The Impact of Oral Health on Respiratory Viral Infection
Abstract
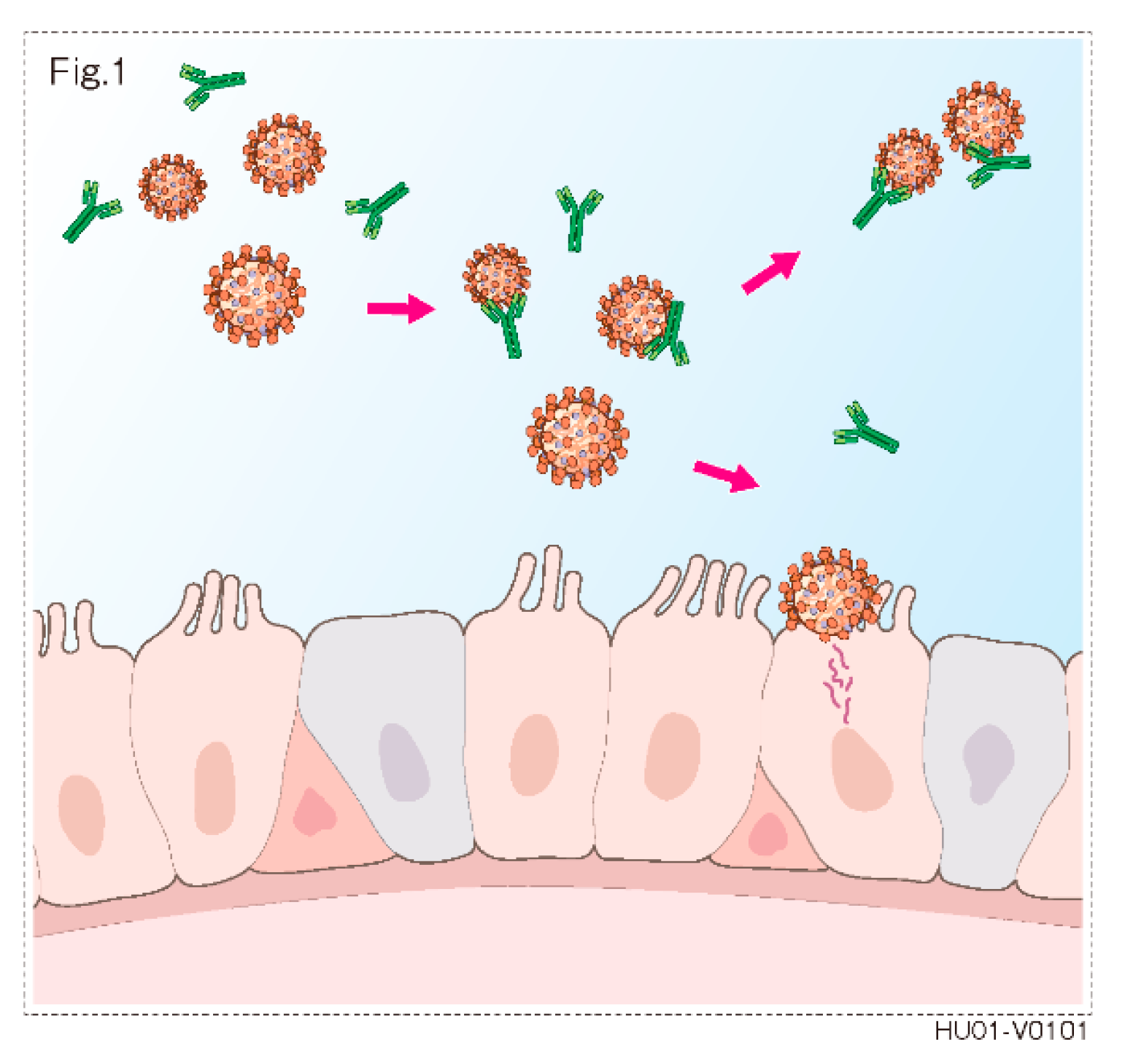
:1. Introduction
2. Methods
3. The Influence of Oral Health on Influenza Virus Infection
3.1. Direct Influence of Oral Bacteria
3.1.1. Apoptosis Induced by Porphyromonas gingivalis
3.1.2. Increase of Influenza Virus Proliferation Induced by Oral Bacteria
3.2. Depression of Immunity Induced by Periodontal Disease
3.3. Inhibition of Influenza Virus Proliferation by Salivary Immunity
3.3.1. Innate Immunity
3.3.2. Humoral Immunity
3.4. Epidemiological Study
4. COVID-19 and Oral Health
4.1. Saliva and SARS-CoV-2
4.1.1. Anti-Viral Activity of Saliva
4.1.2. Possibility of the Inhibition of SARS-CoV2 by Saliva
4.1.3. The Use of Saliva as a Possible Way of COVID-19 Diagnosis
4.2. Association between COVID-19 and Periodontitis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Joseph, C.; Togawa, Y.; Shindo, N. Bacterial and viral infections associated with influenza. Influenza Other Respir. Viruses 2013, 7 (Suppl. 2), 105–113. [Google Scholar] [CrossRef] [Green Version]
- Bao, L.; Zhang, C.; Dong, J.; Zhao, L.; Li, Y.; Sun, J. Oral Microbiome and SARS-CoV-2: Beware of Lung Co-infection. Front Microbiol. 2020, 11, 1840. [Google Scholar] [CrossRef]
- Sampson, V.; Kamona, N.; Sampson, A. Could there be a link between oral hygiene and the severity of SARS-CoV-2 infections? Br. Dent. J. 2020, 228, 971–975. [Google Scholar] [CrossRef] [PubMed]
- Reichhardt, M.P.; Meri, S. SALSA: A Regulator of the Early Steps of Complement Activation on Mucosal Surfaces. Front. Immunol. 2016, 7, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmgren, J.; Czerkinsky, C. Mucosal immunity and vaccines. Nat. Med. 2005, 11 (Suppl. 4), S45–S53. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, R.; Yi, Z.; Li, Y.; Fu, Y.; Zhang, Y.; Li, P.; Li, X.; Pan, Y. Porphyromonas gingivalis induced inflammatory responses and promoted apoptosis in lung epithelial cells infected with H1N1 via the Bcl-2/Bax/Caspase-3 signaling pathway. Mol. Med. Rep. 2018, 18, 97–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Li, C.; Liu, J.C.; Pan, Y.P.; Li, Y.G. In vitro effect of Porphyromonas gingivalis combined with influenza A virus on respiratory epithelial cells. Arch. Oral Biol. 2018, 95, 125–133. [Google Scholar] [CrossRef]
- Nishioka, K.; Kyo, M.; Nakaya, T.; Shime, N. Proteins produced by Streptococcus species in the lower respiratory tract can modify antiviral responses against influenza virus in respiratory epithelial cells. Microbes Infect. 2020, 23, 104764. [Google Scholar] [CrossRef]
- Kamio, N.; Imai, K.; Shimizu, K.; Cueno, M.E.; Tamura, M.; Saito, Y.; Ochiai, K. Neuraminidase-producing oral mitis group streptococci potentially contribute to influenza viral infection and reduction in antiviral efficacy of zanamivir. Cell Mol. Life Sci. 2015, 72, 357–366. [Google Scholar] [CrossRef]
- Kurita-Ochiai, T.; Ochiai, K. Immunosuppressive factor from Actinobacillus actinomycetemcomitans down regulates cytokine production. Infect. Immun. 1996, 64, 50–54. [Google Scholar] [CrossRef] [Green Version]
- Ochiai, K.; Senpuku, H.; Kurita-Ochiai, T. Purification of immunosuppressive factor from Capnocytophaga ochracea. J. Med. Microbiol. 1998, 47, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Kurita-Ochiai, T.; Fukushima, K.; Ochiai, K. Volatile fatty acids, metabolic by-products of periodontopathic bacteria and cytokine production. J. Dent. Res. 1995, 74, 1367–1773. [Google Scholar] [CrossRef]
- Malamud, D.; Wahl, S.M. The mouth: A gateway or a trap for HIV? AIDS 2010, 24, 5–16. [Google Scholar] [CrossRef]
- White, M.R.; Helmerhorst, E.J.; Ligtenberg, A.; Karpel, M.; Tecle, T.; Siqueira, W.L.; Oppenheim, F.G.; Hartshorn, K.L. Multiple components contribute to ability of saliva to inhibit influenza viruses. Oral Microbiol. Immunol. 2009, 24, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Ivinson, K.; Deliyannis, G.; McNabb, L.; Grollo, L.; Gilbertson, B.; Jackson, D.; Brown, L.E. Salivary Blockade Protects the Lower Respiratory Tract of Mice from Lethal Influenza Virus Infection. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [Green Version]
- Malamud, D.; Abrams, W.R.; Barber, C.A.; Weissman, D.; Rehtanz, M.; Golub, E. Antiviral activities in human saliva. Adv. Dent. Res. 2011, 23, 34–37. [Google Scholar] [CrossRef]
- Limsuwat, N.; Suptawiwat, O.; Boonarkart, C.; Puthavathana, P.; Wiriyarat, W.; Auewarakul, P. Sialic acid content in human saliva and anti-influenza activity against human and avian influenza viruses. Arch. Virol. 2016, 161, 649–656. [Google Scholar] [CrossRef]
- Zhong, Y.; Qin, Y.; Yu, H.; Yu, J.; Wu, H.; Chen, L.; Zhang, P.; Wang, X.; Jia, Z.; Guo, Y.; et al. Avian influenza virus infection risk in humans with chronic diseases. Sci. Rep. 2015, 5, 8971. [Google Scholar] [CrossRef] [Green Version]
- Gilbertson, B.; Edenborough, K.; McVernon, J.; Brown, L.E. Inhibition of Influenza A Virus by Human Infant Saliva. Viruses 2019, 11, 766. [Google Scholar] [CrossRef] [Green Version]
- Paixão, V.; Almeida, E.B.; Amaral, J.B.; Roseira, T.; Monteiro, F.R.; Foster, R.; Sperandio, A.; Rossi, M.; Amirato, G.R.; Santos, C.A.F.; et al. Elderly Subjects Supplemented with L-Glutamine Shows an Improvement of Mucosal Immunity in the Upper Airways in Response to Influenza Virus Vaccination. Vaccines 2021, 9, 107. [Google Scholar] [CrossRef]
- Gianchecchi, E.; Manenti, A.; Kistner, O.; Trombetta, C.; Manini, I.; Montomoli, E. How to assess the effectiveness of nasal influenza vaccines? Role and measurement of sIgA in mucosal secretions. Influenza Other Respir. Viruses 2019, 13, 429–437. [Google Scholar] [CrossRef]
- Langley, J.M.; Aoki, F.; Ward, B.J.; McGeer, A.; Angel, J.B.; Stiver, G.; Gorfinkel, I.; Shu, D.; White, L.; Lasko, B.; et al. A nasally administered trivalent inactivated influenza vaccine is well tolerated, stimulates both mucosal and systemic immunity, and potentially protects against influenza illness. Vaccine 2011, 29, 1921–1928. [Google Scholar] [CrossRef] [PubMed]
- Abe, S.; Ishihara, K.; Adachi, M.; Sasaki, H.; Tanaka, K.; Okuda, K. Professional oral care reduces influenza infection in elderly. Arch. Gerontol. Geriatr. 2006, 43, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Li, M.Y.; Li, L.; Zhang, Y.; Wang, X.S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect. Dis. Poverty 2020, 9, 45. [Google Scholar] [CrossRef]
- Li, Y.C.; Bai, W.Z.; Hashikawa, T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J. Med. Virol. 2020. [CrossRef]
- Zhou, L.; Xu, Z.; Castiglione, G.M.; Soiberman, U.S.; Eberhart, C.G.; Duh, E.J. ACE2 and TMPRSS2 are expressed on the human ocular surface, suggesting susceptibility to SARS-CoV-2 infection. Ocul. Surf 2020. [Google Scholar] [CrossRef]
- Xu, J.; Li, Y.; Gan, F.; Du, Y.; Yao, Y. Salivary Glands: Potential Reservoirs for COVID-19 asymptomatic infection. J. Dent. Res. 2020, 99, 989. [Google Scholar] [CrossRef] [Green Version]
- Xu, R.; Cui, B.; Duan, X.; Zhang, P.; Zhou, X.; Yuan, Q. Saliva: Potential diagnostic value and transmission of 2019-nCoV. Int. J. Oral Sci. 2020, 12, 1–6. [Google Scholar] [CrossRef]
- Zhong, M.; Lin, B.; Pathak, J.L.; Gao, H.; Young, A.J.; Wang, X.; Liu, C.; Wu, K.; Liu, M.; Chen, J.M.; et al. ACE2 and Furin Expressions in Oral Epithelial Cells Possibly Facilitate COVID-19 Infection via Respiratory and Fecal-Oral Routes. Front. Med 2020, 7, 580796. [Google Scholar] [CrossRef]
- Queiroz-Junior, C.M.; Santos, A.C.P.M.; Galvão, I.; Souto, G.R.; Mesquita, R.A.; Sá, M.A.; Ferreira, A.J. The angiotensin converting enzyme 2/angiotensin-(1-7)/Mas Receptor axis as a key player in alveolar bone remodeling. Bone 2019, 128, 115041. [Google Scholar] [CrossRef]
- Bunyavanich, S.; Do, A.; Vicencio, A. Nasal Gene Expression of Angiotensin-Converting Enzyme 2 in Children and Adults. JAMA 2020. [Google Scholar] [CrossRef]
- Nagashunmugam, T.; Malamud, D.; Davis, C.; Abrams, W.R.; Friedman, H.M. Human submandibular saliva inhibits human immunodeficiency virus type 1 infection by displacing envelope glycoprotein gp120 from the virus. J. Infect. Dis. 1998, 178, 1635–1641. [Google Scholar] [CrossRef] [Green Version]
- White, M.R.; Crouch, E.; van Eijk, M.; Hartshorn, M.; Pemberton, L.; Tornoe, I.; Holmskov, U.; Hartshorn, K.L. Cooperative anti-influenza activities of respiratory innate immune proteins and neuraminidase inhibitor. Am. J. Physiol. Lung Cell Mol. Physiol. 2005, 288, L831–L840. [Google Scholar] [CrossRef]
- Wu, Z.; Van Ryk, D.; Davis, C.; Abrams, W.R.; Chaiken, I.; Magnani, J.; Malamud, D. Salivary agglutinin inhibits HIV type 1 infectivity through interaction with viral glycoprotein 120. AIDS Res. Hum. Retrovir. 2003, 19, 201–209. [Google Scholar] [CrossRef]
- Hardestam, J.; Petterson, L.; Ahlm, C.; Evander, M.; Lundkvist, A.; Klingström, J. Antiviral effect of human saliva against Hantavirus. J. Med. Viol. 2008, 80, 2122–2126. [Google Scholar] [CrossRef] [PubMed]
- Varadhachary, A.; Chatterjee, D.; Garza, J.; Garr, R.P.; Foley, C.; Letkeman, A.F.; Dean, J.; Haug, D.; Breeze, J.; Traylor, R.; et al. Salivary anti-SARS-CoV-2 IgA as an accessible biomarker of mucosal immunity against COVID-19. MedRxiv 2020. [Google Scholar] [CrossRef]
- Sterlin, D.; Mathian, A.; Miyara, M.; Mohr, A.; Anna, F.; Claër, L.; Quentric, P.; Fadlallah, J.; Devilliers, H.; Ghillani, P.; et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci. Transl. Med. 2021, 13, eabd2223. [Google Scholar] [CrossRef]
- Ejemel, M.; Li, Q.; Hou, S.; Schiller, Z.A.; Tree, J.A.; Wallace, A.; Amcheslavsky, A.; Kurt Yilmaz, N.; Buttigieg, K.R.; Elmore, M.J.; et al. Cross-reactive human IgA monoclonal antibody blocks SARS-CoV-2 spike-ACE2 interaction. Nat. Commun. 2020, 11, 4198. [Google Scholar] [CrossRef]
- Lu, B.; Huang, Y.; Huang, L.; Li, B.; Zheng, Z.; Chen, Z.; Chen, J.; Hu, Q.; Wang, H. Effect of mucosal and systemic immunization with virus-like particles of severe acute respiratory syndrome coronavirus in mice. Immunology 2010, 130, 254–261. [Google Scholar] [CrossRef] [Green Version]
- Ketas, T.J.; Chaturbhuj, D.; Cruz-Portillo, V.M.; Francomano, E.; Golden, E.; Chandrasekhar, S.; Debnath, G.; Diaz-Tapia, R.; Yasmeen, A.; Leconet, W.; et al. Antibody responses to SARS-CoV-2 mRNA vaccines are detectable in saliva. BioRxiv 2021. [Google Scholar] [CrossRef]
- Miletic, I.D.; Schiffman, S.S.; Miletic, V.D.; Sattely-Miller, E.A. Salivary IgA secretion rate in young and elderly persons. Physiol. Behav. 1996, 60, 243–248. [Google Scholar] [CrossRef]
- Evans, P.; Der, G.; Ford, G.; Hucklebridge, F.; Hunt, K.; Lambert, S. Social class, sex, and age differences in mucosal immunity in a large community sample. Brain Behav. Immun. 2000, 14, 41–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanida, T.; Ueta, E.; Tobiume, A.; Hamada, T.; Rao, F.; Osaki, T. Influence of aging on candidal growth and adhesion regulatory agents in saliva. J. Oral Pathol. Med. 2001, 30, 328–335. [Google Scholar] [CrossRef] [PubMed]
- To, K.K.; Tsang, O.T.; Leung, W.S.; Tam, A.R.; Wu, T.C.; Lung, D.C.; Yip, C.C.; Cai, J.P.; Chan, J.M.; Chik, T.S.; et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-Cov-2 detection: An observational cohort study. Lancet Infect. Dis. 2020, 20, 565–574. [Google Scholar] [CrossRef] [Green Version]
- Yeh, C.K.; Johnson, D.A.; Dodds, M.W.; Sakai, S.; Rugh, J.D.; Hatch, J.P. Association of salivary flow rates with maximal bite force. J. Dent. Res. 2000, 79, 1560–1565. [Google Scholar] [CrossRef]
- Jenkins, G.N.; Edgar, W.M. The effect of daily gum-chewing on salivary flow rates in man. J. Dent. Res. 1989, 68, 786–790. [Google Scholar] [CrossRef]
- Dodds, M.W.; Hsieh, S.C.; Johnson, D.A. The effect of increased mastication by daily gum-chewing on salivary gland output and dental plaque acidogenicity. J. Dent. Res. 1991, 70, 1474–1478. [Google Scholar] [CrossRef] [PubMed]
- Dodds, M.W.; Johnson, D.A. Influence of mastication on saliva, plaque pH and masseter muscle activity in man. Arch. Oral Biol. 1993, 38, 623–626. [Google Scholar] [CrossRef]
- Akimoto, T.; Kumai, Y.; Akama, T.; Hayashi, E.; Murakami, H.; Soma, R.; Kuno, S.; Kono, I. Effects of 12 months of exercise training on salivary secretory IgA levels in elderly subjects. Br. J. Sports Med. 2003, 37, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Kimura, F.; Akimoto, T.; Akama, T.; Kuno, S.; Kono, I. Effect of free-living daily physical activity on salivary secretory IgA in elderly. Med. Sci. Sports Exerc. 2007, 39, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Robson, B. Bioinformatics studies on a function of the SARS-CoV-2 spike glycoprotein as the binding of host sialic acid glycans. Comput. Biol. Med. 2020, 122, 103849. [Google Scholar] [CrossRef]
- Seyran, M.; Takayama, K.; Uversky, V.N.; Lundstrom, K.; Palù, G.; Sherchan, S.P.; Attrish, D.; Rezaei, N.; Aljabali, A.A.A.; Ghosh, S.; et al. The structural basis of accelerated host cell entry by SARS-CoV-2. FEBS J. 2020. [Google Scholar] [CrossRef]
- Fernandes, L.L.; Pacheco, V.B.; Borges, L.; Athwal, H.K.; de Paula Eduardo, F.; Bezinelli, L.; Correa, L.; Jimenez, M.; Dame-Teixeira, N.; Lombaert, I.M.A.; et al. Saliva in the Diagnosis of COVID-19: A Review and New Research Directions. J. Dent. Res. 2020, 99, 1435–1443. [Google Scholar] [CrossRef]
- Butler-Laporte, G.; Lawandi, A.; Schiller, I.; Yao, M.; Dendukuri, N.; McDonald, E.G.; Lee, T.C. Comparison of Saliva and Nasopharyngeal Swab Nucleic Acid Amplification Testing for Detection of SARS-CoV-2: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2021, 181, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Marouf, N.; Cai, W.; Said, K.N.; Daas, H.; Diab, H.; Chinta, V.R.; Hssain, A.A.; Nicolau, B.; Sanz, M.; Tamimi, F. Association between periodontitis and severity of COVID-19 infection: A case-control study. J. Clin. Periodontol 2021. [Google Scholar] [CrossRef] [PubMed]
- Mancini, L.; Quinzi, V.; Mummolo, S.; Marzo, G.; Marchetti, E. Angiotensin-Converting Enzyme 2 as a possible correlation between COVID-19 and periodontal disease. Appl. Sci. 2020, 10, 6224. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Van Dyke, T.E. Working group 1 of the joint EFP/AAP workshop. Periodontitis and atherosclerotic cardiovascular disease: Consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J. Periodontol. 2013, 84 (Suppl. 4), S24–S29. [Google Scholar] [CrossRef]
- 58. LaMonte, M.J.; Genco, R.J.; Hovey, K.M.; Wallace, R.B.; Freudenheim, J.L.; Michaud, D.S.; Mai, X.; Tinker, L.F.; Salazar, C.R.; Andrews, C.A.; et al. History of Periodontitis Diagnosis and Edentulism as Predictors of Cardiovascular Disease, Stroke, and Mortality in Post-menopausal Women. J. Am. Heart Assoc. 2017, 6, e004518. [Google Scholar] [CrossRef]
- Sanz, M.; Marco Del Castillo, A.; Jepsen, S.; Gonzalez-Juanatey, J.R.; D’Aiuto, F.; Bouchard, P.; Chapple, I.; Dietrich, T.; Gotsman, I.; Graziani, F.; et al. Periodontitis and cardiovascular diseases: Consensus report. J. Clin. Periodontol. 2020, 47, 268–288. [Google Scholar] [CrossRef] [PubMed]
- 60. Chapple, I.L.; Genco, R. Working group 2 of joint EFP/AAP workshop. Diabetes and periodontal diseases: Consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J. Clin. Periodontol. 2013, 40 (Suppl. 14), S106–S112. [Google Scholar] [CrossRef] [PubMed]
- Suvan, J.E.; Petrie, A.; Nibali, L.; Darbar, U.; Rakmanee, T.; Donos, N.; D’Aiuto, F. Association between overweight/obesity and increased risk of periodontitis. J. Clin. Periodontol. 2015, 42, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Ceriello, A.; Buysschaert, M.; Chapple, I.; Demmer, R.T.; Graziani, F.; Herrera, D.; Jepsen, S.; Lione, L.; Madianos, P.; et al. Scientific evidence on the links between periodontal diseases and diabetes: Consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International Diabetes Federation and the European Federation of Periodontology. J. Clin. Periodontol. 2018, 45, 138–149. [Google Scholar] [CrossRef]
- Muñoz Aguilera, E.; Suvan, J.; Buti, J.; Czesnikiewicz-Guzik, M.; Barbosa Ribeiro, A.; Orlandi, M.; Guzik, T.J.; Hingorani, A.D.; Nart, J.; D’Aiuto, F. Periodontitis is associated with hypertension: A systematic review and meta-analysis. Cardiovasc. Res. 2020, 116, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Dietrich, T.; Ferro, C.J.; Cockwell, P.; Chapple, I.L. Association between periodontitis and mortality in stages 3-5 chronic kidney disease: NHANES III and linked mortality study. J. Clin. Periodontol. 2016, 43, 104–113. [Google Scholar] [CrossRef] [Green Version]
- Gomes-Filho, I.S.; Cruz, S.S.D.; Trindade, S.C.; Passos-Soares, J.S.; Carvalho-Filho, P.C.; Figueiredo, A.C.M.G.; Lyrio, A.O.; Hintz, A.M.; Pereira, M.G.; Scannapieco, F. Periodontitis and respiratory diseases: A systematic review with meta-analysis. Oral Dis 2020, 26, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Nwizu, N.; Wactawski-Wende, J.; Genco, R.J. Periodontal disease and cancer: Epidemiologic studies and possible mechanisms. Periodontol. 2000 2020, 83, 213–233. [Google Scholar] [CrossRef]
- Schenkein, H.A.; Papapanou, P.N.; Genco, R.; Sanz, M. Mechanisms underlying the association between periodontitis and atherosclerotic disease. Periodontol. 2000 2020, 83, 90–106. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tada, A.; Senpuku, H. The Impact of Oral Health on Respiratory Viral Infection. Dent. J. 2021, 9, 43. https://doi.org/10.3390/dj9040043
Tada A, Senpuku H. The Impact of Oral Health on Respiratory Viral Infection. Dentistry Journal. 2021; 9(4):43. https://doi.org/10.3390/dj9040043
Chicago/Turabian StyleTada, Akio, and Hidenobu Senpuku. 2021. "The Impact of Oral Health on Respiratory Viral Infection" Dentistry Journal 9, no. 4: 43. https://doi.org/10.3390/dj9040043
APA StyleTada, A., & Senpuku, H. (2021). The Impact of Oral Health on Respiratory Viral Infection. Dentistry Journal, 9(4), 43. https://doi.org/10.3390/dj9040043




