Comparing Three Types of Mandarin Powders Prepared via Microfluidic-Jet Spray Drying: Physical Properties, Phenolic Retention and Volatile Profiling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Mandarin Juice and Feed Solution for Spray Drying
2.3. Spray Drying of Juice Samples
2.4. Properties of the Mandarin Juice Powders
2.4.1. Water Activity
2.4.2. Bulk and Trapped Density
2.4.3. Solubility and Hygroscopicity
2.4.4. Colour Measurement
2.5. Morphological Observation by Scanning Electron Microscopy (SEM)
2.6. Determination of Phenolic Compounds by High Performance Liquid Chromatography (HPLC)
2.7. Characterization of Aroma Compounds by Headspace Solid-Phase Micro-Extraction and Gas Chromatography-Mass Spectrometry (HS-SPME-GC-MS)
2.8. Statistical Analysis
3. Results and Discussion
3.1. Physical Properties of Mandarin Juice Powders
3.1.1. Colour Differences among Powder Samples
3.1.2. Water Activity of the Powders
3.1.3. Density of the Powders
3.1.4. Flowability and Cohesiveness of Powders
3.1.5. Solubility of Powders
3.1.6. Hygroscopicity of Powders
3.2. SEM Imaging Analysis
3.3. Retention of Phenolic Compounds during Spray Drying
3.4. Volatile Compounds in Mandarin Juices and Juice Powders
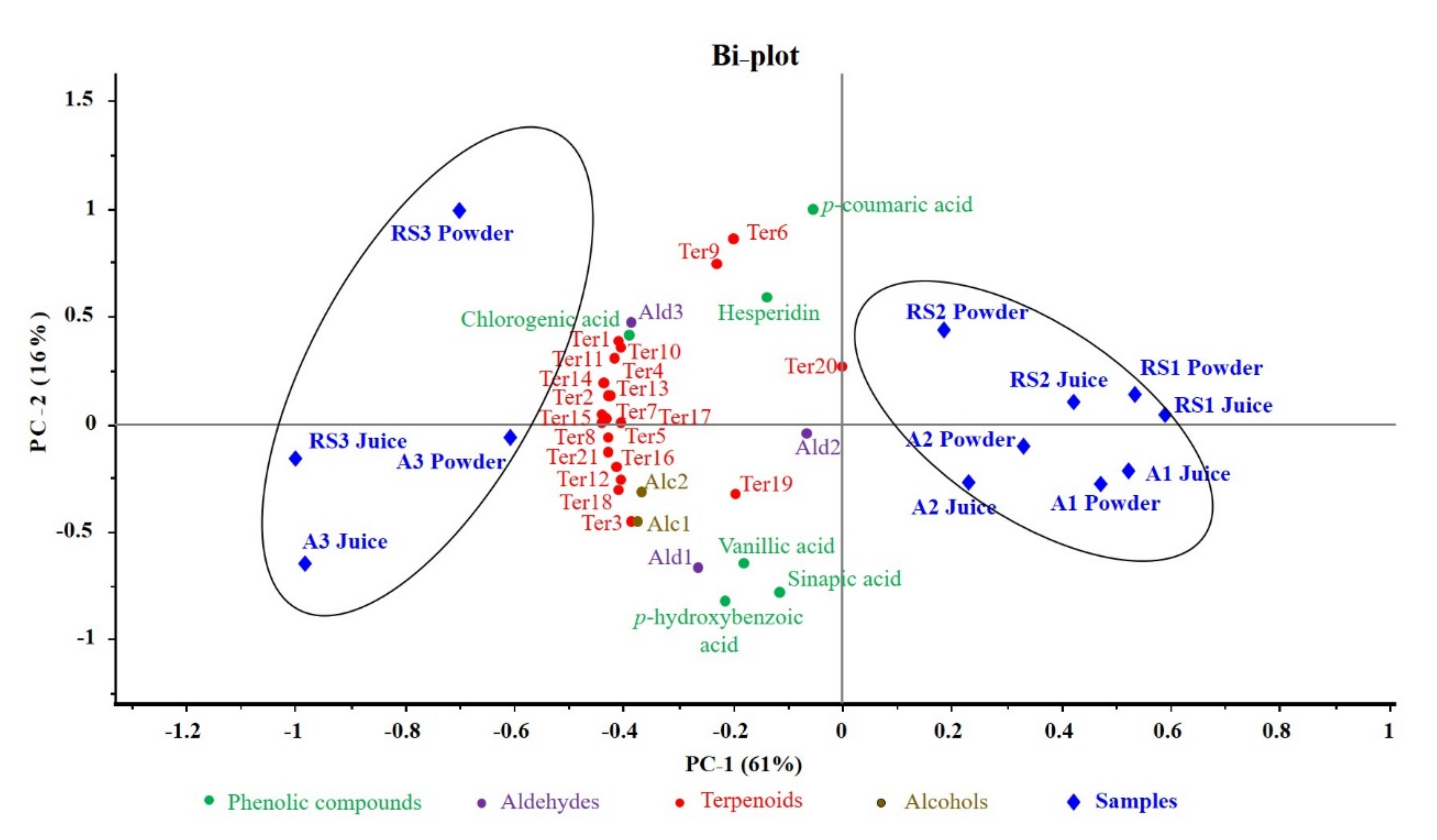
3.5. Distribution of Phenolic and Aromatic Compounds in Different Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Putnik, P.; Barba, F.J.; Lorenzo, J.M.; Gabrić, D.; Shpigelman, A.; Cravotto, G.; Bursać Kovačević, D. An integrated approach to mandarin processing: Food safety and nutritional quality, consumer preference, and nutrient bioaccessibility. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1345–1358. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Heying, E.; Tanumihardjo, S.A. History, global distribution, and nutritional importance of citrus fruits. Compr. Rev. Food Sci. Food Saf. 2012, 11, 530–545. [Google Scholar] [CrossRef]
- Lado, J.; Cuellar, F.; Rodrigo, M.J.; Zacarías, L. Nutritional Composition of Mandarins. In Nutritional Compsition of Fruit Cultivars; Academic Press: Amsterdam, The Netherlands, 2016; pp. 419–443. [Google Scholar]
- Tripoli, E.; Guardia, M.L.; Giammanco, S.; Majo, D.D.; Giammanco, M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 2007, 104, 466–479. [Google Scholar] [CrossRef]
- Song, M.; Charoensinphon, N.; Wu, X.; Zheng, J.; Gao, Z.; Xu, F.; Wang, M.; Xiao, H. Inhibitory effects of metabolites of 5-demethylnobiletin on human nonsmall cell lung cancer cells. J. Agric. Food Chem. 2016, 64, 4943–4949. [Google Scholar] [CrossRef]
- Babbar, N.; Oberoi, H.S.; Uppal, D.S.; Patil, R.T. Total phenolic content and antioxidant capacity of extracts obtained from six important fruit residues. Food Res. Int. 2011, 44, 391–396. [Google Scholar] [CrossRef]
- Wahyuono, R.A.E.; Hesse, J.; Hipler, U.-C.; Elsner, P.; Böhm, V. Carotenoids of indigenous citrus species from Aceh and its in vitro antioxidant, antidiabetic and antibacterial activities. Eur. Food Res. Technol. 2016, 242, 1869–1881. [Google Scholar]
- Liu, L.; Xu, X.; Cheng, D.; Yao, X.; Pan, S. Preparative separation of polymethoxylated flavones from Ponkan (Citrus reticulata Blanco cv. Ponkan) peel by high-speed countercurrent chromatography and their antifungal activities against Aspergillus niger. Eur. Food Res. Technol. 2012, 235, 631–635. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Mohamed, A.K. Novel derived pectin hydrogel from mandarin peel based metal-organic frameworks composite for enhanced Cr (VI) and Pb (II) ions removal. Int. J. Biol. Macromol. 2020, 164, 920–931. [Google Scholar] [CrossRef]
- Zhao, S.; Gao, W.; Tian, G.; Zhao, C.; DiMarco-Crook, C.; Fan, B.; Li, C.; Xiao, H.; Lian, Y.; Zheng, J. Citrus oil emulsions stabilized by citrus pectin: The influence mechanism of citrus variety and acid treatment. J. Agric. Food Chem. 2018, 66, 12978–12988. [Google Scholar] [CrossRef]
- Espina, L.; Somolinos, M.; Lorán, S.; Conchello, P.; García, D.; Pagán, R. Chemical composition of commercial citrus fruit essential oils and evaluation of their antimicrobial activity acting alone or in combined processes. Food Control 2011, 22, 896–902. [Google Scholar] [CrossRef]
- Ojha, P.; Thapa, S. Quality evaluation of biscuit incorporated with mandarin peel powder. Sci. Study Research. Chem. Chem. Eng. Biotechnol. Food Ind. 2017, 18, 19. [Google Scholar]
- Betoret, E.; Sentandreu, E.; Betoret, N.; Codoñer-Franch, P.; Valls-Bellés, V.; Fito, P. Technological development and functional properties of an apple snack rich in flavonoid from mandarin juice. Innov. Food Sci. Emerg. Technol. 2012, 16, 298–304. [Google Scholar] [CrossRef]
- Phisut, N. Spray drying technique of fruit juice powder: Some factors influencing the properties of product. Int. Food Res. J. 2012, 19, 1297–1306. [Google Scholar]
- Zhang, C.; Chen, X.; Zhang, J.; Kilmartin, P.A.; Quek, S.Y. Exploring the effects of microencapsulation on odour retention of fermented noni juice. J. Food Eng. 2020, 273, 109892. [Google Scholar] [CrossRef]
- Zhang, C.; Khoo, S.L.A.; Swedlund, P.; Ogawa, Y.; Shan, Y.; Quek, S.Y. Fabrication of spray-dried microcapsules containing noni juice using blends of maltodextrin and gum acacia: Physicochemical properties of powders and bioaccessibility of bioactives during in vitro digestion. Foods 2020, 9, 1316. [Google Scholar] [CrossRef]
- Anandharamakrishnan, C. Spray Drying Techniques for Food Ingredient Encapsulation; John Wiley & Sons: Chicago, IL, USA, 2015. [Google Scholar]
- Wu, W.D.; Patel, K.C.; Rogers, S.; Chen, X.D. Monodisperse droplet generators as potential atomizers for spray drying technology. Dry. Technol. 2007, 25, 1907–1916. [Google Scholar] [CrossRef]
- Lee, K.C.; Eun, J.B.; Hwang, S.J. Physicochemical properties and sensory evaluation of mandarin (Citrus unshiu) beverage powder spray-dried at different inlet air temperatures with different amounts of a mixture of maltodextrin and corn syrup. Food Sci. Biotechnol. 2016, 25, 1345–1351. [Google Scholar] [CrossRef]
- Saikia, S.; Mahnot, N.K.; Mahanta, C.L. Effect of spray drying of four fruit juices on physicochemical, phytochemical and antioxidant properties. J. Food Process. Preserv. 2015, 39, 1656–1664. [Google Scholar] [CrossRef]
- Zhang, C.; Ada Khoo, S.L.; Chen, X.D.; Quek, S.Y. Microencapsulation of fermented noni juice via micro-fluidic-jet spray drying: Evaluation of powder properties and functionalities. Powder Technol. 2020, 361, 995–1005. [Google Scholar] [CrossRef]
- Frascareli, E.C.; Silva, V.M.; Tonon, R.V.; Hubinger, M.D. Effect of process conditions on the microencapsulation of coffee oil by spray drying. Food Bioprod. Process. 2012, 90, 413–424. [Google Scholar] [CrossRef]
- Santhalakshmy, S.; Bosco, S.J.D.; Francis, S.; Sabeena, M. Effect of inlet temperature on physicochemical properties of spray-dried jamun fruit juice powder. Powder Technol. 2015, 274, 37–43. [Google Scholar] [CrossRef]
- Rigon, R.T.; Zapata Norena, C.P. Microencapsulation by spray-drying of bioactive compounds extracted from blackberry (rubus fruticosus). J. Food Sci. Technol. 2016, 53, 1515–1524. [Google Scholar] [CrossRef] [Green Version]
- Elez Garofulić, I.; Zorić, Z.; Pedisić, S.; Dragović-Uzelac, V. Optimization of sour cherry juice spray drying as affected by carrier material and temperature. Food technology and biotechnology. Food Technol. Biotechnol. 2016, 54, 441–449. [Google Scholar] [CrossRef]
- Chen, Z.G.; Buchanan, P.; Quek, S.Y. Development and validation of an HPLC-DAD-MS method for determination of four nucleoside compounds in the New Zealand native mushroom Hericium sp. Food Chem. 2019, 278, 729–737. [Google Scholar] [CrossRef]
- Chen, X.; Fedrizzi, B.; Kilmartin, P.A.; Quek, S.Y. Development of volatile organic compounds and their glycosylated precursors in tamarillo (Solanum betaceum Cav.) during fruit ripening: A prediction of biochemical pathway. Food Chem. 2021, 339, 128046. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Quek, S.Y.; Fedrizzi, B.; Kilmartin, P.A. Characterization of free and glycosidically bound volatile compounds from tamarillo (Solanum betaceum Cav.) with considerations on hydrolysis strategies and incubation time. LWT—Food Sci. Technol. 2020, 124, 109178. [Google Scholar] [CrossRef]
- Chen, X.; Yu, J.; Cui, H.; Xia, S.; Zhang, X.; Yang, B. Effect of temperature on flavor compounds and sensory characteristics of maillard reaction products derived from mushroom hydrolysate. Molecules 2018, 23, 247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbosa-Cánovas, G.V.; Fontana Jr, A.J.; Schmidt, S.J.; Labuza, T.P. Water Activity in Foods: Fundamentals and Applications; John Wiley & Sons: Chicago, IL, USA, 2020. [Google Scholar]
- Janiszewska-Turak, E.; Dellarosa, N.; Tylewicz, U.; Laghi, L.; Romani, S.; Dalla Rosa, M.; Witrowa-Rajchert, D. The influence of carrier material on some physical and structural properties of carrot juice microcapsules. Food Chem. 2017, 236, 134–141. [Google Scholar] [CrossRef]
- Naohara, J.; Manabe, M. Molecular mass and solubility changes in pectins during storage of satsuma mandarin fruits (Citrus unshiu Marc.). J. Food Sci. 1994, 59, 578–580. [Google Scholar] [CrossRef]
- Cortés-Rojas, D.F.; Souza, C.R.F.; Oliveira, W.P. Optimization of spray drying conditions for production of Bidens pilosa L. dried extract. Chem. Eng. Res. Des. 2015, 93, 366–376. [Google Scholar] [CrossRef]
- Ma, Y.Q.; Ye, X.Q.; Fang, Z.X.; Chen, J.C.; Xu, G.H.; Liu, D.H. Phenolic compounds and antioxidant activity of extracts from ultrasonic treatment of Satsuma mandarin (Citrus unshiu Marc.) peels. J. Agric. Food Chem. 2008, 56, 5682–5690. [Google Scholar] [CrossRef] [PubMed]
- Sansone, F.; Mencherini, T.; Picerno, P.; d’Amore, M.; Aquino, R.P.; Lauro, M.R. Maltodextrin/pectin microparticles by spray drying as carrier for nutraceutical extracts. J. Food Eng. 2011, 105, 468–476. [Google Scholar] [CrossRef]
- Zhang, C.; Quek, S.Y.; Fu, N.; Liu, B.; Kilmartin, P.A.; Chen, X.D. A study on the structure formation and properties of noni juice microencapsulated with maltodextrin and gum acacia using single droplet drying. Food Hydrocoll. 2019, 88, 199–209. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Y.; Xi, W.; Shen, Y.; Qiao, L.; Zhong, L.; Ye, X.; Zhou, Z. Phenolic compositions and antioxidant capacities of Chinese wild mandarin (Citrus reticulata Blanco) fruits. Food Chem. 2014, 145, 674–680. [Google Scholar] [CrossRef]
- Dhuique-Mayer, C.; Tbatou, M.; Carail, M.; Caris-Veyrat, C.; Dornier, M.; Amiot, M.J. Thermal degradation of antioxidant micronutrients in citrus juice: Kinetics and newly formed compounds. J. Agric. Food Chem. 2007, 55, 4209–4216. [Google Scholar] [CrossRef]
- Zhao, X.J.; Xing, T.T.; Li, Y.F.; Jiao, B.N. Analysis of phytochemical contributors to antioxidant capacity of the peel of Chinese mandarin and orange varieties. Int. J. Food Sci. Nutr. 2019, 70, 825–833. [Google Scholar] [CrossRef]
- Gershenzon, J.; Dudareva, N. The function of terpene natural products in the natural world. Nat. Chem. Biol. 2007, 3, 408–414. [Google Scholar] [CrossRef]
- Ren, J.N.; Tai, Y.N.; Dong, M.; Shao, J.H.; Yang, S.Z.; Pan, S.Y.; Fan, G. Characterisation of free and bound volatile compounds from six different varieties of citrus fruits. Food Chem. 2015, 185, 25–32. [Google Scholar] [CrossRef]
- Pérez-López, A.J.; Saura, D.; Lorente, J.; Carbonell-Barrachina, Á.A. Limonene, linalool, α-terpineol, and terpinen-4-ol as quality control parameters in mandarin juice processing. Eur. Food Res. Technol. 2005, 222, 281–285. [Google Scholar] [CrossRef]
- Ravichandran, C.; Badgujar, P.C.; Gundev, P.; Upadhyay, A. Review of toxicological assessment of d-limonene, a food and cosmetics additive. Food Chem. Toxicol. 2018, 120, 668–680. [Google Scholar] [CrossRef] [PubMed]


| L* | a* | b* | Chroma | Hue Angle | |
|---|---|---|---|---|---|
| A1 powder | 88.30 ± 0.56 a | 8.04 ± 0.65 b | 30.40 ± 0.59 f | 29.54 ± 2.53 e | 74.94 ± 2.71 e |
| A2 powder | 86.04 ± 0.29 cd | 5.69 ± 0.45 d | 50.26 ± 0.40 c | 48.08 ± 2.77 b | 83.28 ± 0.81 c |
| A3 powder | 87.10 ± 0.39 b | 3.39 ± 0.34 e | 52.57 ± 0.70 b | 52.71 ± 0.79 a | 86.50 ± 0.28 a |
| RS1 powder | 85.11 ± 1.43 d | 9.26 ± 0.10 a | 37.09 ±0.37 e | 37.95 ±1.32 d | 73.69 ±2.53 e |
| RS2 powder | 85.22 ± 0.50 d | 8.97 ± 0.85 a | 42.85 ± 0.80 d | 43.54 ± 0.85 c | 78.67 ± 0.75 d |
| RS3 powder | 86.33 ± 0.73 bc | 6.79 ± 0.11 c | 54.68 ± 0.24 a | 54.28 ± 1.23 a | 84.69 ± 1.51 bc |
| A1 Powder | A2 Powder | A3 Powder | RS1 Powder | RS2 Powder | RS3 Powder | |
|---|---|---|---|---|---|---|
| Water Activity (Aw) | 0.25 ± 0.00 a | 0.17 ± 0.01 b | 0.20 ± 0.01 a | 0.17 ± 0.01 b | 0.20 ± 0.003 a | 0.14 ± 0.01 c |
| Bulk density, ρbulk (g/mL) | 0.56 ± 0.01 c | 0.61 ± 0.01 a | 0.60 ± 0.01 a | 0.57 ± 0.01 c | 0.58 ± 0.01 bc | 0.60 ± 0.01 ab |
| Tapped density, ρtap (g/mL) | 0.71 ± 0.00 cd | 0.75 ± 0.02 ab | 0.73 ± 0.02 bc | 0.69 ± 0.00 d | 0.72 ± 0.02 c | 0.76 ± 0.02 a |
| Carr’s index (%) | 21.48 ± 0.02 a | 18.37 ± 0.33 bc | 19.00 ± 0.31 c | 17.14 ± 0.00 c | 21.02 ± 1.41 ab | 22.05 ± 1.38 a |
| Hausner’s ratio | 1.27 ± 0.02 a | 1.23 ± 0.01 b | 1.22 ± 0.01 b | 1.21 ± 0.00 b | 1.27 ± 0.02 a | 1.28 ± 0.02 a |
| Solubility (%) | 74.19 ± 1.13 a | 74.07 ± 1.62 a | 73.82 ± 1.02 a | 75.30 ± 1.56 a | 74.82 ± 1.40 a | 73.84 ± 1.28 a |
| Hygroscopicity (g moisture/100 g solids) | 17.45 ± 0.14 c | 17.71 ± 0.21 bc | 18.20 ± 0.19 ab | 18.02 ± 0.40 bc | 17.53 ± 0.48 abc | 18.53 ± 0.46 a |
| Concentrations (µg/100 mg DW) A | ||||||
|---|---|---|---|---|---|---|
| Chlorogenic Acid | Hesperidin | p-Coumaric Acid | p-Hydroxybenzoic Acid | Sinapic Acid | Vanillic Acid | |
| A1 juice | 9.6 ± 0.17 b | 44.8 ± 0.72 b | ND C | 65.9 ± 0.92 f | 489.7 ± 7.6 c | 44.3 ± 1.1 e |
| A1 powder | 14.1 ± 0.95 c | 105.9 ± 0.46 h | ND | 69.7 ± 0.96 g | 406.9 ± 15.4 b | 56.7 ± 2.4 g |
| A1 Retention/% B | 145.8 | 236.4 | N/A D | 105.8 | 83.1 | 128.0 |
| A2 juice | 13.68 ± 0.14 c | 46.7 ± 0.19 b | ND | 70.0 ± 0.46 g | 554.1 ± 22.2 d | 48.1 ± 0.15 f |
| A2 powder | 17.2 ± 0.88 d | 147.6 ± 3.0 i | ND | 57.5 ± 2.8 e | 529.2 ± 25.2 d | 57.1 ± 0.58 g |
| A2 Retention/% | 125.55 | 316.06 | N/A | 82.26 | 95.51 | 118.71 |
| A3 juice | 32.8 ± 0.82 g | 66.4 ± 1.5 e | ND | 91.5 ± 1.5 i | 912.6 ± 9.6 f | 94.5 ± 1.3 h |
| A3 powder | 28.8 ± 1.2 f | 148.5 ± 0.76 i | ND | 77.9 ± 4.1 h | 664.5 ± 43.9 e | 103.6 ± 1.0 i |
| A3 Retention/% | 87.80 | 223.64 | N/A | 85.14 | 72.81 | 109.63 |
| RS1 juice | 8.4 ± 0.05 a | 15.0 ± 0.27 a | 0.80 ± 0.05 a | 19.3 ± 0.72 bc | 8.7 ± 0.46 a | 4.6 ± 0.09 a |
| RS1 powder | 18.9 ± 0.37 e | 88.6 ± 0.64 f | 2.4 ± 0.03 d | 14.7 ± 0.28 a | 13.3 ± 1.7 a | 8.4 ± 0.53 b |
| RS1 Retention/% | 225.00 | 594.63 | 287.50 | 131.29 | 65.41 | 54.76 |
| RS2 juice | 14.3 ± 0.21 c | 61.7 ± 0.46 d | 1.06 ± 0.03 b | 22.2 ± 0.60 c | 17.8 ± 0.17 a | 7.4 ± 0.2 b |
| RS2 powder | 28.3 ± 0.42 f | 98.9 ± 4.5 g | 2.8 ± 0.04 e | 18.1 ± 0.98 b | 16.2 ± 0.66 a | 14.7 ± 0.17 c |
| RS2 Retention/% | 197.90 | 160.45 | 254.55 | 81.53 | 91.01 | 198.65 |
| RS3 juice | 55.8 ± 0.32 i | 56.4 ± 0.75 c | 1.25 ± 0.04 c | 67.9 ± 2.4 fg | 28.2 ± 0.29 a | 18.5 ± 0.82 d |
| RS3 powder | 51.7 ± 0.38 h | 180.5 ± 3.6 j | 3.6 ± 0.16 f | 29.5 ± 0.51 d | 30.2 ± 2.4 a | 18.6 ± 0.80 d |
| RS3 Retention/% | 92.65 | 320.04 | 276.92 | 43.45 | 106.74 | 100.54 |
| ARI | BCompound | CCode | Samples | Concentration (µg/L) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 | A2 | A3 | RS1 | RS2 | RS3 | ||||||||||
| Mean | STD% | Mean | STD% | Mean | STD% | Mean | Std% | Mean | Std% | Mean | Std% | ||||
| Terpenoids | |||||||||||||||
| 1018 | *α-Pinene | Ter1 | Juice | 257.7 f | 9.1 | 4648.1 de | 3.4 | 11,477.3 c | 16.5 | 218.4 f | 5.7 | 688.1 f | 10.4 | 20,751.9 a | 8.6 |
| Powder | 118.6 f | 9.6 | 3624.4 e | 9.9 | 15,933.8 b | 1.5 | 112.2 f | 9.7 | 6613.1 d | 1.2 | 23,330.0 a | 12.8 | |||
| 1116 | β-Pinene | Ter2 | Juice | 122.0 f | 9.5 | 2166.2 e | 3.2 | 15,454.9 b | 3.7 | N.D. | N/A | 350.6 f | 6.5 | 19,847.5 a | 9.4 |
| Powder | DN.D. | EN/A | 1951.6 ef | 12.4 | 14,497.5 c | 8.7 | 9.8 f | 9.8 | 4694.2 d | 10.3 | 13,876.9 c | 11.8 | |||
| 1160 | *β-Myrcene | Ter3 | Juice | 721.9 de | 2.4 | 8959.4 c | 9.5 | 36,170.8 a | 8.1 | 87.1 e | 8.1 | 1498.8 de | 4.1 | 31,926.8 b | 10.8 |
| Powder | 573.4 de | 6.5 | 1743.2 de | 4.2 | 10,819.7 c | 5.9 | 118.8 e | 6.5 | 2684.5 d | 4.0 | 7685.4 c | 10.8 | |||
| 1180 | *d-Limonene | Ter4 | Juice | 11617.3 d | 19.4 | 107,098.5 cd | 3.1 | 587,867.0 b | 6.8 | 12,860.3 d | 2.8 | 27,705.9 d | 2.6 | 699,261.6 a | 9.4 |
| Powder | 11128.2 d | 1.0 | 113,068.2 cd | 8.3 | 635,341.3 ab | 7.7 | 21,746.7 d | 6.6 | 264,961.4 cd | 10.9 | 672,178.4 a | 0.7 | |||
| 1213 | *γ-Terpinene | Ter5 | Juice | 961.6 de | 0.5 | 6578.5 de | 7.4 | 155,084.6 a | 10.6 | 60.1 d | 7.0 | 3695.6 de | 11 | 67,276.4 c | 8.8 |
| Powder | N.D. | N/A | 536.4 de | 8.7 | 73,418.7 c | 7.9 | N.D. | N/A | 122.4 d | 13.2 | 117,129.2 b | 11.2 | |||
| 1296 | p-Mentha-1,5,8-triene | Ter6 | Juice | 0.8 e | 17.2 | 14.0 d | 18.2 | 25.2 cd | 16.9 | N.D. | N/A | 30.3 c | 7.8 | 24.6 cd | 6.9 |
| Powder | N.D. | N/A | 16.7 d | 7.4 | 53.6 b | 2.1 | N.D. | N/A | 29.6 cd | 0.9 | 364.1 a | 6.0 | |||
| 1451 | α-Cubebene | Ter7 | Juice | 117.5 d | 2.4 | 290.6 c | 2.9 | 672.8 b | 8.5 | 116.0 d | 0.1 | 127.2 d | 0.8 | 812.8 a | 9.0 |
| Powder | 115.6 d | 2.4 | 265.5 c | 2.6 | 810.1 a | 8.9 | 119.4 d | 6.9 | 266.7 c | 18.2 | 644.8 b | 7.7 | |||
| 1537 | *Linalool | Ter8 | Juice | 65.4 f | 14.9 | 908.7 d | 2.5 | 8106.1 a | 2.4 | 73.4 f | 12 | 781.2 de | 2.2 | 8530.5 a | 4.4 |
| Powder | N.D. | N/A | 258.1 ef | 4 | 3319.0 c | 6.3 | 10.5 f | 2.2 | 820.7 de | 6.7 | 5774.0 b | 18.5 | |||
| 1585 | cis-β-Elemene | Ter9 | Juice | 119.3 f | 2.1 | 133.1 ef | 6.7 | 256.3 e | 8.7 | 123.2 f | 1.9 | 152.1 ef | 1.7 | 228.6 ef | 5.9 |
| Powder | 116.1 f | 0.4 | 414.1 d | 4.3 | 1603.4 b | 3.1 | 127.8 f | 11 | 639.0 c | 5.9 | 2011.2 a | 6.7 | |||
| 1624 | Terpinen-4-ol | Ter10 | Juice | 30.7 f | 12.9 | 161.2 d | 10.4 | 313.3 b | 5.1 | 38.1 ef | 3.3 | 170.7 d | 9.3 | 345.7 b | 9.8 |
| Powder | 6.1 f | 3.4 | 77.8 e | 11.6 | 289.2 c | 2.7 | 22.9 f | 7 | 220.5 d | 3.2 | 414.4 a | 8.3 | |||
| 1652 | p-Menth-1-en-9-al | Ter11 | Juice | 6.7 e | 8.6 | 50.9 d | 19 | 164.7 b | 11 | 4.3 e | 4.5 | 21.6 e | 9.5 | 175.1 b | 11.9 |
| Powder | 3.9e | 13.8 | 46.8d | 13.7 | 206.4a | 2.2 | 2.2e | 2.0 | 100.3c | 9.8 | 225.0a | 12.7 | |||
| 1665 | *β-Famesene | Ter12 | Juice | 141.1c | 12.6 | 1282.4 b | 8.6 | 5029.2 a | 3.1 | 131.6 c | 1.8 | 164.6 c | 3.2 | 5662.4 a | 8.8 |
| Powder | 121.6 c | 0.3 | 1121.4 b | 10.2 | 5795.8 a | 9.9 | 177.9 c | 11.4 | 1467.0 b | 16.6 | 1962.4 b | 2.3 | |||
| 1700 | *α-Terpineol | Ter13 | Juice | 24.2 g | 17.6 | 148.9 e | 1.6 | 579.8 b | 3.1 | 53.6 fg | 5.9 | 261.2 d | 5.5 | 712.7 a | 10 |
| Powder | 13.2 g | 8.2 | 83.6 f | 9.8 | 376.0 c | 2.8 | 22.4 g | 1.4 | 205.2 e | 6.2 | 568.4 b | 3.9 | |||
| 1748 | *α-Farnesene | Ter14 | Juice | 96.8 de | 3.8 | 1698.0 c | 14 | 6332.1 a | 1.4 | 93.7 d | 1.7 | 167.8 de | 0.7 | 6142.2 b | 9.4 |
| Powder | 89.1 de | 1.8 | 1355.8 c | 11.7 | 7520.7 a | 5.5 | 129.7 d | 2.0 | 1918.3 c | 1.2 | 6859.0 a | 6.6 | |||
| 1754 | Geranyl acetate | Ter15 | Juice | 12.9 e | 19.6 | 220.4 de | 14 | 2135.0 b | 4.3 | 9.2 e | 10.5 | 27.3 e | 9.7 | 2456.8 a | 10.1 |
| Powder | 10.9 e | 1.0 | 194.9 de | 10.4 | 1322.0 c | 2.5 | 14.1 e | 10.4 | 435.1 d | 9.5 | 1743.0 bc | 5.4 | |||
| 1810 | *Nerol | Ter16 | Juice | 5.8 e | 8.5 | 16.8 e | 7.1 | 146.1 b | 2.7 | 4.1 e | 6.9 | 11.2 ef | 14.4 | 173.6 a | 6.6 |
| Powder | 3.6 e | 0.6 | 5.0 e | 9.2 | 56.4 d | 8.3 | 3.8 e | 5.3 | 10.3 e | 17.1 | 77.8 c | 12 | |||
| 1814 | cis-Carveol | Ter17 | Juice | 4.8 de | 0.8 | 21.5 c | 4.5 | 60.7 a | 6.7 | 2.5 de | 11.5 | 7.2 de | 12 | 59.0 a | 8.0 |
| Powder | 0.8 e | 2.1 | 11.3 d | 7.8 | 37.0 b | 5.4 | 1.4 de | 21.4 | 25.2 c | 7.7 | 49.6 a | 17.7 | |||
| 1855 | *Geraniol | Ter18 | Juice | 17.1 f | 15.1 | 30.9 de | 8.3 | 116.8 a | 4.9 | 20.7 ef | 2.6 | 27.6 de | 6.6 | 99.5 a | 5.2 |
| Powder | 10.7 f | 4.3 | 19.0 ef | 9.9 | 73.9 b | 7.8 | 21.1 ef | 5.8 | 40.4 cd | 16.7 | 44.1 c | 12.7 | |||
| 1903 | p-Menth-1-en-9-ol | Ter19 | Juice | N.D. | N/A | 4.0 d | 7.3 | 15.1 b | 18.5 | N.D. | N/A | 0.4 e | 16 | 11.8 b | 11.2 |
| Powder | 27.8 a | 0.3 | N.D. | N/A | 13.0 b | 4.2 | N.D. | N/A | N.D. | N/A | 9.3 c | 14.4 | |||
| 1921 | p-Mentha-1,8-dien-7-yl acetate | Ter20 | Juice | 9.2 bc | 15.5 | 23.7 bc | 13.4 | 31.3 bc | 0.4 | 7.5 bc | 13 | 22.0 bc | 11.7 | 22.6 bc | 1.0 |
| Powder | 12.0 bc | 6.8 | 24.0 bc | 1.8 | 71.2 b | 7.0 | 28.3 bc | 2.0 | 237.3 a | 7.0 | N.D. | N/A | |||
| 1992 | Nerolidol | Ter21 | Juice | 4.1 f | 6.2 | 11.7 d | 10.5 | 31.9 b | 14 | 3.5 f | 2.4 | 4.5 f | 15 | 35.4 a | 14.6 |
| Powder | 3.7 f | 1.1 | 8.8 ef | 10.4 | 35.6 ab | 6.2 | 3.5 f | 1.9 | 11.4 ef | 12.7 | 21.4 c | 12.3 | |||
| Total | Juice | 14,336.8 | 13,4467.4 | 830,071 | 13,907.2 | 35,915.9 | 864,557.6 | ||||||||
| Powder | 12,355.4 | 12,4826.5 | 771,594.3 | 22,672.3 | 285,502.6 | 854,968.4 | |||||||||
| Aldehydes | |||||||||||||||
| 1078 | *Hexanal | Ald1 | Juice | 7.3 d | 14.4 | 28.9 b | 4.3 | 55.6 a | 12.3 | 4.5 d | 8 | 7.9 d | 8.2 | 66.4 a | 10.5 |
| Powder | 16.1 c | 9.4 | 23.9 b | 0.8 | 21.9 bc | 12.9 | 30.2 b | 7.9 | 7.7 d | 12.3 | N.D. | N/A | |||
| 1392 | Nonanal | Ald2 | Juice | 11.5 ef | 7.8 | 20.0 de | 7.1 | 63.5 b | 15.9 | N.D. | N/A | 153.6 a | 6.2 | 29.3 d | 12.6 |
| Powder | 14.0 ef | 20.5 | 26.5 d | 28.3 | 53.7 c | 13.7 | 6.6 fg | 8.6 | 27.7 d | 9.4 | 25.2 d | 7.7 | |||
| 1474 | Decanal | Ald3 | Juice | 81.5 g | 11 | 894.6 de | 0.7 | 5019.0 b | 14.8 | 83.0 g | 9.9 | 864.7 de | 4.5 | 5356.7 b | 15.3 |
| Powder | 197.2 fg | 6.5 | 717.4 def | 8.4 | 3735.8 c | 14.4 | 185.4 fg | 4.9 | 1179.0 d | 4.3 | 9710.3 a | 7.2 | |||
| Total | Juice | 100.3 | 943.5 | 5138.1 | 87.5 | 1026.2 | 5452.3 | ||||||||
| Powder | 227.3 | 772 | 3811.5 | 222.3 | 1214.3 | 9735.5 | |||||||||
| Alcohols | |||||||||||||||
| 1460 | 1-Heptanol | Alc1 | Juice | 3.3 c | 8.7 | 3.9 c | 7.9 | 17.9 a | 17.4 | 2.4 c | 14.2 | 3.4 c | 20.5 | 19.8 a | 13.3 |
| Powder | 2.6 c | 2.9 | 2.9 c | 10.3 | 7.8 b | 9.5 | 3.4 c | 26.8 | 3.0 c | 22.6 | 4.5 c | 12.2 | |||
| 1522 | *1-Octanol | Alc2 | Juice | 20.5 fg | 4 | 64.3 e | 5.7 | 937.7 a | 4.1 | 10.2 g | 10.2 | 125.1 d | 23.6 | 460.9 b | 1.8 |
| Powder | 5.1 g | 7.1 | 16.0 fg | 15.3 | 144.1 d | 13.2 | 3.9 g | 11.2 | 43.2 ef | 8.9 | 314.8 c | 6.3 | |||
| Total | Juice | 23.8 | 68.2 | 955.6 | 12.6 | 128.5 | 480.7 | ||||||||
| Powder | 7.6 | 18.9 | 151.9 | 7.3 | 46.2 | 319.3 | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Ting, J.L.H.; Peng, Y.; Tangjaidee, P.; Zhu, Y.; Li, Q.; Shan, Y.; Quek, S.Y. Comparing Three Types of Mandarin Powders Prepared via Microfluidic-Jet Spray Drying: Physical Properties, Phenolic Retention and Volatile Profiling. Foods 2021, 10, 123. https://doi.org/10.3390/foods10010123
Chen X, Ting JLH, Peng Y, Tangjaidee P, Zhu Y, Li Q, Shan Y, Quek SY. Comparing Three Types of Mandarin Powders Prepared via Microfluidic-Jet Spray Drying: Physical Properties, Phenolic Retention and Volatile Profiling. Foods. 2021; 10(1):123. https://doi.org/10.3390/foods10010123
Chicago/Turabian StyleChen, Xiao, Joanna Le Hoong Ting, Yaoyao Peng, Pipat Tangjaidee, Yongchao Zhu, Qili Li, Yang Shan, and Siew Young Quek. 2021. "Comparing Three Types of Mandarin Powders Prepared via Microfluidic-Jet Spray Drying: Physical Properties, Phenolic Retention and Volatile Profiling" Foods 10, no. 1: 123. https://doi.org/10.3390/foods10010123
APA StyleChen, X., Ting, J. L. H., Peng, Y., Tangjaidee, P., Zhu, Y., Li, Q., Shan, Y., & Quek, S. Y. (2021). Comparing Three Types of Mandarin Powders Prepared via Microfluidic-Jet Spray Drying: Physical Properties, Phenolic Retention and Volatile Profiling. Foods, 10(1), 123. https://doi.org/10.3390/foods10010123







