Antioxidant Efficacy of Olive By-Product Extracts in Human Colon HCT8 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Extraction of Phenolic Compounds from By-Products
2.2.1. Olive Wastewater (OWW)
2.2.2. Olive Pomace (OP)
2.2.3. Olive Leaves (OLs)
2.3. Chemical Characterization of Extracts
2.3.1. Olive Wastewater (OWW) and Olive Pomace (OP)
2.3.2. Olive Leaves (OLs)
2.4. Cell Culture and Treatment
2.5. Crystal Violet Assay
2.6. Reactive Oxygen Species (ROS) Detection
2.7. Statistical Analysis
3. Results
3.1. Characterization of Olive By-Products and Waste
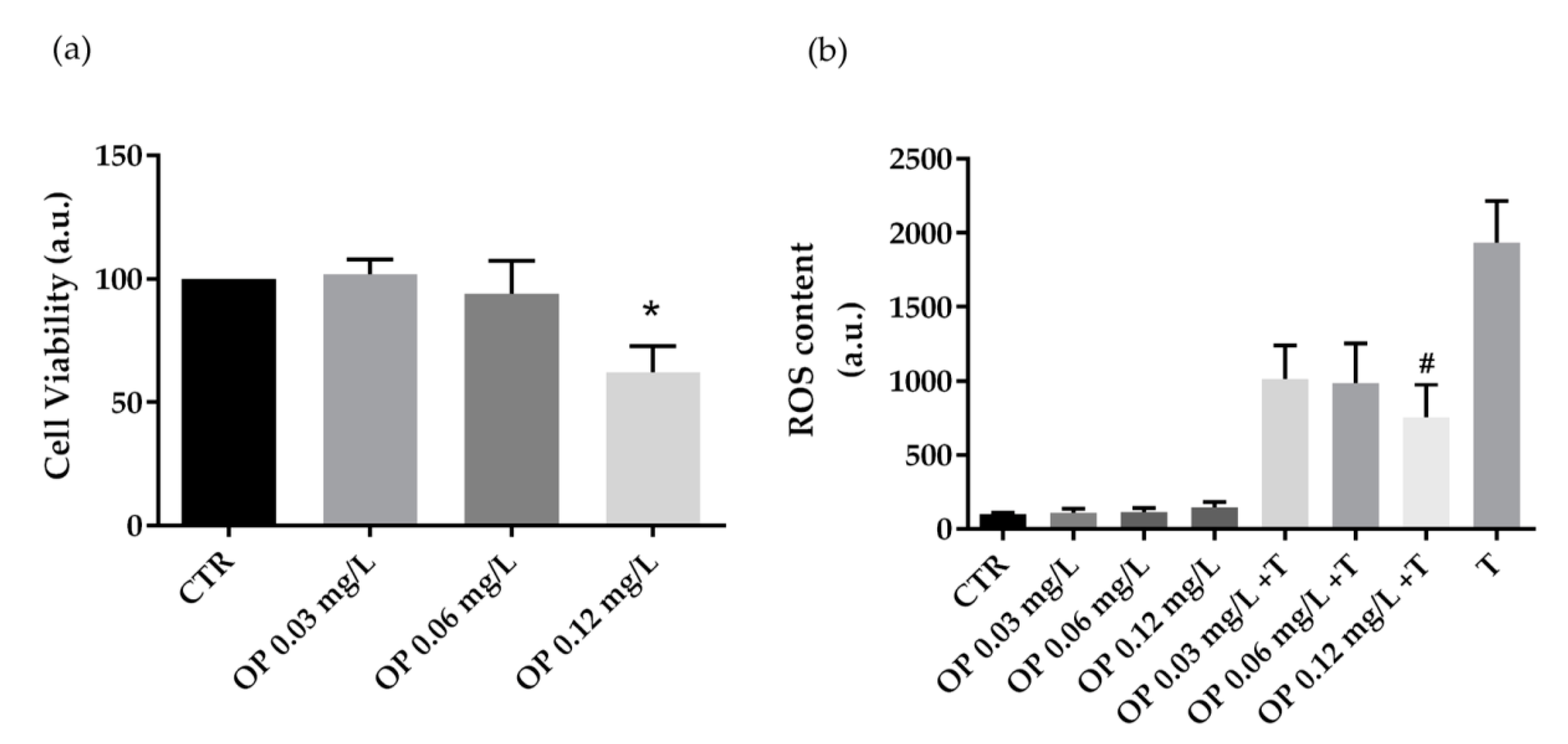
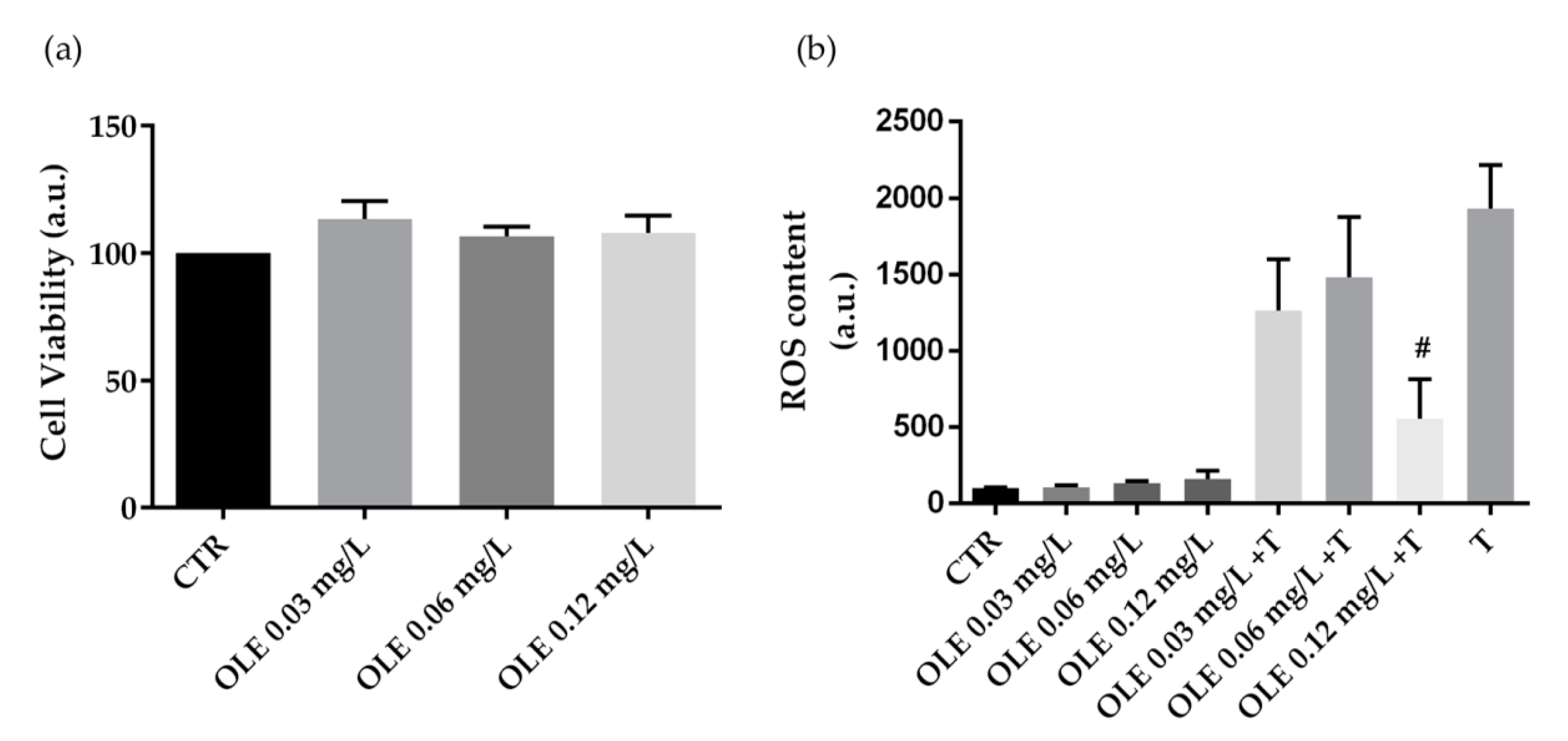
3.2. Biological Characterization of Olive By-Products
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kouka, P.; Tsakiri, G.; Tzortzi, D.; Dimopoulou, S.; Sarikaki, G.; Stathopoulos, P.; Veskoukis, A.S.; Halabalaki, M.; Skaltsounis, A.L.; Kouretas, D. The Polyphenolic Composition of Extracts Derived from Different Greek Extra Virgin Olive Oils Is Correlated with Their Antioxidant Potency. Oxidative Med. Cell. Longev. 2019, 2019, 1870965. [Google Scholar] [CrossRef] [PubMed]
- Saez, J.A.; Perez-Murcia, M.D.; Vico, A.; Martinez-Gallardo, M.R.; Andreu-Rodriguez, F.J.; Lopez, M.J.; Bustamante, M.A.; Sanchez-Hernandez, J.C.; Moreno, J.; Moral, R. Olive mill wastewater-evaporation ponds long term stored: Integrated assessment of in situ bioremediation strategies based on composting and vermicomposting. J. Hazard. Mater. 2020, 402, 123481. [Google Scholar] [CrossRef] [PubMed]
- Babic, S.; Malev, O.; Pflieger, M.; Lebedev, A.T.; Mazur, D.M.; Kuzic, A.; Coz-Rakovac, R.; Trebse, P. Toxicity evaluation of olive oil mill wastewater and its polar fraction using multiple whole-organism bioassays. Sci. Total Environ. 2019, 686, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Sahin, S.; Bilgin, M. Olive tree (Olea europaea L.) leaf as a waste by-product of table olive and olive oil industry: A review. J. Sci. Food Agric. 2018, 98, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Bonaccorsi, I.L.; Cacciola, F.; Dugo, L.; De Gara, L.; Dugo, P.; Mondello, L. Distribution of bioactives in entire mill chain from the drupe to the oil and wastes. Nat. Prod. Res. 2020, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ochando-Pulido, J.M.; Martinez-Ferez, A. Novel micro/ultra/nanocentrifugation membrane process assessment for revalorization and reclamation of agricultural wastewater. J. Environ. Manag. 2018, 222, 447–453. [Google Scholar] [CrossRef]
- Sygouni, V.; Pantziaros, A.G.; Iakovides, I.C.; Sfetsa, E.; Bogdou, P.I.; Christoforou, E.A.; Paraskeva, C.A. Treatment of Two-Phase Olive Mill Wastewater and Recovery of Phenolic Compounds Using Membrane Technology. Membranes 2019, 9, 27. [Google Scholar] [CrossRef] [Green Version]
- De Bruno, A.; Romeo, R.; Piscopo, A.; Poiana, M. Antioxidant quantification in different portions obtained during olive oil extraction process in an olive oil press mill. J. Sci. Food Agric. 2020. [Google Scholar] [CrossRef]
- Romani, A.; Ieri, F.; Urciuoli, S.; Noce, A.; Marrone, G.; Nediani, C.; Bernini, R. Health Effects of Phenolic Compounds Found in Extra-Virgin Olive Oil, By-Products, and Leaf of Olea europaea L. Nutrients 2019, 11, 1776. [Google Scholar] [CrossRef] [Green Version]
- Difonzo, G.; Russo, A.; Trani, A.; Paradiso, V.M.; Ranieri, M.; Pasqualone, A.; Summo, C.; Tamma, G.; Silletti, R.; Caponio, F. Green extracts from Coratina olive cultivar leaves: Antioxidant characterization and biological activity. J. Funct. Foods 2017, 31, 63–70. [Google Scholar] [CrossRef]
- Ranieri, M.; Di Mise, A.; Difonzo, G.; Centrone, M.; Venneri, M.; Pellegrino, T.; Russo, A.; Mastrodonato, M.; Caponio, F.; Valenti, G.; et al. Green olive leaf extract (OLE) provides cytoprotection in renal cells exposed to low doses of cadmium. PLoS ONE 2019, 14, e0214159. [Google Scholar] [CrossRef] [PubMed]
- Asgharzade, S.; Sheikhshabani, S.H.; Ghasempour, E.; Heidari, R.; Rahmati, S.; Mohammadi, M.; Jazaeri, A.; Amini-Farsani, Z. The effect of oleuropein on apoptotic pathway regulators in breast cancer cells. Eur. J. Pharmacol. 2020, 886, 173509. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.Z.; Li, L.; Espe, M.; Lu, K.L.; Rahimnejad, S. Hydroxytyrosol Attenuates Hepatic Fat Accumulation via Activating Mitochondrial Biogenesis and Autophagy through the AMPK Pathway. J. Agric. Food Chem. 2020, 68, 9377–9386. [Google Scholar] [CrossRef]
- D’Evoli, L.; Morroni, F.; Lombardi-Boccia, G.; Lucarini, M.; Hrelia, P.; Cantelli-Forti, G.; Tarozzi, A. Red chicory (Cichorium intybus L. cultivar) as a potential source of antioxidant anthocyanins for intestinal health. Oxidative Med. Cell. Longev. 2013, 2013, 704310. [Google Scholar] [CrossRef] [Green Version]
- Owen, R.W.; Giacosa, A.; Hull, W.E.; Haubner, R.; Spiegelhalder, B.; Bartsch, H. The antioxidant/anticancer potential of phenolic compounds isolated from olive oil. Eur. J. Cancer 2000, 36, 1235–1247. [Google Scholar] [CrossRef]
- Tang, X.; Kuhlenschmidt, T.B.; Li, Q.; Ali, S.; Lezmi, S.; Chen, H.; Pires-Alves, M.; Laegreid, W.W.; Saif, T.A.; Kuhlenschmidt, M.S. A mechanically-induced colon cancer cell population shows increased metastatic potential. Mol. Cancer 2014, 13, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lievin-Le Moal, V.; Servin, A.L. Pathogenesis of human enterovirulent bacteria: Lessons from cultured, fully differentiated human colon cancer cell lines. Microbiol. Mol. Biol. Rev. 2013, 77, 380–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romeo, R.; De Bruno, A.; Imeneo, V.; Piscopo, A.; Poiana, M. Evaluation of enrichment with antioxidants from olive oil mill wastes in hydrophilic model system. J. Food Process. Preserv. 2019, 43, e14211. [Google Scholar] [CrossRef]
- De Bruno, A.; Romeo, R.; Fedele, F.L.; Sicari, A.; Piscopo, A.; Poiana, M. Antioxidant activity shown by olive pomace extracts. J. Environ. Sci. Health 2018, 53, 526–533. [Google Scholar] [CrossRef]
- Marroncini, G.; Fibbi, B.; Errico, A.; Grappone, C.; Maggi, M.; Peri, A. Effects of low extracellular sodium on proliferation and invasive activity of cancer cells in vitro. Endocrine 2020, 67, 473–484. [Google Scholar] [CrossRef]
- Romeo, R.; De Bruno, A.; Imeneo, V.; Piscopo, A.; Poiana, M. Impact of Stability of Enriched Oil with Phenolic Extract from Olive Mill Wastewaters. Foods 2020, 9, 856. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, S.; Koohi, D.E.; Emmamzadehhashemi, M.S.B.; Khamas, S.S.; Moazen, M.; Hashemi, A.K.; Amin, G.; Golfakhrabadi, F.; Yousefi, Z.; Yousefbeyk, F. Investigation of phenolic compounds and antioxidant activity of leaves extracts from seventeen cultivars of Iranian olive (Olea europaea L.). J. Food Sci. Technol. 2018, 55, 4600–4607. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.; Allen, P.; Brunton, N.; O’Grady, M.; Kerry, J.P. Phenolic composition and in vitro antioxidant capacity of four commercial phytochemical products: Olive leaf extract (Olea europaea L.), lutein, sesamol and ellagic acid. Food Chem. 2011, 126, 948–955. [Google Scholar] [CrossRef]
- Benavente-Garcıa, O.; Castillo, J.; Lorente, J.; Ortuño, A.D.R.J.; Del Rio, J.A. Antioxidant activity of phenolics extracted from Olea europaea L. leaves. Food Chem. 2000, 68, 457–462. [Google Scholar] [CrossRef]
- Tun, S.; Spainhower, C.J.; Cottrill, C.L.; Lakhani, H.V.; Pillai, S.S.; Dilip, A.; Chaudhry, H.; Shapiro, J.I.; Sodhi, K. Therapeutic Efficacy of Antioxidants in Ameliorating Obesity Phenotype and Associated Comorbidities. Front. Pharmacol. 2020, 11, 1234. [Google Scholar] [CrossRef] [PubMed]
- Ordovas, J.M.; Kaput, J.; Corella, D. Nutrition in the genomics era: Cardiovascular disease risk and the Mediterranean diet. Mol. Nutr. Food Res. 2007, 51, 1293–1299. [Google Scholar] [CrossRef]
- Waterman, E.; Lockwood, B. Active components and clinical applications of olive oil. Altern. Med. Rev. A J. Clin. Ther. 2007, 12, 331–342. [Google Scholar]
- D’Amore, S.; Vacca, M.; Cariello, M.; Graziano, G.; D’Orazio, A.; Salvia, R.; Sasso, R.C.; Sabba, C.; Palasciano, G.; Moschetta, A. Genes and miRNA expression signatures in peripheral blood mononuclear cells in healthy subjects and patients with metabolic syndrome after acute intake of extra virgin olive oil. Biochim. Et Biophys. Acta 2016, 1861, 1671–1680. [Google Scholar] [CrossRef]
- Lama, A.; Pirozzi, C.; Mollica, M.P.; Trinchese, G.; Di Guida, F.; Cavaliere, G.; Calignano, A.; Mattace Raso, G.; Berni Canani, R.; Meli, R. Polyphenol-rich virgin olive oil reduces insulin resistance and liver inflammation and improves mitochondrial dysfunction in high-fat diet fed rats. Mol. Nutr. Food Res. 2017, 61, 1600418. [Google Scholar] [CrossRef]
- Cariello, M.; Contursi, A.; Gadaleta, R.M.; Piccinin, E.; De Santis, S.; Piglionica, M.; Spaziante, A.F.; Sabba, C.; Villani, G.; Moschetta, A. Extra-Virgin Olive Oil from Apulian Cultivars and Intestinal Inflammation. Nutrients 2020, 12, 1084. [Google Scholar] [CrossRef] [Green Version]
- Gong, J.; Zhou, S.; Yang, S. Vanillic Acid Suppresses HIF-1alpha Expression via Inhibition of mTOR/p70S6K/4E-BP1 and Raf/MEK/ERK Pathways in Human Colon Cancer HCT116 Cells. Int. J. Mol. Sci. 2019, 20, 456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldsmith, C.D.; Bond, D.R.; Jankowski, H.; Weidenhofer, J.; Stathopoulos, C.E.; Roach, P.D.; Scarlett, C.J. The Olive Biophenols Oleuropein and Hydroxytyrosol Selectively Reduce Proliferation, Influence the Cell Cycle, and Induce Apoptosis in Pancreatic Cancer Cells. Int. J. Mol. Sci. 2018, 19, 1937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palombo, R.; Savini, I.; Avigliano, L.; Madonna, S.; Cavani, A.; Albanesi, C.; Mauriello, A.; Melino, G.; Terrinoni, A. Luteolin-7-glucoside inhibits IL-22/STAT3 pathway, reducing proliferation, acanthosis, and inflammation in keratinocytes and in mouse psoriatic model. Cell Death Dis. 2016, 7, e2344. [Google Scholar] [CrossRef] [PubMed]
- Hassan, Z.K.; Elamin, M.H.; Omer, S.A.; Daghestani, M.H.; Al-Olayan, E.S.; Elobeid, M.A.; Virk, P. Oleuropein induces apoptosis via the p53 pathway in breast cancer cells. Asian Pac. J. Cancer Prev. 2013, 14, 6739–6742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardeno, A.; Sanchez-Hidalgo, M.; Rosillo, M.A.; Alarcon de la Lastra, C. Oleuropein, a secoiridoid derived from olive tree, inhibits the proliferation of human colorectal cancer cell through downregulation of HIF-1alpha. Nutr. Cancer 2013, 65, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Karanovic, D.; Mihailovic-Stanojevic, N.; Miloradovic, Z.; Ivanov, M.; Vajic, U.J.; Grujic-Milanovic, J.; Markovic-Lipkovski, J.; Dekanski, D.; Jovovic, D. Olive leaf extract attenuates adriamycin-induced focal segmental glomerulosclerosis in spontaneously hypertensive rats via suppression of oxidative stress, hyperlipidemia, and fibrosis. Phytother. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Lins, P.G.; Marina Piccoli Pugine, S.; Scatolini, A.M.; de Melo, M.P. In vitro antioxidant activity of olive leaf extract (Olea europaea L.) and its protective effect on oxidative damage in human erythrocytes. Heliyon 2018, 4, e00805. [Google Scholar] [CrossRef] [Green Version]
- Visioli, F.; Bellomo, G.; Galli, C. Free radical-scavenging properties of olive oil polyphenols. Biochem. Biophys. Res. Commun. 1998, 247, 60–64. [Google Scholar] [CrossRef]
- Deiana, M.; Serra, G.; Corona, G. Modulation of intestinal epithelium homeostasis by extra virgin olive oil phenolic compounds. Food Funct. 2018, 9, 4085–4099. [Google Scholar] [CrossRef]
- Huguet-Casquero, A.; Xu, Y.; Gainza, E.; Pedraz, J.L.; Beloqui, A. Oral delivery of oleuropein-loaded lipid nanocarriers alleviates inflammation and oxidative stress in acute colitis. Int. J. Pharm. 2020, 586, 119515. [Google Scholar] [CrossRef]
- Azaizeh, H.; Halahlih, F.; Najami, N.; Brunner, D.; Faulstich, M.; Tafesh, A. Antioxidant activity of phenolic fractions in olive mill wastewater. Food Chem. 2012, 134, 2226–2234. [Google Scholar] [CrossRef] [PubMed]
- Martinez, L.; Ros, G.; Nieto, G. Hydroxytyrosol: Health Benefits and Use as Functional Ingredient in Meat. Medicines 2018, 5, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sotler, R.; Poljsak, B.; Dahmane, R.; Jukic, T.; Pavan Jukic, D.; Rotim, C.; Trebse, P.; Starc, A. Prooxidant Activities of Antioxidants and Their Impact on Health. Acta Clin. Croat. 2019, 58, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Luo, C.; Liu, J. Hydroxytyrosol induces apoptosis in human colon cancer cells through ROS generation. Food Funct. 2014, 5, 1909–1914. [Google Scholar] [CrossRef]
- Toric, J.; Markovic, A.K.; Brala, C.J.; Barbaric, M. Anticancer effects of olive oil polyphenols and their combinations with anticancer drugs. Acta Pharm. 2019, 69, 461–482. [Google Scholar] [CrossRef] [Green Version]

| Parameter | OWW | OP | OLE |
|---|---|---|---|
| TPC | 8.61 | 4.46 | 190 |
| ABTS | 3579 | 451 | 780 |
| Hydroxytyrosol | 1.43 | 0.23 | nd |
| Tyrosol | 0.25 | 0.16 | 3.61 |
| Caffeic acid | 0.07 | 0.01 | nd |
| Apigenin-7-O-glucoside | 0.03 | 0.03 | 6.92 |
| Vanillic acid | 0.01 | 0.03 | nd |
| Oleuropein | 0.02 | 0.09 | 137 |
| Verbascoside | nd | nd | 20 |
| Luteolin-7-O-glucoside | 0.008 | 0.012 | 3.14 |
| Rutin | nd | nd | 3.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Centrone, M.; D’Agostino, M.; Difonzo, G.; De Bruno, A.; Di Mise, A.; Ranieri, M.; Montemurro, C.; Valenti, G.; Poiana, M.; Caponio, F.; et al. Antioxidant Efficacy of Olive By-Product Extracts in Human Colon HCT8 Cells. Foods 2021, 10, 11. https://doi.org/10.3390/foods10010011
Centrone M, D’Agostino M, Difonzo G, De Bruno A, Di Mise A, Ranieri M, Montemurro C, Valenti G, Poiana M, Caponio F, et al. Antioxidant Efficacy of Olive By-Product Extracts in Human Colon HCT8 Cells. Foods. 2021; 10(1):11. https://doi.org/10.3390/foods10010011
Chicago/Turabian StyleCentrone, Mariangela, Mariagrazia D’Agostino, Graziana Difonzo, Alessandra De Bruno, Annarita Di Mise, Marianna Ranieri, Cinzia Montemurro, Giovanna Valenti, Marco Poiana, Francesco Caponio, and et al. 2021. "Antioxidant Efficacy of Olive By-Product Extracts in Human Colon HCT8 Cells" Foods 10, no. 1: 11. https://doi.org/10.3390/foods10010011
APA StyleCentrone, M., D’Agostino, M., Difonzo, G., De Bruno, A., Di Mise, A., Ranieri, M., Montemurro, C., Valenti, G., Poiana, M., Caponio, F., & Tamma, G. (2021). Antioxidant Efficacy of Olive By-Product Extracts in Human Colon HCT8 Cells. Foods, 10(1), 11. https://doi.org/10.3390/foods10010011