A Reliable Surgical Procedure for Sinus Floor Augmentation with Antral Pseudocysts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection and Ethical Statements
2.2. CBCT Evaluation
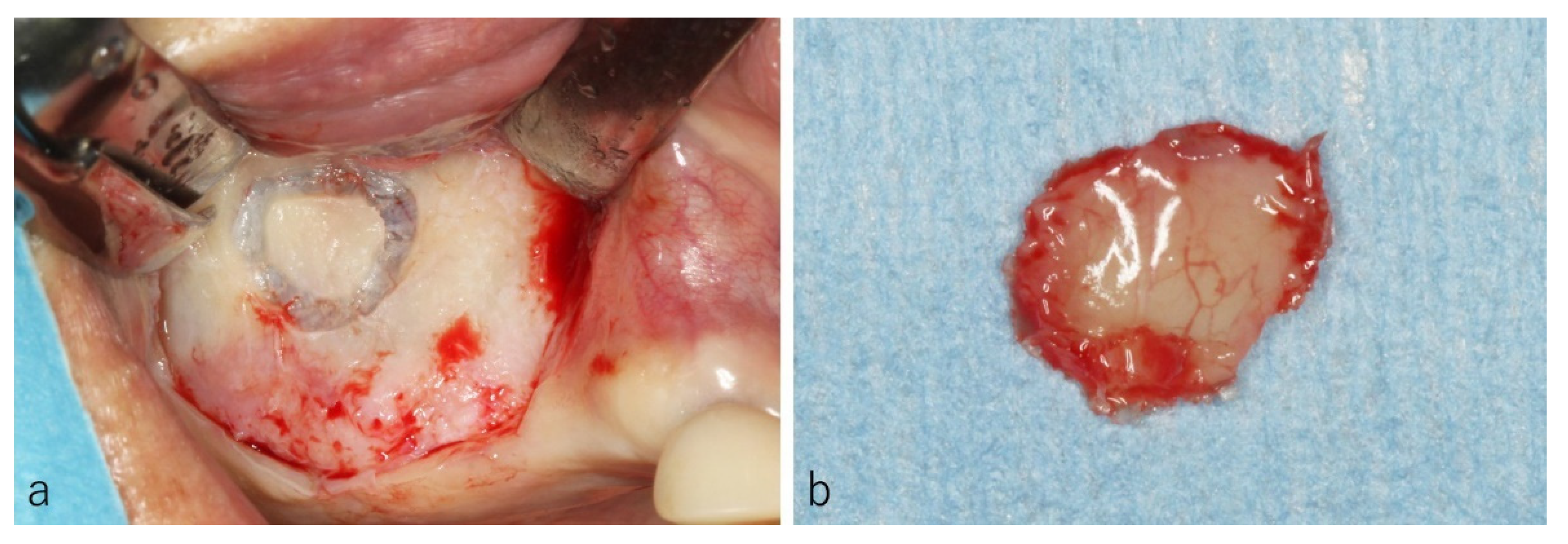
2.3. Surgical Procedure
3. Results
3.1. Histology of the Frontal Wall
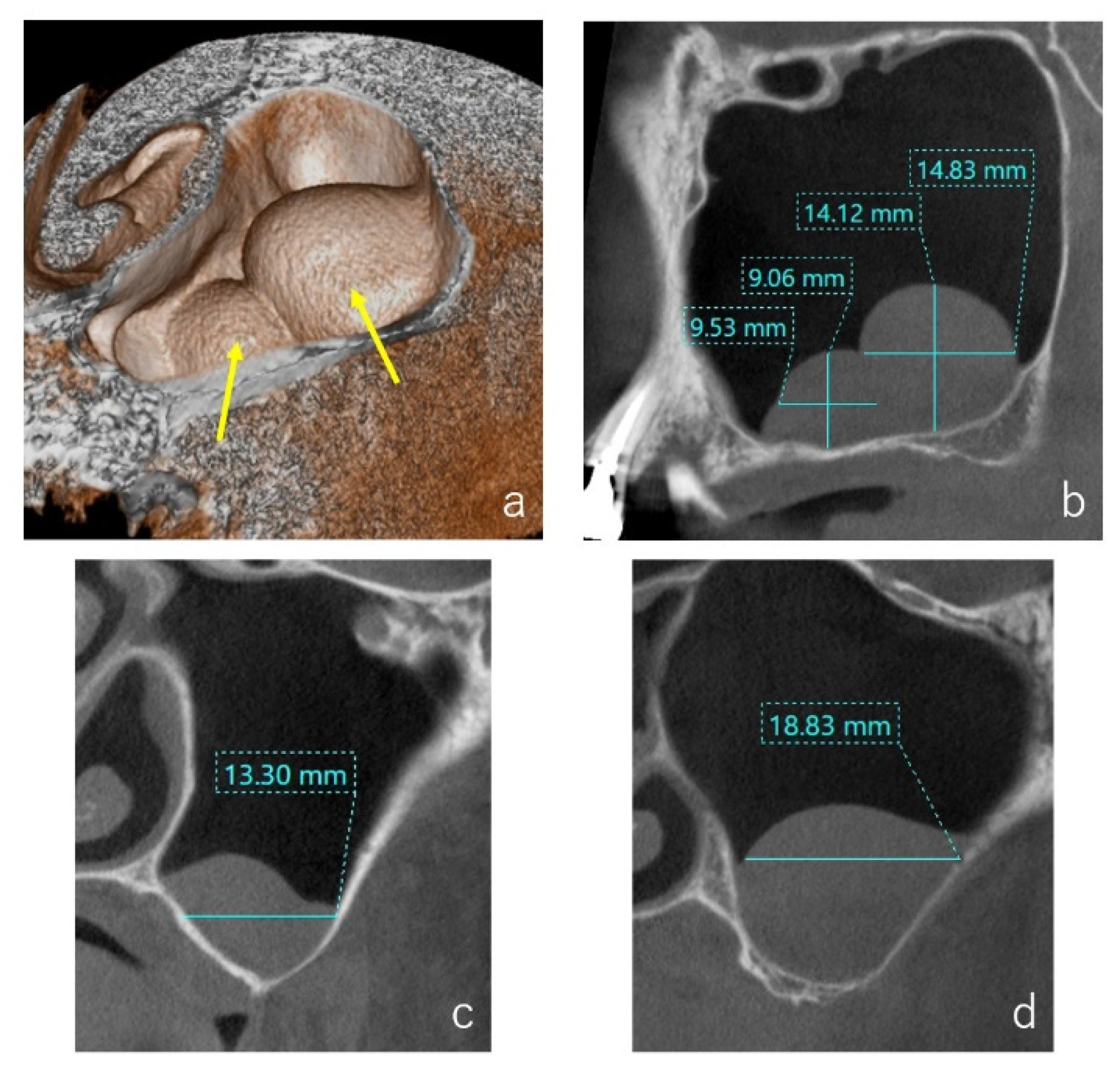
3.2. CBCT Evaluation of the Cystic Lesions
3.3. Types of Colors and Contents of the Cystic Lesions
3.4. Histopathological Evaluations of the Cystic Lesions
3.5. Surgery and Clinical Progress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jensen, O.T.; Shulman, L.B. Report of the Sinus Consensus Conference of 1996. Int. J. Oral Maxillofac. Implant. 1998, 13, 11–45. [Google Scholar]
- Pjetursson, B.E.; Tan, W.C.; Zwahlen, M. A systematic review of the success of sinus floor elevation and survival of implants inserted in combination with sinus floor elevation. J. Clin. Periodontol. 2008, 35, 216–240. [Google Scholar] [CrossRef]
- Gardner, D.G. Pseudocysts and retention cysts of the maxillary sinus. Oral Surg. Oral Med. Oral Pathol. 1984, 58, 561–567. [Google Scholar] [CrossRef]
- Hadar, T.; Shvero, J. Mucus retention cyst of the maxillary sinus: The endoscopic approach. Br. J. Oral Maxillofac. Surg. 2000, 38, 227–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allard, R.H.; Kwast, W.A. Mucosal antral cysts. Review of the literature and report of a radiographic survey. Oral Surg. Oral Med. Oral Pathol 1981, 51, 2–9. [Google Scholar] [CrossRef]
- Beaumont, C.; Zafiropoulos, G.G. Prevalence of maxillary sinus disease and abnormalities in patients scheduled for sinus lift procedures. J. Periodontol. 2005, 76, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Pignataro, L.; Mantovani, M. ENT assessment in the integrated management of candidate for (maxillary) sinus lift. Acta Otorhinolaryngol. Ital. 2008, 28, 110–119. [Google Scholar] [PubMed]
- Gothberg, K.A.; Little, J.W.; King, D.R. A clinical study of cysts arising from mucosa of the maxillary sinus. Oral Surg. Oral Med. Oral Pathol. 1976, 41, 52–58. [Google Scholar] [CrossRef]
- Perfetti, G.; Rossi, F. Sinus augmentation procedure of the jaw sinus in patients with mucocele. Int. J. Immunopathol. Pharmacol. 2008, 21, 243–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mardinger, O.; Manor, I. Maxillary sinus augmentation in the presence of antral pseudocyst: A clinical approach. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007, 103, 180–184. [Google Scholar] [CrossRef]
- Kim, S.B.; Yun, P.Y. Clinical evaluation of sinus bone graft in patients with mucous retention cyst. Maxillofac. Plast. Reconstr. Surg. 2016, 38, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiapasco, M.; Palombo, D. Sinus grafting and simultaneous removal of large antral pseudocysts of the maxillary sinus with a micro-invasive intraoral access. Int. J. Oral Maxillofac. Surg. 2015, 44, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Hu, X. Maxillary sinus augmentation following removal of a maxillary sinus pseudocyst after a shortened healing period. J. Oral Maxillofac. Surg. 2010, 68, 2856–2860. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, H. Complications of postoperative swelling of the maxillary sinus membrane after sinus floor augmentation. J. Oral Sci. Rehabil. 2015, 1, 26–33. [Google Scholar]
- Kawakami, S.; Lang, N.P.; Iida, T.; Ferri, M.; Apaza Alccayhuaman, K.A.; Botticelli, D. Influence of the position of the antrostomy in sinus floor elevation assessed with cone-beam computed tomography: A randomized clinical trial. J. Investig. Clin. Dent. 2018, 9, e12362. [Google Scholar] [CrossRef]
- Kawakami, S.; Lang, N.P.; Ferri, M.; Apaza Alccayhuaman, K.A.; Botticelli, D. Influence of the height of the antrostomy in sinus floor elevation assessed by cone beam computed tomography- a randomized clinical trial. Int. J. Oral Maxillofac. Implants 2019, 34, 223–232. [Google Scholar] [CrossRef]
- Hirota, A.; Lang, N.P.; Ferri, M.; Fortich Mesa, N.; Apaza Alccayhuaman, K.A.; Botticelli, D. Tomographic evaluation of the influence of the placement of a collagen membrane subjacent to the sinus mucosa during maxillary sinus floor augmentation: A randomized clinical trial. Int. J. Implant. Dent. 2019, 5, 31. [Google Scholar] [CrossRef]
- Imai, H.; Lang, N.P.; Ferri, M.; Hirota, A.; Apaza Alccayhuaman, A.A.; Botticelli, D. Tomographic assessment on the influence on dimensional variations of the use of a collagen membrane to protect the antrostomy after maxillary sinus floor augmentation. A randomized clinical trial. Int. J. Oral Maxillofac. Implants. 2020, 35, 350–356. [Google Scholar] [CrossRef]
- Sakuma, S.; Ferri, M.; Imai, H.; Fortich Mesa, N.; Blanco Victorio, D.J.; Apaza Alccayhuaman, K.A.; Botticelli, D. Involvement of the maxillary sinus ostium (MSO) in the edematous processes after sinus floor augmentation: A cone-beam computed tomographic study. Int. J. Implant Dent. 2020, 6, 35. [Google Scholar] [CrossRef]
- Kato, S.; Omori, Y.; Kanayama, M.; Hirota, A.; Ferri, M.; Apaza Alccayhuaman, K.A.; Botticelli, D. Sinus Mucosa Thickness Changes and Ostium Involvement After Maxillary Sinus Floor Elevation in Sinus with Septa. A Cone Beam Computed Tomography Study. Dent. J. 2021, 9, 82. [Google Scholar] [CrossRef]
- Kawakami, S.; Botticelli, D.; Nakajima, Y.; Sakuma, S.; Baba, S. Anatomical analyses for maxillary sinus floor augmentation with a lateral approach: A cone beam computed tomography study. Ann. Anat. 2019, 226, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Omori, Y.; Nakajima, Y.; Imai, H.; Yonezawa, D.; Ferri, M.; Apaza Alccayhuaman, K.A.; Botticelli, D. Influence of Anatomical Parameters on the Dimensions of the Subantral Space and Sinus Mucosa Thickening after Sinus Floor Elevation. A Retrospective Cone Beam Computed Tomography Study. Dent. J. 2021, 9, 76. [Google Scholar] [CrossRef]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nosaka, Y.; Nosaka, H.; Nakajima, Y.; Tanioka, T.; Botticelli, D.; Baba, S. A Reliable Surgical Procedure for Sinus Floor Augmentation with Antral Pseudocysts. Dent. J. 2021, 9, 122. https://doi.org/10.3390/dj9100122
Nosaka Y, Nosaka H, Nakajima Y, Tanioka T, Botticelli D, Baba S. A Reliable Surgical Procedure for Sinus Floor Augmentation with Antral Pseudocysts. Dentistry Journal. 2021; 9(10):122. https://doi.org/10.3390/dj9100122
Chicago/Turabian StyleNosaka, Yasuhiro, Hitomi Nosaka, Yasushi Nakajima, Tadasuke Tanioka, Daniele Botticelli, and Shunsuke Baba. 2021. "A Reliable Surgical Procedure for Sinus Floor Augmentation with Antral Pseudocysts" Dentistry Journal 9, no. 10: 122. https://doi.org/10.3390/dj9100122
APA StyleNosaka, Y., Nosaka, H., Nakajima, Y., Tanioka, T., Botticelli, D., & Baba, S. (2021). A Reliable Surgical Procedure for Sinus Floor Augmentation with Antral Pseudocysts. Dentistry Journal, 9(10), 122. https://doi.org/10.3390/dj9100122






