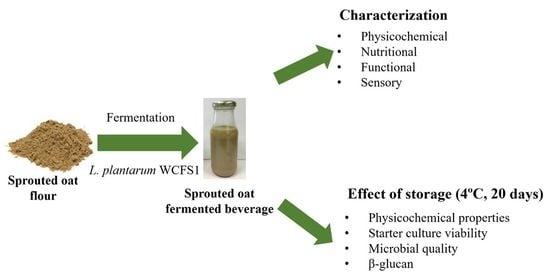
Production and Characterization of a Novel Gluten-Free Fermented Beverage Based on Sprouted Oat Flour
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical, Reagents and Standards
2.2. Oat Substrate
2.3. Starter Culture
2.4. Preparation of Sprouted Oat Fermented Beverage (SOFB)
2.5. pH and Titratable Acidity
2.6. Organic Acids
2.7. Water Holding Capacity
2.8. Microbiological Analysis
2.9. Total Protein, Thiamine, Riboflavin and β-glucan Content
2.10. Fatty Acids
2.11. Total Phenolic Compounds
2.12. Oxygen Radical Absorbance Capacity (ORAC)
2.13. Estimation of SOFB Stability during Refrigerated Storage
2.14. Sensory Evaluation
2.15. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characteristics of SOFB
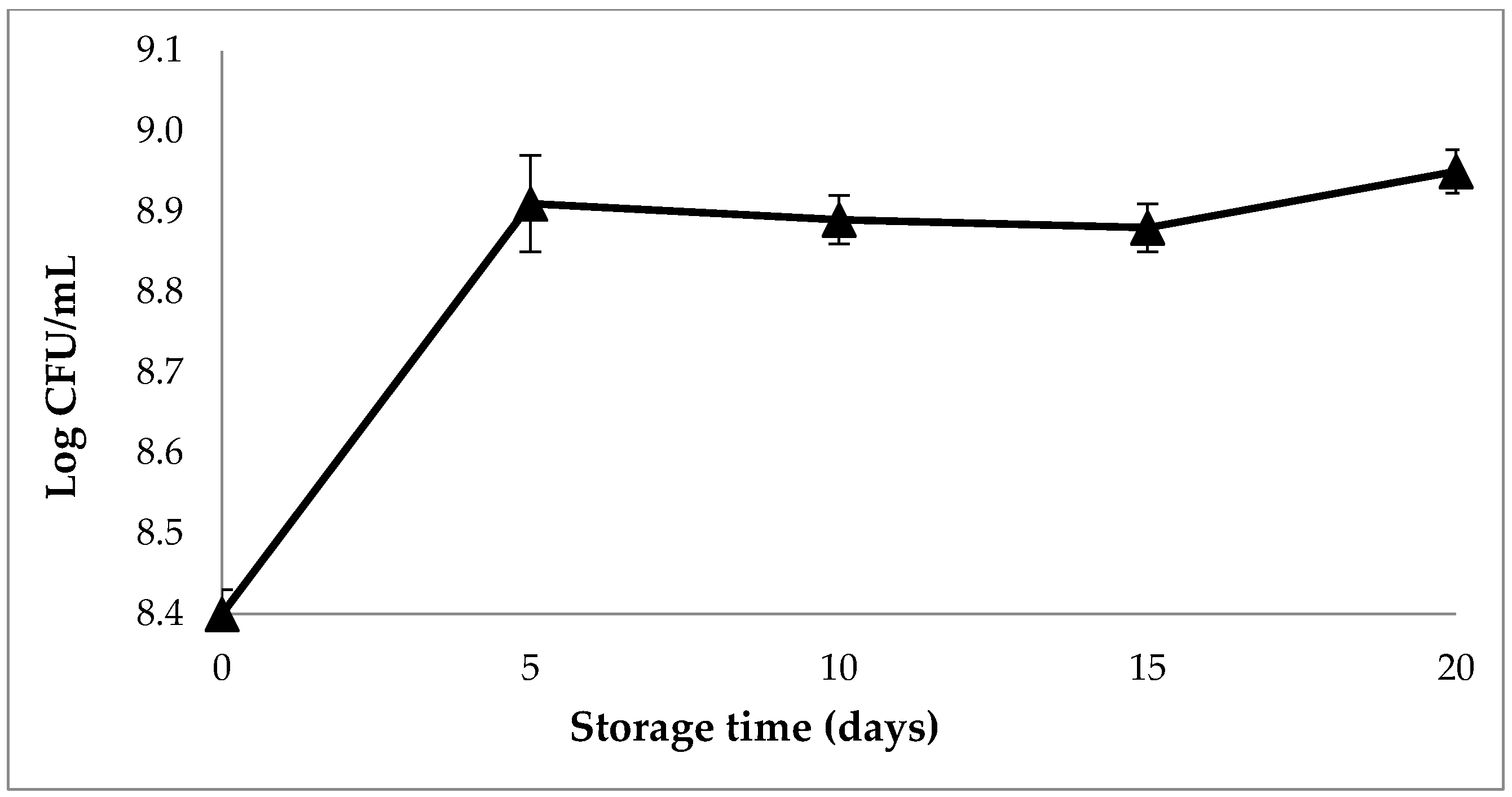
3.2. Viability of L. plantarum in SOFB
3.3. Microbiological Quality of SOFB
3.4. Nutritional Characteristics of SOFB
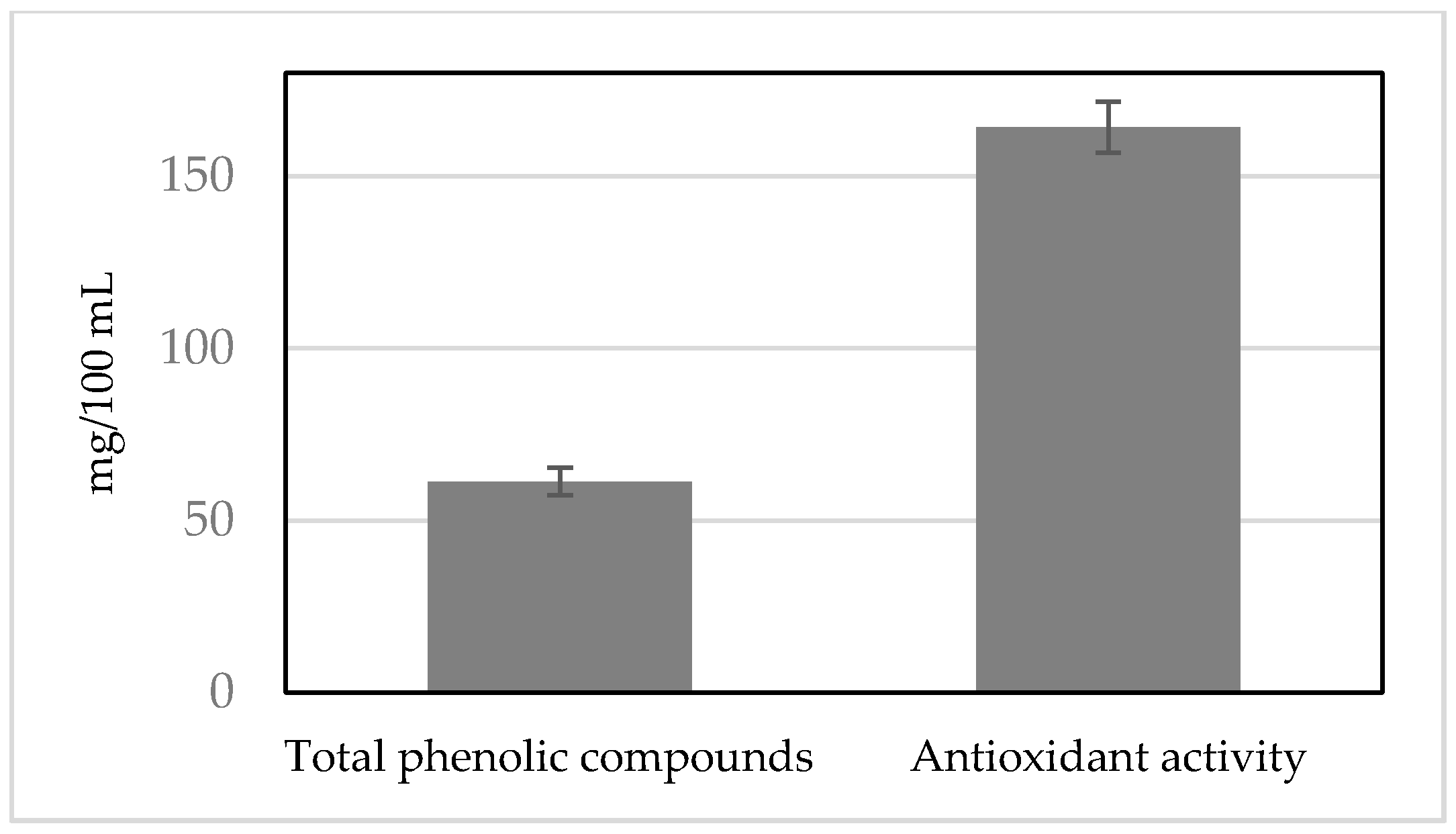
3.5. Content of Total Phenolic Compounds in SOFB
3.6. Oxygen Radical Antioxidant Activity (ORAC)
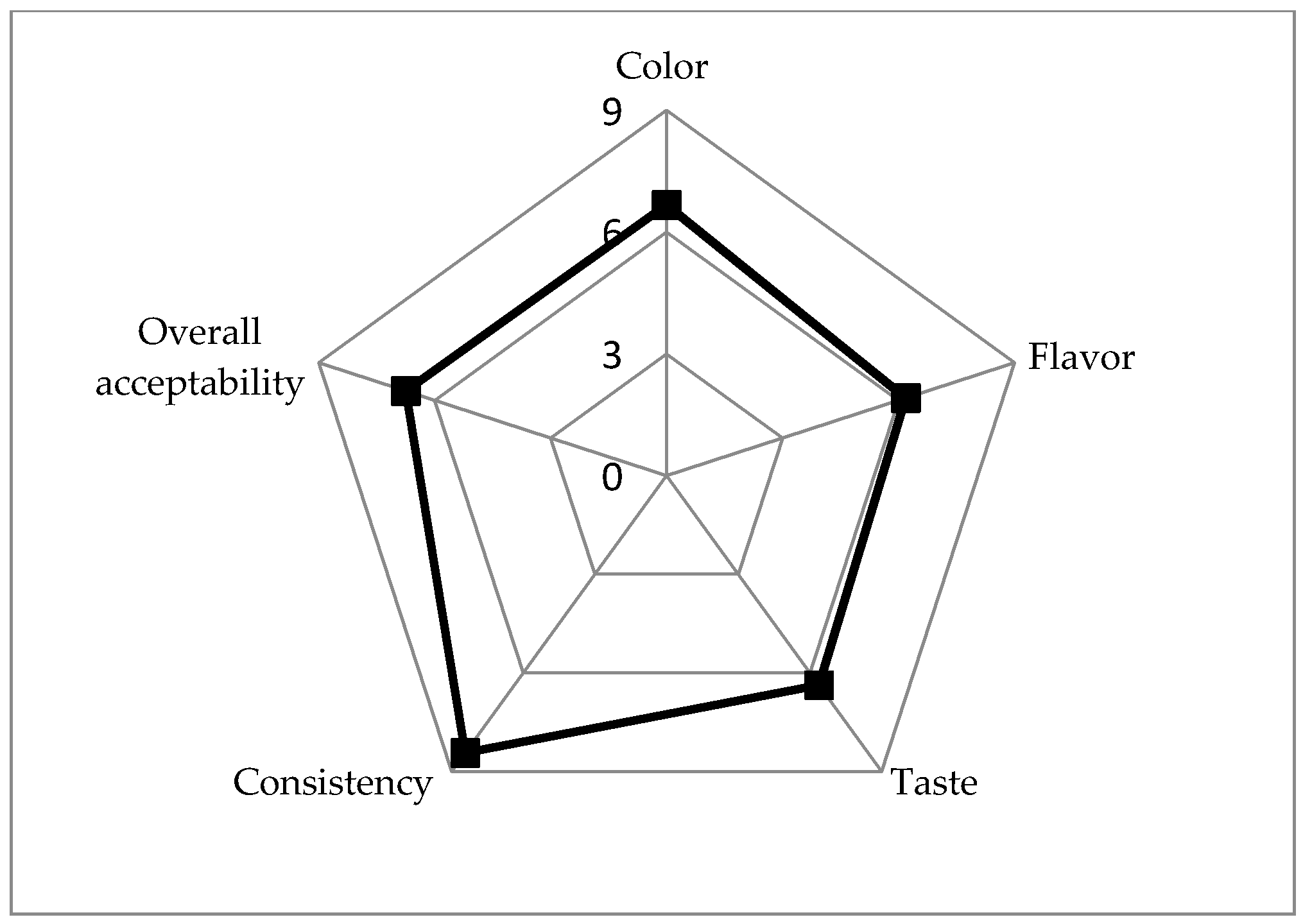
3.7. Acceptability of SOFB
3.8. Effect of Refrigerated Storage on SOFB Quality
3.8.1. Viability of L. plantarum Starter Culture during Refrigerated Storage
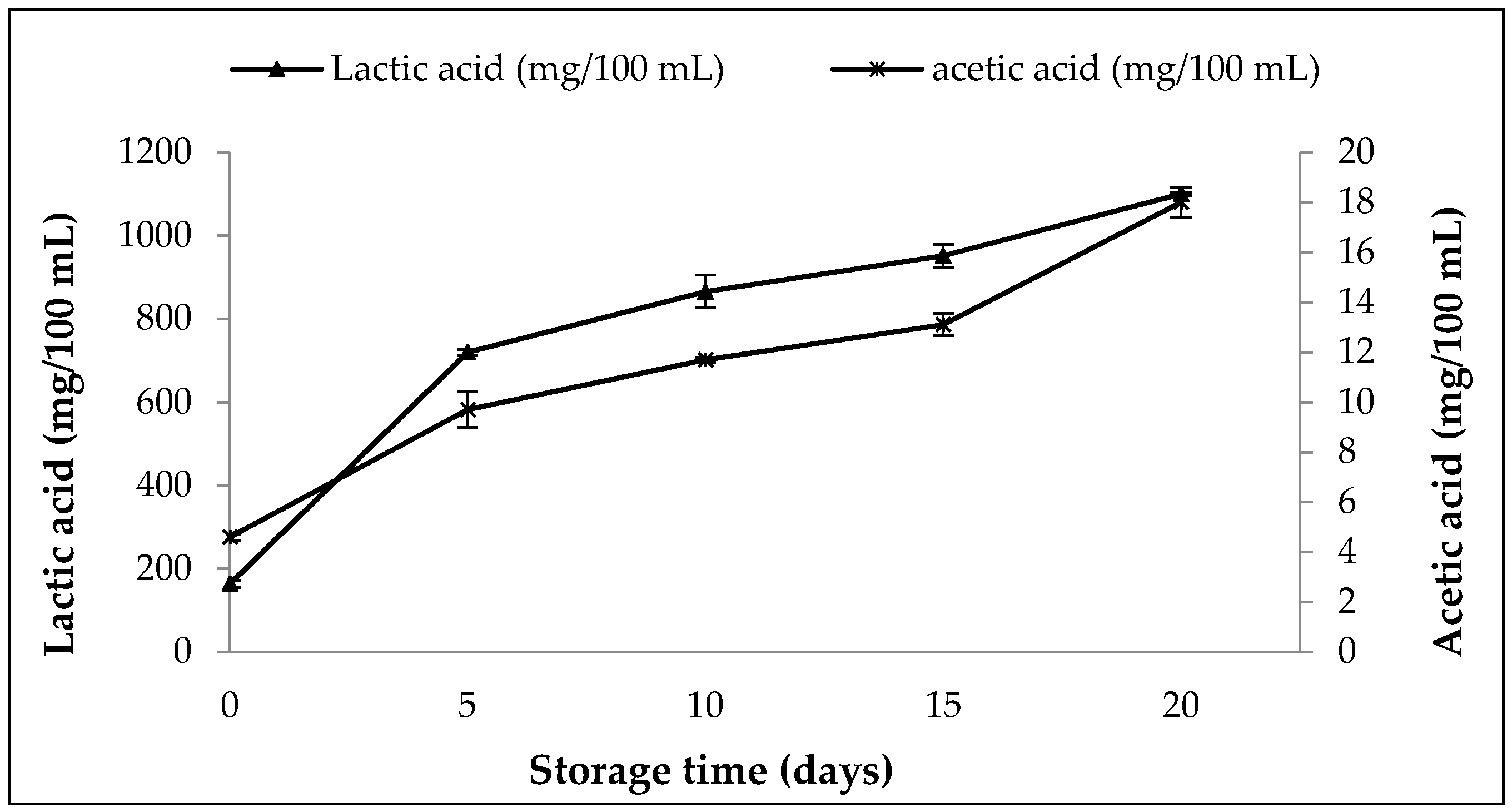
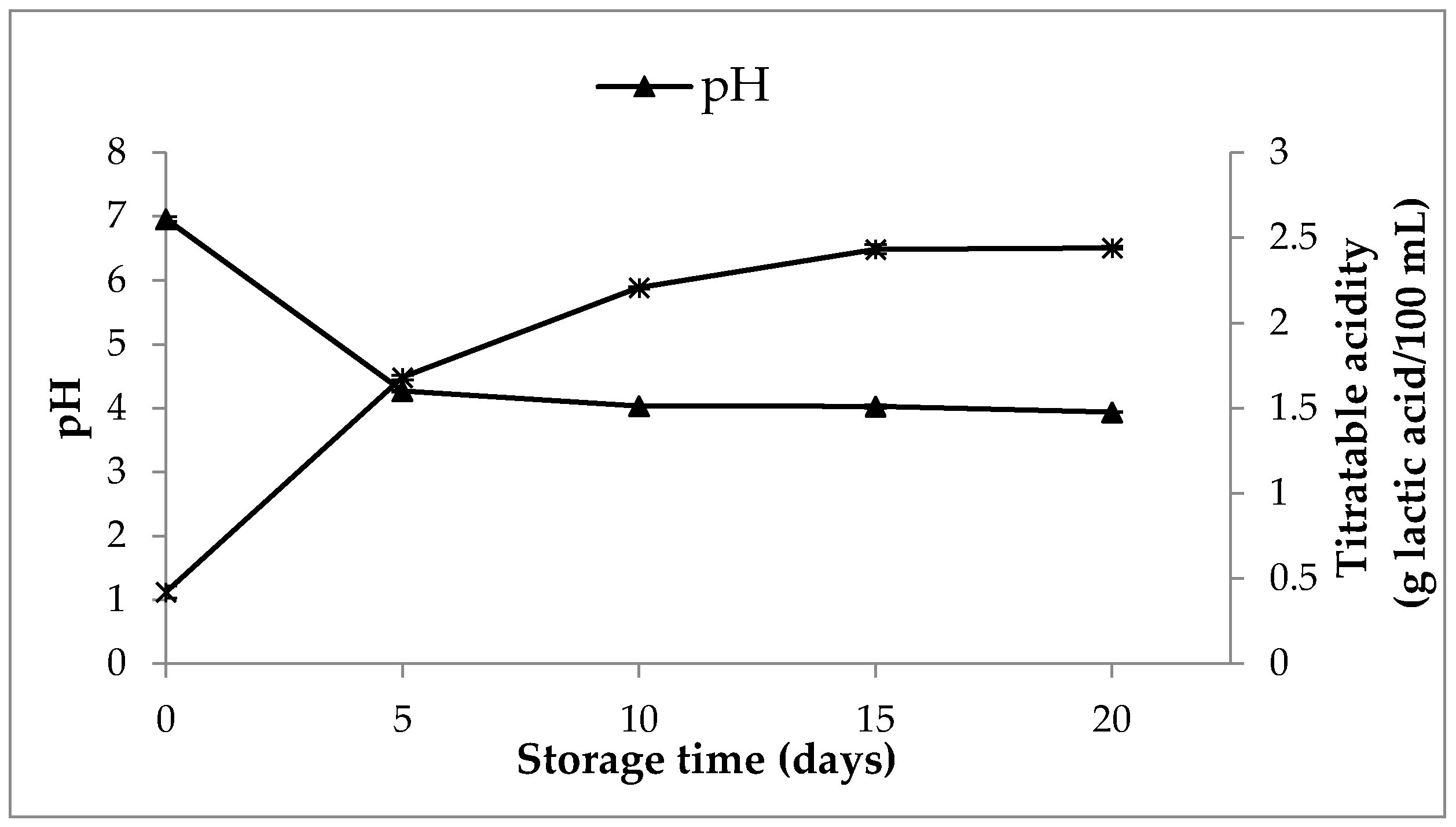
3.8.2. Changes in Content of Organic Acids, pH, Titratable Activity and WHC in SOFB during Refrigerated Storage
3.8.3. Changes in β-glucan Content of SOFB during Refrigerated Storage
3.8.4. Microbial Quality of SOFB during Refrigerated Storage
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ragudin, R.F.; Itodo, O.A.; Stoyanov, J.; Dejanovic, G.M.; Gamba, M.; Asllanaj, E.; Bussler, W.; Metzger, B.; Muka, T.; Glisic, M.; et al. A Systematic Review of Phytochemicals in Oat and Buckwheat. Food Chem. 2021, 338, 127982. [Google Scholar] [CrossRef]
- Li, X.; Cai, X.; Ma, X.; Jing, L.; Gu, J.; Bao, L.; Li, J.; Xu, M.; Zhang, Z.; Li, Y. Short- and Long-Term Effects of Wholegrain Oat Intake on Weight Management and Glucolipid Metabolism in Overweight Type-2-diabetics: A Randomized Control Trial. Nutrients 2016, 8, 549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, L.X.; Zhao, J.; Huang, Y.S.; Li, Y. The Difference between Oats and Beta-glucan Extract Intake in the Management of HbA1c Fasting Glucose and Insulin Sensitivity: A Meta-Analysis of Randomized Controlled Trials. Food Func. 2016, 7, 1413. [Google Scholar] [CrossRef] [PubMed]
- Fritz, R.D.; Chen, Y.; Contreras, V. Gluten-Containing Grains Skew Gluten Assessment in Oats Due to Sample Grind Non-Homogeneity. Food Chem. 2017, 216, 170–175. [Google Scholar] [CrossRef]
- Aydar, E.F.; Tutuncu, S.; Ozcelik, B. Plant-Based Milk Substitutes: Bioactive Compounds, Conventional and Novel Processes, Bioavailability Studies and Health Effects. J. Func. Foods 2020, 70, 103975. [Google Scholar] [CrossRef]
- Corbo, M.R.; Bevilacqua, A.; Petruzzi, L.; Casanova, F.P.; Sinigaglia, M. Functional Beverages: The Emerging Side of Functional Foods. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1192–1206. [Google Scholar] [CrossRef]
- Petrova, P.; Petrov, K. Lactic Acid Fermentation of Cereals and Pseudocereals: Ancient Nutritional Biotechnologies with Modern Applications. Nutrients 2020, 12, 1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cáceres, P.J.; Peñas, E.; Martínez-Villaluenga, C.; García-Mora, P.; Frias, J. Development of a Multifunctional Yogurt-Like Product from Germinated Brown Rice. LWT 2019, 99, 306–312. [Google Scholar] [CrossRef] [Green Version]
- Galli, V.; Mazzoli, L.; Luti, S.; Venturi, M.; Guerrini, S.; Paoli, P.; Vincenzini, M.; Granchi, L.; Pazzagli, L. Effect of Selected Strains of Lactobacilli on the Antioxidant and Anti-inflammatory Properties of Sourdough. Int. J. Food Microbiol. 2018, 286, 55–65. [Google Scholar] [CrossRef]
- Sidari, R.; Martorana, A.; Zappia, C.; Mincione, A.; Giuffrè, A.M. Persistence and Effect of a Multistrain Starter Culture on Antioxidant and Rheological Properties of Novel Wheat Sourdoughs and Bread. Foods 2020, 9, 1258. [Google Scholar] [CrossRef]
- Angelov, A.; Gotcheva, V.; Kuncheva, R.; Hristozova, T. Development of a New Oat-Based Probiotic Drink. Int. J. Food Microbiol. 2006, 112, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Chavan, M.; Gat, Y.; Harmalkar, M.; Waghmare, R. Development of non-dairy fermented probiotic drink based on germinated and ungerminated cereals and legumes. LWT 2018, 91, 339–344. [Google Scholar] [CrossRef]
- Gupta, M.; Bajaj, B.K. Development of fermented oat flour beverage as a potential probiotic vehicle. Food Biosci. 2017, 20, 107–109. [Google Scholar] [CrossRef]
- Nionelli, L.; Coda, R.; Curiel, J.A.; Kaisa, P.; Gobetti, M.; Rizzello, C.G. Manufacture and characterization of a yogurt-like beverage made with oat flakes fermented by selected lactic acid bacteria. Int. J. Food Microbiol. 2014, 185, 17–26. [Google Scholar] [CrossRef]
- Yépez, A.; Russo, P.; Spano, G.; Khomenko, I.; Biasioli, F.; Capozzi, V.; Aznar, R. In situ riboflavin fortification of different kefir-like cereal-based beverages using selected Andean LAB strains. Food Microbiol. 2019, 77, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Salmerón, I.; Thomas, K.; Pandiella, S.S. Effect of substrate composition and inoculum on the fermentation kinetics and flavor compound profiles of potentially non-dairy probiotic formulations. LWT 2014, 55, 240–247. [Google Scholar] [CrossRef]
- Wang, C.; Liang, S.; Wang, H.; Guo, M. Physicochemical properties and probiotic survivability of symbiotic oat-based beverage. Food Sci. Biotechnol. 2018, 27, 735–743. [Google Scholar] [CrossRef]
- Rico, D.; Peñas, E.; García, M.C.; Martínez-Villaluenga, C.; Rai, D.K.; Birsan, R.I.; Frias, J.; Martin-Diana, A.B. Sprouted Barley Flour as a Nutritious and Functional Ingredient. Foods 2020, 9, 296. [Google Scholar] [CrossRef] [Green Version]
- Tomé-Sánchez, I.; Martín-Diana, A.B.; Peñas, E.; Bautista-Expósito, S.; Frias, J.; Rico, D.; González-Maillo, L.; Martinez-Villaluenga, C. Soluble Phenolic Composition Tailored by Germination Conditions Accompany Antioxidant and Anti-Inflammatory Properties of Wheat. Antioxidants 2020, 9, 426. [Google Scholar] [CrossRef]
- Aparicio-García, N.; Martínez-Villaluenga, C.; Frias, J.; Peñas, E. Changes in Protein Profile, Bioactive Potential and Enzymatic Activities of Gluten-Free Flours Obtained from Hulled and Dehulled Oat Varieties as Affected by Germination Conditions. LWT 2020, 134, 109955. [Google Scholar] [CrossRef]
- Aparicio-García, N.; Martínez-Villaluenga, C.; Frias, J.; Peñas, E. Sprouted oat as a potential gluten-free ingredient with enhanced nutritional and bioactive properties. Food Chem. 2021, 338, 127972. [Google Scholar] [CrossRef] [PubMed]
- De Vries, M.C.; Vaughan, E.E.; Kleerebezem, M.; de Vos, W.M. Lactobacillus plantarum-Survival, Functional and Potential Probiotic Properties in the Human Intestinal Tract. Int. Dairy J. 2006, 16, 1018–1028. [Google Scholar] [CrossRef]
- Mäkinen, O.E.; Zannini, E.; Arendt, E.K. Germination of oat and quinoa and evaluation of the malts as gluten free baking ingredients. Plant Food Hum. Nutr. 2013, 68, 90–95. [Google Scholar] [CrossRef]
- ISO 4833-1:2013. Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 1: Colony Count at 30 °C by the Pour Plate Technique. Available online: https://www.iso.org/standard/53728.html (accessed on 2 March 2020).
- ISO 21528-2:2017. Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Enterobacteriaceae—Part 2: Colony-Count Technique. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma?c=N0059874 (accessed on 2 March 2020).
- ISO 16649-1:2018. Microbiology of the Food Chain—Horizontal Method for the Enumeration of Beta-Glucuronidase-Positive Escherichia coli—Part 1: Colony-Count Technique at 44 Degrees C Using Membranes and 5-bromo-4-chloro-3-indolyl Beta-D-Glucuronide. Available online: https://www.iso.org/standard/64951.html (accessed on 2 March 2020).
- NF V08-059:2002. Microbiologie des Aliments—Dénombrement des Levures et Moisissures par Comptage des Colonies à 25° C—Méthode de Routine. Available online: https://www.boutique.afnor.org/norme/nf-v08-059/microbiologie-des-aliments-denombrement-des-levures-et-moisissures-par-comptage-des-colonies-a-25-c-methode-de-routine/article/767939/fa120539 (accessed on 2 March 2020).
- ISO 7932:2004. Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Presumptive Bacillus cereus—Colony-Count Technique at 30 Degrees C. Available online: https://www.iso.org/standard/38219.html (accessed on 2 March 2020).
- ISO 6579-1:2017. Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella—Part 1: Detection of Salmonella spp. Available online: https://www.aenor.com/normas-y-libros/buscador-de-normas/une/?c=N0058760 (accessed on 2 March 2020).
- ISO 11290-1:2017. Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Listeria monocytogenes and of Listeria spp.—Part 1: Detection Method. Available online: https://www.aenor.com/normas-y-libros/buscador-de-normas/une?c=N0059546 (accessed on 2 March 2020).
- Canaviri Paz, P.; Janny, R.J.; Håkansson, A. Safeguarding of quinoa beverage production by fermentation with Lactobacillus plantarum DSM 9843. Int. J. Food Microbiol. 2020, 324, 108630. [Google Scholar] [CrossRef] [PubMed]
- Rathore, S.; Salmerón, I.; Pandiella, S.S. Production of Potentially Probiotic Beverages using Single and Mixed Cereal Substrates Fermented with Lactic Acid Bacteria Cultures. Food Microbiol. 2012, 30, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Petersson, K.; Nordlund, E.; Tornberg, E.; Eliasson, A.C.; Buchert, J. Impact of cell wall-degrading enzymes on water-holding capacity and solubility of dietary fibre in rye and wheat bran. J. Sci. Food Agric. 2013, 93, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Ludena Urquizo, F.E.; García Torres, S.M.; Tolonen, T.; Jaakkola, M.; Pena-Niebuhr, M.G.; von Wright, A.; Repo-Carrasco-Valencia, R.; Korhonen, H.; Plumed-Ferrer, C. Development of a fermented quinoa-based beverage. Food Sci. Nutr. 2017, 5, 602–608. [Google Scholar] [CrossRef] [Green Version]
- Bautista-Expósito, S.; Martínez-Villaluenga, C.; Dueñas, M.; Silván, J.M.; Frias, J.; Peñas, E. Combination of pH-controlled fermentation in mild acidic conditions and enzymatic hydrolysis of Savinase to improve metabolic health-promoting properties of lentil. J. Func. Foods 2018, 48, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Coda, R.; Lanera, A.; Trani, A.; Gobbettti, M.; Di Cagno, R. Yogurt-Like Beverages Made of a Mixture of Cereals, Soy and Grape Must: Microbiology, Texture, Nutritional and Sensory Properties. Int. J. Food Microbiol. 2012, 155, 120–127. [Google Scholar] [CrossRef]
- Kleerebezem, M.; Boekhorst, J.; van Kranenburg, R.; Molenaar, D.; Kuipers, O.P.; Leer, R.; Tarchini, R.; Peters, S.A.; Sandbrink, H.M.; Fiers, M.W.E.; et al. Complete Genome Sequence of Lactobacillus plantarum WCFS1. Proc. Natl. Acad. Sci. USA 2003, 100, 1990–1995. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, H.; Curiel, J.A.; Landete, J.M.; de las Rivas, B.; de Felipe, F.L.; Gómez-Cordovés, C.; Mancheño, J.M.; Muñoz, R. Food Phenolics and Lactic Acid Bacteria. Int. J. Food Microbiol. 2009, 132, 79–90. [Google Scholar] [CrossRef] [Green Version]
- Siezen, R.J.; Tzeneva, V.A.; Castioni, A.; Wels, M.; Phan, H.T.K.; Rademaker, J.L.W.; Starrenburg, M.J.C.; Kleerebezem, M.; Molenaar, D.; van Hylckama Vlieg, J.E.T. Phenotypic and genomic diversity of Lactobacillus plantarum strains isolated from various environmental niches. Environ. Microbiol. 2010, 12, 758–773. [Google Scholar] [CrossRef]
- Bochnak-Niedźwiecka, J.; Świeca, M. Quality of New functional Powdered Beverages Enriched with Lyophilized Fruits-Potentially Bioaccessible Antioxidant Properties, Nutritional Value, and Consumer Analysis. Appl. Sci. 2020, 10, 3668. [Google Scholar] [CrossRef]
- Kumar, A.; Kaur, A.; Tomer, V. Process Optimization for the Development of a Symbiotic Beverage Based on Lactic Acid Fermentation of Nutricereals and Milk-Based Beverage. LWT 2020, 131, 109774. [Google Scholar] [CrossRef]
- Muthuramalingam, K.; Singh, V.; Choi, S.I.; Kim, Y.M.; Unno, T. Dietary intervention using (1,3)/(1,6)-β-glucan, a fungus-derived soluble prebiotic ameliorates high-fat diet-induced metabolic distress and alters beneficially the gut microbiota in mice model. Eur. J. Nutr. 2020, 59, 2617–2629. [Google Scholar] [CrossRef] [PubMed]
- Yingying, Z.; Lianger, D.; Lu, H.; Zhenxing, S.; Julin, D.; Yang, Y.; Ruiling, S. Effects of Oat β-glucan, Oat Resistant Starch, and the Whole Oat Flour on Insulin Resistance, Inflammation, and Gut Microbiota in High-Fat-Diet-Induced Type 2 Diabetic Rats. J. Func. Foods 2020, 69, 103939. [Google Scholar] [CrossRef]
- De Arcangelis, E.; Djurle, S.; Andersson, A.A.M.; Marconi, E.; Messia, M.C.; Andersson, R. Structure analysis of β-glucan in barley and effects of wheat β-glucanase. J. Cereal Sci. 2019, 85, 175–181. [Google Scholar] [CrossRef]
- Ziarno, M.; Zaręba, D.; Maciejak, M.; Veber, A.L. The Impact of Dairy Starter Cultures on Selected Qualitative Properties of Functional Fermented Beverage Prepared from Germinated White Kidney Beans. J. Food Nutr. Res. 2019, 58, 167–176. [Google Scholar]
- Russo, P.; de Chiara, M.L.V.; Capozzi, V.; Arena, M.P.; Amodio, M.L.; Rascón, A.; Dueñas, M.T.; López, P.; Spano, G. Lactobacillus plantarum strains for multifunctional oat-based foods. LWT 2016, 68, 288–294. [Google Scholar] [CrossRef] [Green Version]
- Burgess, C.M.; Smid, E.J.; Rutten, G.; van Sinderen, D. A general method for selection of riboflavin-overproducing food grade micro-organisms. Microb. Cell Fact. 2006, 5, 24. [Google Scholar] [CrossRef] [Green Version]
- Giuffrè, A.M.; Tellah, S.; Capocasale, M. Seed Oil from Ten Algerian Peanut Landraces for Edible Use and Biodiesel Production. J. Oleo Sci. 2016, 65, 9–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FAO. Fats and Fatty Acids in Human Nutrition: Report of an Expert Consultation; FAO Food and Nutrition Paper No. 91; FAO: Rome, Italy, 2010. [Google Scholar]
- Šertović, E.; Saric, Z.; Barać, M.; Barukčić, I.; Kostić, A.; Božanic, R. Physical, Chemical, Microbiological and Sensory Characteristics of a Probiotic Beverage Produced from Different Mixtures of Cow’s Milk and Soy Beverage by Lactobacillus acidophilus La5 and Yoghurt culture. Food Technol. Biotechnol. 2019, 57, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Pasqualone, A.; Summo, C.; Laddomada, B.; Mudura, E.; Coldea, T.E. Effect of Processing Variables on the Physico-chemical characteristics and aroma of Borş, a Tradicional Beverage Derived from Wheat Bran. Food Chem. 2018, 265, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Fernandes de Araújo, F.; de Paulo Farias, D.; Neri-Numa, I.A.; Pastore, G.M. Polyphenols and Their Applications: An Approach in Food Chemistry and Innovation Potential. Food Chem. 2021, 338, 127535. [Google Scholar] [CrossRef]
- Valero-Cases, E.; Cerdá-Bernad, D.; Pastor, J.J.; Frutos, M.J. Non-Dairy Fermented Beverages as Potential Carriers to Ensure Probiotics, Prebiotics, and Bioactive Compounds Arrival to the Gut and Their Health Benefits. Nutrients 2020, 12, 1666. [Google Scholar] [CrossRef]
- Voss, G.B.; Monteiro, M.J.P.; Jauregi, P.; Valente, L.M.P.; Pintado, M.E. Functional Characterisation and Sensory Evaluation of a Novel Synbiotic Okara Beverage. Food Chem. 2021, 340, 127793. [Google Scholar] [CrossRef]
- Kwaw, E.; Ma, Y.; Tchabo, W.; Apaliya, M.T.; Wu, M.; Sackle Sackey, A.; Tahir, H.W. Effect of Lactobacillus Strains on Phenolic Profile, Color Attributes and Antioxidant Activities of Lactic-Acid-Fermented Mulberry Juice. Food Chem. 2018, 250, 148–154. [Google Scholar] [CrossRef]
- Makinen, O.E.; Uniacke-Lowe, T.; O’mahony, J.A.; Arendt, E.K. Physicochemical and acid gelation properties of commercial UHT-treated plant based milk substitutes and lactose free bovine milk. Food Chem. 2015, 68, 630–638. [Google Scholar] [CrossRef]
- Pineli, L.L.O.; Botelho, R.B.A.; Zandonadi, R.P.; Solorzano, J.L.; de Oliveira, G.T.; Reis, C.E.G.; Teixeira, D.S. Low Glycemic Index and Increased Protein Content in a Novel Quinoa Milk. LWT 2015, 63, 1261–1267. [Google Scholar] [CrossRef]
- Rincon, L.; Botelho, R.B.A.; de Alencar, E.R. Development of Novel Plant-Based Milk Based on Chickpea and Coconut. LWT 2020, 128, 109479. [Google Scholar] [CrossRef]
- Grasso, N.; Alonso-Miravalles, L.; O’Mahony, J.A. Composition, Physicochemical and Sensorial Properties of Commercial Plant-Based Yogurts. Foods 2020, 9, 252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amanda, E.; Choo, W.S. Effect of Refrigerated Storage on the Physicochemical Characteristics and Viability of Lactobacillis Plantarum in Fermented Watermelon Juice with or Without Supplementation with Inulin or Fructooligosaccharide. Food Process. Preserv. 2018, e13831. [Google Scholar] [CrossRef]
- Mesquita, M.C.; Dos Santos Leandro, E.; de Alencar, E.R.; Botlho, R.B.A. Fermentation of Chickpea (Cicer arietinum L.) and coconut (Coccus nucifera L.) Beverages by Lactobacillus paracasei subsp. Paracasei LBC 81: The Influence of Sugar Content on Growth and Stability during Storage. LWT 2020, 132, 109834. [Google Scholar] [CrossRef]
- Mantzourani, I.; Terpeou, A.; Bekatorou, A.; Mallouchos, A.; Alexopoulos, A.; Kimbaris, A.; Bezirtzoglu, E.; Koutinas, A.A. Functional Pomegrade Beverage Production by Fermentation with a novel Symbiotic L. paracasei Biocatalyst. Food Chem. 2020, 308, 125658. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO. Probiotics in Food. Health and Nutritional Properties and Guidelines Evaluation; FAO Food and Nutrition Paper 85; FAO: Rome, Italy, 2006. [Google Scholar]
- Tripathi, M.K.; Giri, S.K. Probiotic Functional Foods: Survival of Probiotics during Processing and Storage. J. Func. Foods 2014, 9, 225–241. [Google Scholar] [CrossRef]
- Ranadheera, S.; Vidanarachchi, J.; Rocha, R.S.; Cruz, A.G.; Ajlouni, S. Probiotic Delivery through Fermentation: Dairy vs. Non-Dairy Beverages. Fermentation 2017, 3, 67. [Google Scholar] [CrossRef] [Green Version]
- Bancalari, E.; Castellone, V.; Bottari, B.; Gatti, M. Wild Lactobacillus casei Group Strains: Potentiality to Ferment Plant Derived Juices. Foods 2020, 9, 314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Oliveira Vogado, C.; Dos Santos Leandro, E.; Zandonadi, R.P.; de Alencar, E.R.; Ginani, V.C.; Nakano, E.Y.; Habú, S.; Aguiar, P.A. Enrichment of Probiotic Fermetned Milk with Green Banana Pulp: Characterization Microbiological, Physicochemical and Sensory. Nutrients 2018, 10, 427. [Google Scholar] [CrossRef] [Green Version]
- Mårtensson, O.; Öste, R.; Holst, O. The Effect of Yoghurt Culture on the Survival of Probiotic Bacteria in Oat-based, non-dairy products. Food Res. Int. 2002, 35, 775–784. [Google Scholar] [CrossRef]
| SOFB | |
|---|---|
| pH | 6.96 ± 0.04 |
| Titratable acidity (g lactic acid/100 mL) | 0.42 ± 0.01 |
| Lactic acid (mg/L) | 1634.4 ± 9.1 |
| Acetic acid (mg/L) | 89.54 ± 0.23 |
| WHC (%) | 34.93 ± 0.27 |
| SOFB | |
|---|---|
| L. plantarum WCFS1 | 8.85 ± 0.004 |
| Aerobic mesophilic bacteria | 8.82 ± 0.005 |
| Enterobacteriaceae | <1 |
| Total coliforms | <1 |
| Molds and yeasts | <1 |
| Escherichia coli | <1 |
| Bacillus cereus | <1 |
| Salmonella spp. | ND * |
| Listeria monocytogenes | ND * |
| SOFB | |
|---|---|
| Protein (g/100 mL) | 1.67 ± 0.009 |
| β-glucan (mg/100 mL) | 79.0 ± 6.0 |
| Thiamine (μg/100 mL) | 676.0 ± 4.0 |
| Riboflavin (μg/100 mL) | 28.12 ± 1.04 |
| Fatty Acids | SOFB |
|---|---|
| 14:0 (Myristic) | 0.34 ± 0.009 |
| 16:0 (Palmitic) | 19.18 ± 0.03 |
| 16:1n7 (Palmitoleic) | 0.36 ± 0.005 |
| 18:0 (Stearic) | 1.64 ± 0.006 |
| 18:1n7c (Asclepic) | 1.01 ± 0.003 |
| 18:1n9c (Oleic) | 32.10 ± 0.03 |
| 18:2n6c (Linoleic) | 42.56 ± 0.03 |
| 18:3n3 (α-Linolenic) | 1.58 ± 0.01 |
| 20:0 (Arachidic) | 0.15 ± 0.01 |
| 20:1n9 (cis-11-Eicosenoic) | 0.75 ± 0.003 |
| 20:5n3 (EPA) | 0.16 ± 0.01 |
| 22:0 (Behenic) | 0.16 ± 0.008 |
| SFA | 21.47 ± 0.04 |
| MUFA | 34.22 ± 0.02 |
| PUFA | 44.30 ± 0.03 |
| Storage Days | |||||
|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 20 | |
| WHC | 34.9 ± 0.3 a | 35.3 ± 0.1 a | 33.7 ± 1.0 a | 34.3 ± 0.6 a | 34.5 ± 0.5 a |
| β-glucan | 79.0 ± 6.0 a | 74.0 ± 1.0 a | 71.0 ± 2.0 a | 71.0 ± 1.0 a | 72.0 ± 3.0 a |
| Aerobic mesophilic bacteria | 8.82 ± 0.01 a | 8.91 ± 0.04 b | 9.04 ± 0.01 c | 8.93 ± 0.07 c | 9.02 ± 0.03 c |
| Enterobacteriaceae | <1 | <1 | <1 | <1 | <1 |
| Total coliforms | <1 | <1 | <1 | <1 | <1 |
| Molds and yeasts | <1 | <1 | <1 | <1 | <1 |
| Escherichia coli | <1 | <1 | <1 | <1 | <1 |
| Bacillus cereus | <1 | <1 | <1 | <1 | <1 |
| Salmonella spp.* | ND | ND | ND | ND | ND |
| Listeria monocytogenes * | ND | ND | ND | ND | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aparicio-García, N.; Martínez-Villaluenga, C.; Frias, J.; Peñas, E. Production and Characterization of a Novel Gluten-Free Fermented Beverage Based on Sprouted Oat Flour. Foods 2021, 10, 139. https://doi.org/10.3390/foods10010139
Aparicio-García N, Martínez-Villaluenga C, Frias J, Peñas E. Production and Characterization of a Novel Gluten-Free Fermented Beverage Based on Sprouted Oat Flour. Foods. 2021; 10(1):139. https://doi.org/10.3390/foods10010139
Chicago/Turabian StyleAparicio-García, Natalia, Cristina Martínez-Villaluenga, Juana Frias, and Elena Peñas. 2021. "Production and Characterization of a Novel Gluten-Free Fermented Beverage Based on Sprouted Oat Flour" Foods 10, no. 1: 139. https://doi.org/10.3390/foods10010139
APA StyleAparicio-García, N., Martínez-Villaluenga, C., Frias, J., & Peñas, E. (2021). Production and Characterization of a Novel Gluten-Free Fermented Beverage Based on Sprouted Oat Flour. Foods, 10(1), 139. https://doi.org/10.3390/foods10010139