal dynamics of dental, tongue, and salivary microbiota in the healthy oral cavity. NPJ Biofilms Microb. 2017, 3, 2. [Google Scholar] [CrossRef]

| Strain | Origin | Agar/Broth | Cultivation Time, Temperature, and Air Composition |
|---|---|---|---|
| Lactobacillus rhamnosus GG ATCC 1 53103 (LGG) | Valio Ltd., Helsinki, Finland | MRS 2 | 24 h, 37 °C, 5% CO2 |
| Aggregatibacter actinomycetemcomitans ATCC 43718 | ATCC | BHI 3 | 24 h, 37 °C, 5% CO2 |
| Candida albicans ATCC 10231 | ATCC | Sabouraud | 24 h, 37 °C, air |
| Fusobacterium nucleatum ATCC 25586 | ATCC | Brucella | 48 h, 37 °C, mixture of 0.2% O2, 5% CO2, 9.9% H2, 84.9% N2 |
| Streptococcus mutans ATCC 27351 | ATCC | BHI | 24 h, 37 °C, 5% CO2 |
| Streptococcus sanguinis ATCC 10556 | ATCC | BHI | 24 h, 37 °C, 5% CO2 |
| Trade Name | Main Active Component | Manufacturer |
|---|---|---|
| Corsodyl® | 0.2% (or 2 mg/mL) chlorhexidine gluconate | GlaxoSmithKline, UK |
| Listerine® Total Care | Essential oils: eucalyptol 0.092%, methyl salicylate 0.060%, thymol 0.064% and menthol 0.042% | Johnson & Johnson, UK |
| Meridol® | Amine fluoride and stannous fluoride (250 ppm F−) | GABA, Switzerland |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Q.; Kainulainen, V.; Stamatova, I.; Janket, S.-J.; Meurman, J.H.; Korpela, R. Mouthwash Effects on LGG-Integrated Experimental Oral Biofilms. Dent. J. 2020, 8, 96. https://doi.org/10.3390/dj8030096
Jiang Q, Kainulainen V, Stamatova I, Janket S-J, Meurman JH, Korpela R. Mouthwash Effects on LGG-Integrated Experimental Oral Biofilms. Dentistry Journal. 2020; 8(3):96. https://doi.org/10.3390/dj8030096
Chicago/Turabian StyleJiang, Qingru, Veera Kainulainen, Iva Stamatova, Sok-Ja Janket, Jukka H. Meurman, and Riitta Korpela. 2020. "Mouthwash Effects on LGG-Integrated Experimental Oral Biofilms" Dentistry Journal 8, no. 3: 96. https://doi.org/10.3390/dj8030096
APA StyleJiang, Q., Kainulainen, V., Stamatova, I., Janket, S.-J., Meurman, J. H., & Korpela, R. (2020). Mouthwash Effects on LGG-Integrated Experimental Oral Biofilms. Dentistry Journal, 8(3), 96. https://doi.org/10.3390/dj8030096