Antioxidant Potential of Pine Needles: A Systematic Study on the Essential Oils and Extracts of 46 Species of the Genus Pinus †
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Plant Material
2.3. Isolation of Essential Oils
2.4. Preparation of Extracts
2.5. Extraction and Isolation of Secondary Metabolites from P. nigra subsp. nigra
2.6. Evaluation of Antioxidant Activity Using the Peroxy-Oxalate Chemiluminescence Assay
2.7. Evaluation of Antioxidant Activity Using the Luminol Chemiluminescence Assay
2.8. Determination of Antioxidant Activity and Statistical Analysis
3. Results and Discussion
3.1. Evaluation of the Antioxidant Activity of Essential Oils
3.2. Evaluation of the Antioxidant Activity of Extracts
3.3. Phytochemical Analysis of P. nigra subsp. nigra and Evaluation of the Antioxidant Activity of the Isolated Metabolites
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Augustyniak, A.; Bartosz, G.; Čipak, A.; Duburs, G.; Horáková, L.U.; Łuczaj, W.; Majekova, M.; Odysseos, A.D.; Rackova, L.; Skrzydlewska, E.; et al. Natural and synthetic antioxidants: An updated overview. Free Radic. Res. 2010, 44, 1216–1262. [Google Scholar] [CrossRef] [PubMed]
- Jennings, B.H.; Akoh, C.C. Effectiveness of natural versus synthetic antioxidants in a rice bran oil-based structured lipid. Food Chem. 2009, 114, 1456–1461. [Google Scholar] [CrossRef]
- Caleja, C.; Barros, L.; Antonio, A.L.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. A comparative study between natural and synthetic antioxidants: Evaluation of their performance after incorporation into biscuits. Food Chem. 2017, 216, 342–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farjon, A. Pines: Drawings and Description of the Genus Pinus, 2nd ed.; Brill: Leiden, The Netherlands, 2005. [Google Scholar]
- Gernandt, D.S.; Geada López, G.; Ortiz García, S.; Liston, A. Phylogeny and classification of Pinus. Taxon 2005, 54, 29–42. [Google Scholar] [CrossRef] [Green Version]
- Packer, L.; Rimbach, G.; Virgili, F. Antioxidant activity and biologic properties of a procyanidin-rich extract from pine (Pinus maritima) bark, Pycnogenol. Free Radic. Biol. Med. 1999, 27, 704–724. [Google Scholar] [CrossRef]
- Ferreira-Santos, P.; Zanuso, E.; Genisheva, Z.; Rocha, C.M.R.; Teixeira, J.A. Green and sustainable valorization of bioactive phenolic compounds from Pinus by-products. Molecules 2020, 25, 2931. [Google Scholar] [CrossRef]
- Dridi, W.; Bordenave, N. Pine bark phenolic extracts, current uses, and potential food applications: A review. Curr. Pharm. Des. 2020, 26, 1866–1879. [Google Scholar] [CrossRef]
- Mármol, I.; Quero, J.; Jiménez-Moreno, N.; Rodríguez-Yoldi, M.J.; Ancín-Azpilicueta, C. A systematic review of the potential uses of pine bark in food industry and health care. Trends Food Sci. Technol. 2019, 88, 558–566. [Google Scholar] [CrossRef]
- El Omari, N.; Ezzahrae Guaouguaou, F.; El Menyiy, N.; Benali, T.; Aanniz, T.; Chamkhi, I.; Balahbib, A.; Taha, D.; Shariati, M.A.; Zengin, G.; et al. Phytochemical and biological activities of Pinus halepensis mill., and their ethnomedicinal use. J. Ethnopharmacol. 2020, 268, 113661. [Google Scholar] [CrossRef]
- Jeong, M.S.; Park, S.J.; Han, E.J.; Park, S.Y.; Kim, M.J.; Jung, K.; Cho, S.H.; Kim, S.Y.; Yoon, W.J.; Ahn, G.; et al. Pinus thunbergii PARL leaf protects against alcohol-induced liver disease by enhancing antioxidant defense mechanism in BALB/c mice. J. Funct. Foods 2020, 73, 104116. [Google Scholar] [CrossRef]
- Chiu, H.F.; Wang, H.M.; Shen, Y.C.; Venkatakrishnan, K.; Wang, C.K. Anti-inflammatory properties of fermented pine (Pinus morrisonicola Hay.) needle on lipopolysaccharide-induced inflammation in RAW 264.7 macrophage cells. J. Food Biochem. 2019, 43, e12994. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, H.G.; Lee, H.W.; Han, J.M.; Lee, S.K.; Kim, D.W.; Saravanakumar, A.; Son, C.G. Hippocampal memory enhancing activity of pine needle extract against scopolamine-induced amnesia in a mouse model. Sci. Rep. 2015, 5, 9651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Kim, H.; Choue, R.; Lim, H. Evaluation of the effects of Pinus koraiensis needle extracts on serum lipid and oxidative stress in adults with borderline dyslipidemia: A randomized, double-blind, and placebo-controlled clinical trial. Evid. Based Complement. Altern. Med. 2016, 2016, 9594251. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, Z.; Song, W.; Zhao, Z.; Zhao, Y. Isolation of proanthocyanidins from Pinus thunbergii needles and tyrosinase inhibition activity. Process Biochem. 2021, 100, 245–251. [Google Scholar] [CrossRef]
- EU Novel Food Catalogue. Available online: https://ec.europa.eu/food/safety/novel_food/catalogue_en (accessed on 4 January 2021).
- Sak, K.; Jürisoo, K.; Raal, A. Estonian folk traditional experiences on natural anticancer remedies: From past to the future. Pharm. Biol. 2014, 52, 855–866. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.-Y.; Chung, H.-J. Flavor compounds of pine sprout tea and pine needle tea. J. Agric. Food Chem. 2000, 48, 1269–1272. [Google Scholar] [CrossRef]
- How to Make Pine Needle Tea. WikiHow. Available online: www.wikihow.com/Make-Pine-Needle-Tea (accessed on 10 April 2020).
- Pine Needle Recipes. Pinterest. Available online: www.pinterest.ca/arnica1281/pine-needle-recipes/ (accessed on 10 April 2020).
- Ioannou, E.; Koutsaviti, A.; Tzakou, O.; Roussis, V. The genus Pinus: A comparative study on the needle essential oil composition of 46 pine species. Phytochem. Rev. 2014, 13, 741–768. [Google Scholar] [CrossRef]
- Arnous, A.; Petrakis, C.; Makris, D.; Kefalas, P. A peroxyoxalate chemiluminescence-based assay for the evaluation of hydrogen peroxide scavenging activity employing 9,10-diphenylanthracene as the fluorophore. J. Pharmacol. Toxicol. Methods 2002, 48, 171–177. [Google Scholar] [CrossRef]
- Parejo, I.; Petrakis, C.; Kefalas, P. A transition metal enhanced luminol chemiluminescence in the presence of a chelator. J. Pharmacol. Toxicol. Methods 2000, 43, 183–190. [Google Scholar] [CrossRef]
- Parejo, I.; Codina, C.; Petrakis, C.; Kefalas, P. Evaluation of scavenging activity assessed by CoII/EDTA- induced chemiluminescence and DPPH (2,2-diphenyl-1-picrylhydrazyl) free radical assay. J. Pharmacol. Toxicol. Methods 2000, 44, 507–512. [Google Scholar] [CrossRef]
- Ruberto, G.; Baratta, M.T. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem. 2000, 69, 167–174. [Google Scholar] [CrossRef]
- Dar, M.Y.; Shah, W.A.; Mubashir, S.; Rather, M.A. Chromatographic analysis, anti-proliferative and radical scavenging activity of Pinus wallichiana essential oil growing in high altitude areas of Kashmir, India. Phytomedicine 2012, 19, 1228–1233. [Google Scholar] [CrossRef]
- Chen, J.; Gao, Y.; Jin, Y.; Li, S.; Zhang, Y. Chemical composition, antibacterial and antioxidant activities of the essential oil from needles of Pinus parviflora Siebold & Zucc. J. Essent. Oil Bear. Plants 2015, 18, 1187–1196. [Google Scholar] [CrossRef]
- Djerrad, Z.; Djouahri, A.; Kadik, L. Variability of Pinus halepensis Mill. essential oils and their antioxidant activities depending on the stage of growth during vegetative cycle. Chem. Biodivers. 2017, 14, e1600340. [Google Scholar] [CrossRef]
- Djerrad, Z.; Kadik, L.; Djouahri, A. Chemical variability and antioxidant activities among Pinushalepensis Mill. essential oils provenances, depending on geographic variation and environmental conditions. Ind. Crops Prod. 2015, 74, 440–449. [Google Scholar] [CrossRef]
- Postu, P.A.; Sadiki, F.Z.; El Idrissi, M.; Cioanca, O.; Trifan, A.; Hancianu, M.; Hritcu, L. Pinus halepensis essential oil attenuates the toxic Alzheimer’s amyloid beta (1–42)-induced memory impairment and oxidative stress in the rat hippocampus. Biomed. Pharmacother. 2019, 112, 108673. [Google Scholar] [CrossRef]
- Grassmann, J.; Hippeli, S.; Vollmann, R.; Elstner, E.F. Antioxidative properties of the essential oil from Pinus mugo. J. Agric. Food Chem. 2003, 51, 7576–7582. [Google Scholar] [CrossRef]
- Kurti, F.; Giorgi, A.; Beretta, G.; Mustafa, B.; Gelmini, F.; Testa, C.; Angioletti, S.; Giupponi, L.; Zilio, L.E.; Pentimalli, D.; et al. Chemical composition, antioxidant and antimicrobial activities of essential oils of different Pinus species from Kosovo. J. Essent. Oil Res. 2019, 31, 263–275. [Google Scholar] [CrossRef]
- Apetrei, C.L.; Spac, A.; Brebu, M.; Tuchilus, C.; Miron, A. Composition, and antioxidant and antimicrobial activities of the essential oils of a full-grown Pinus cembra L. tree from the Calimani Mountains (Romania). J. Serb. Chem. Soc. 2013, 78, 27–37. [Google Scholar] [CrossRef]
- Park, J.-S.; Lee, G.-H. Volatile compounds and antimicrobial and antioxidant activities of the essential oils of the needles of Pinus densiflora and Pinus thunbergii. J. Sci. Food Agric. 2011, 91, 703–709. [Google Scholar] [CrossRef]
- Xie, Q.; Liu, Z.; Li, Z. Chemical composition and antioxidant activity of essential oil of six Pinus taxa native to China. Molecules 2015, 20, 9380–9392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yener, H.O.; Saygideger, S.D.; Sarikurkcu, C.; Yumrutas, O. Evaluation of antioxidant activities of essential oils and methanol extracts of Pinus species. J. Essent. Oil Bear. Plants 2014, 17, 295–302. [Google Scholar] [CrossRef]
- Ustun, O.; Senola, F.S.; Kurkcuoglu, M.; Orhan, I.E.; Kartal, M.; Baser, K.H.C. Investigation on chemical composition, anticholinesterase and antioxidant activities of extracts and essential oils of Turkish Pinus species and pycnogenol. Ind. Crops Prod. 2012, 38, 115–123. [Google Scholar] [CrossRef]
- Maric, S.; Jukic, M.; Katalinic, V.; Milos, M. Comparison of chemical composition and free radical scavenging ability of glycosidically bound and free volatiles from Bosnian Pine (Pinus heldreichii Christ. var. leucodermis). Molecules 2007, 12, 283–289. [Google Scholar] [CrossRef] [Green Version]
- Tümen, I.; Akkol, E.K.; Taştan, H.; Süntar, I.; Kurtca, M. Research on the antioxidant, wound healing, and anti-inflammatory activities and the phytochemical composition of maritime pine (Pinus pinaster Ait). J. Ethnopharmacol. 2018, 211, 235–246. [Google Scholar] [CrossRef]
- Sacchetti, G.; Maietti, S.; Muzzoli, M.; Scaglianti, M.; Manfredini, S.; Radice, M.; Bruni, R. Comparative evaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobials in foods. Food Chem. 2005, 91, 621–632. [Google Scholar] [CrossRef]
- Salem, M.Z.M.; Ali, H.M.; Basalah, M.O. Essential oils from wood, bark, and needles of Pinus roxburghii Sarg. from Alexandria, Egypt: Antibacterial and antioxidant activities. BioResources 2014, 9, 7454–7466. [Google Scholar] [CrossRef] [Green Version]
- Guri, A.; Kefalas, P.; Roussis, V. Antioxidant potential of six pine species. Phytother. Res. 2006, 20, 263–266. [Google Scholar] [CrossRef]
- Kang, Y.-H.; Howard, L.R. Phenolic composition and antioxidant activities of different solvent extracts from pine needles in Pinus species. Food Sci. Nutr. 2010, 15, 36–43. [Google Scholar] [CrossRef] [Green Version]
- Maimoona, A.; Naeem, I.; Shujaat, S.; Saddique, Z.; Mughal, T.; Mehmood, T. Comparison of radical scavenging capacity of different extracts of barks and needles of Pinus roxburghii and Pinus wallichiana. Asian J. Chem. 2011, 23, 819–822. [Google Scholar]
- Puri, A.; Srivastava, A.K.; Singhal, B.; Mishra, S.K.; Srivastava, S.; Lakshmi, V. Antidyslipidemic and antioxidant activity of Pinus roxburghii needles. Med. Chem. Res. 2011, 20, 1589–1593. [Google Scholar] [CrossRef]
- Jung, M.J.; Chung, H.Y.; Choi, J.H.; Choi, J.S. Antioxidant principles from the needles of red pine, Pinus densiflora. Phytother. Res. 2003, 17, 1064–1068. [Google Scholar] [CrossRef] [PubMed]
- Kwak, C.S.; Moon, S.C.; Lee, M.S. Antioxidant, antimutagenic, and antitumor effects of pine needles (Pinus densiflora). Nutr. Cancer 2006, 56, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, T.; Choi, Y.-W.; Kim, Y.-K. Effect of an extraction solvent on the antioxidant quality of Pinus densiflora needle extract. J. Pharm. Anal. 2019, 9, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Apetrei, C.L.; Tuchilus, C.; Aprotosoaie, A.C.; Oprea, A.; Malterud, K.E.; Miron, A. Chemical, antioxidant and antimicrobial investigations of Pinus cembra L. bark and needles. Molecules 2011, 16, 7773–7788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koutsaviti, A.; Ioannou, E.; Couladis, M.; Tzakou, O.; Roussis, V. 1H and 13C NMR spectral assignments of abietane diterpenes from Pinus heldreichii and Pinus nigra subsp. nigra. Magn. Reson. Chem. 2017, 55, 772–778. [Google Scholar] [CrossRef]
- Koutsaviti, A. Comparative Study of the Essential Oil Composition of the Foliage of 54 Pinus Taxa—Isolation and Structure Elucidation of Secondary Metabolites from the Species Pinus heldreichii Christ., Pinus pinea L. and Pinus nigra subsp. nigra Arn. Ph.D. Thesis, National and Kapodistrian University of Athens, Athens, Greece, October 2016. [Google Scholar]
- Abou-Zaid, M.; Dumas, M.; Chauret, D.; Watson, A.; Thompson, D. C-Methyl flavonols from the fungus Colletotrichum dematium f. sp. epilobii. Phytochemistry 1997, 45, 957–961. [Google Scholar] [CrossRef]
- Harborne, J.B.; Greenham, J.; Williams, C.A.; Eagles, J.; Markham, K.R. Ten isoprenylated and C-methylated flavonoids from the leaves of three Vellozia species. Phytochemistry 1993, 34, 219–226. [Google Scholar] [CrossRef]
- Mabry, T.J.; Markham, K.R.; Thomas, M.B. The Systematic Identification of Flavonoids; Springer: Berlin, Germany, 1970. [Google Scholar]
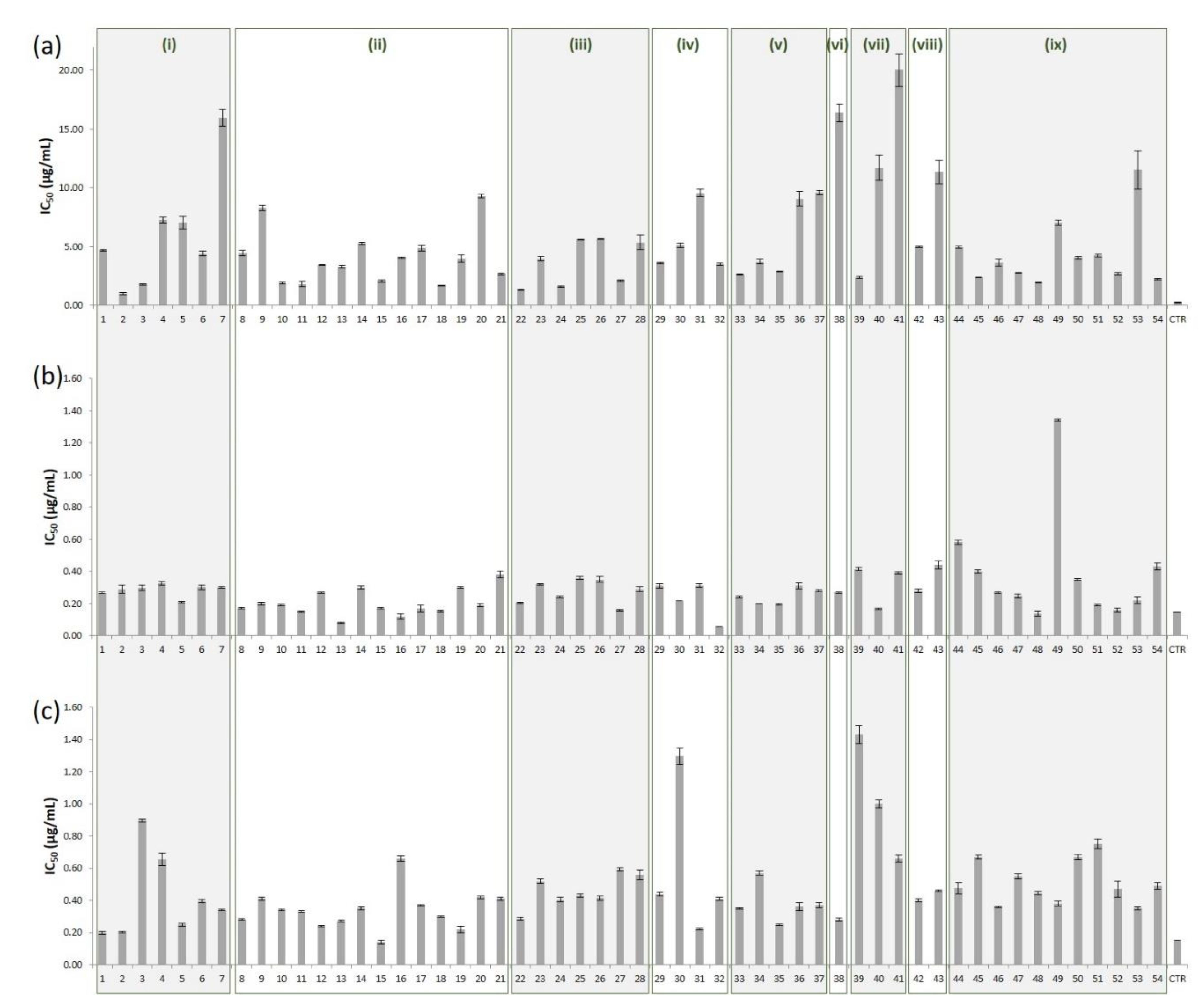

| Taxon | IC50 (μg/mL) | ||||||
|---|---|---|---|---|---|---|---|
| Essential Oil | Organic Extract | Hydroethanolic Extract | |||||
| Subgenus Pinus | |||||||
| Section Pinus | |||||||
| Subsection Pinaster | |||||||
| 1 | P. brutia | 4.67 ± 0.14 | 0.27 ± 0.01 | 0.20 ± 0.02 | |||
| 2 | P. canariensis | 1.00 ± 0.08 | 0.29 ± 0.05 | 0.20 ± 0.01 | |||
| 3 | P. halepensis | 1.78 ± 0.17 | 0.30 ± 0.03 | 0.90 ± 0.02 | |||
| 4 | P. heldreichii | 7.26 ± 0.54 | 0.33 ± 0.02 | 0.66 ± 0.08 | |||
| 5 | P. pinaster | 7.03±1.12 | 0.21 ± 0.01 | 0.25 ± 0.02 | |||
| 6 | P. pinea | 4.40 ± 0.37 | 0.30 ± 0.03 | 0.40 ± 0.02 | |||
| 7 | P. roxburghii | 15.96±1.45 | 0.30 ± 0.01 | 0.34 ± 0.01 | |||
| Subsection Pinus | |||||||
| 8 | P. densiflora | 4.45 ± 0.40 | 0.17 ± 0.01 | 0.28 ± 0.01 | |||
| 9 | P. massoniana | 8.29 ± 0.41 | 0.20 ± 0.02 | 0.41 ± 0.02 | |||
| 10 | P. mugo | 1.89 ± 0.16 | 0.19 ± 0.01 | 0.34 ± 0.01 | |||
| 11 | P. mugo var. prostrata | 1.79 ± 0.21 | 0.15 ± 0.01 | 0.33 ± 0.01 | |||
| 12 | P. mugo var. pumilio | 3.42 ± 0.06 | 0.27 ± 0.01 | 0.24 ± 0.01 | |||
| 13 | P. nigra subsp. caramanica | 3.28 ± 0.27 | 0.08 ± 0.01 | 0.27 ± 0.01 | |||
| 14 | P. nigra subsp. laricio | 5.25 ± 0.19 | 0.30 ± 0.02 | 0.35 ± 0.02 | |||
| 15 | P. nigra subsp. nigra | 2.05 ± 0.20 | 0.17 ± 0.01 | 0.14 ± 0.02 | |||
| 16 | P. nigra subsp. salzmannii | 4.05 ± 0.12 | 0.12 ± 0.03 | 0.66 ± 0.03 | |||
| 17 | P. sylvestris | 4.86 ± 0.48 | 0.17 ± 0.04 | 0.37 ± 0.01 | |||
| 18 | P. sylvestris subsp. scotica | 1.67 ± 0.05 | 0.15 ± 0.01 | 0.30 ± 0.01 | |||
| 19 | P. tabuliformis | 3.97 ± 0.62 | 0.30 ± 0.01 | 0.22 ± 0.04 | |||
| 20 | P. taiwanensis | 9.31 ± 0.29 | 0.19 ± 0.02 | 0.42 ± 0.02 | |||
| 21 | P. thunbergii | 2.68 ± 0.12 | 0.38 ± 0.04 | 0.41 ± 0.02 | |||
| Section Trifoliae | |||||||
| Subsection Australes | |||||||
| 22 | P. attenuata | 1.30 ± 0.02 | 0.20 ± 0.01 | 0.28 ± 0.02 | |||
| 23 | P. elliottii | 3.97 ± 0.35 | 0.32 ± 0.01 | 0.52 ± 0.03 | |||
| 24 | P. muricata | 1.60 ± 0.09 | 0.24 ± 0.01 | 0.40 ± 0.03 | |||
| 25 | P. patula | 5.63 ± 0.02 | 0.36 ± 0.02 | 0.43 ± 0.02 | |||
| 26 | P. radiata | 5.65 ± 0.10 | 0.35 ± 0.04 | 0.41 ± 0.03 | |||
| 27 | P. rigida | 2.09 ± 0.12 | 0.16 ± 0.01 | 0.59 ± 0.02 | |||
| 28 | P. teocote | 5.36±1.23 | 0.29 ± 0.03 | 0.56 ± 0.06 | |||
| Subsection Contortae | |||||||
| 29 | P. banksiana | 3.60 ± 0.14 | 0.31 ± 0.03 | 0.44 ± 0.02 | |||
| 30 | P. contorta var. contorta | 5.11 ± 0.40 | 0.22 ± 0.00 | 1.30 ± 0.10 | |||
| 31 | P. contorta var. latifolia | 9.57 ± 0.64 | 0.31 ± 0.02 | 0.22 ± 0.01 | |||
| 32 | P. contorta var. murrayana | 3.51 ± 0.16 | 0.06 ± 0.00 | 0.41 ± 0.02 | |||
| Subsection Ponderosae | |||||||
| 33 | P. coulteri | 2.64 ± 0.08 | 0.24 ± 0.01 | 0.35 ± 0.01 | |||
| 34 | P. jeffreyi | 3.72 ± 0.39 | 0.20 ± 0.00 | 0.57 ± 0.03 | |||
| 35 | P. ponderosa | 2.86 ± 0.09 | 0.19 ± 0.01 | 0.25 ± 0.01 | |||
| 36 | P. sabineana | 9.05±1.25 | 0.31 ± 0.04 | 0.36 ± 0.05 | |||
| 37 | P. torreyana | 9.58 ± 0.40 | 0.28 ± 0.01 | 0.37 ± 0.03 | |||
| Subgenus Strobus | |||||||
| Section Parrya | |||||||
| Subsection Balfourianae | |||||||
| 38 | P. aristata | 16.39±1.52 | 0.27 ± 0.01 | 0.28 ± 0.02 | |||
| Subsection Cembroides | |||||||
| 39 | P. cembroides | 2.38 ± 0.16 | 0.41 ± 0.02 | 1.43 ± 0.11 | |||
| 40 | P. culminicola | 11.71±2.17 | 0.17 ± 0.01 | 1.00 ± 0.05 | |||
| 41 | P. monophylla | 20.03±2.77 | 0.39 ± 0.01 | 0.66 ± 0.04 | |||
| Section Quinquefoliae | |||||||
| Subsection Gerardianae | |||||||
| 42 | P. bungeana | 4.99 ± 0.14 | 0.28 ± 0.02 | 0.40 ± 0.02 | |||
| 43 | P. gerardiana | 11.35±2.03 | 0.44 ± 0.05 | 0.46 ± 0.01 | |||
| Subsection Strobus | |||||||
| 44 | P. armandii | 4.95 ± 0.17 | 0.58 ± 0.03 | 0.48 ± 0.07 | |||
| 45 | P. cembra | 2.36 ± 0.05 | 0.40 ± 0.02 | 0.67 ± 0.02 | |||
| 46 | P. flexilis | 3.62 ± 0.57 | 0.27 ± 0.01 | 0.36 ± 0.01 | |||
| 47 | P. koraiensis | 2.73 ± 0.07 | 0.25 ± 0.02 | 0.55 ± 0.03 | |||
| 48 | P. monticola | 1.94 ± 0.09 | 0.14 ± 0.03 | 0.45 ± 0.02 | |||
| 49 | P. parviflora | 7.04 ± 0.44 | 1.34 ± 0.01 | 0.38 ± 0.03 | |||
| 50 | P. peuce | 4.04 ± 0.26 | 0.35 ± 0.01 | 0.67 ± 0.03 | |||
| 51 | P. pumila | 4.24 ± 0.27 | 0.19 ± 0.01 | 0.75 ± 0.06 | |||
| 52 | P. strobiformis | 2.68 ± 0.15 | 0.16 ± 0.02 | 0.47 ± 0.10 | |||
| 53 | P. strobus | 11.54±3.27 | 0.22 ± 0.04 | 0.35 ± 0.02 | |||
| 54 | P. wallichiana | 2.23 ± 0.12 | 0.43 ± 0.04 | 0.49 ± 0.04 | |||
| β-carotene | 0.23 ± 0.01 | ||||||
| quercetin | 0.15 ± 0.00 | ||||||
| Species | Extract | Assay | Activity | Reference |
|---|---|---|---|---|
| P. brutia | Fresh needles, CHCl3/MeOH (3:1) extract, organic phase F1 | LCL 1 | 165.8 ± 0.06 μg/mL 9 | [42] |
| Fresh needles, CHCl3/MeOH (3:1) extract, organic phase F2 | LCL 1 | 31.89 ± 0.02 μg/mL 9 | ||
| Dry needles, CHCl3/MeOH (3:1) extract, organic phase F1 | LCL 1 | 327.5 ± 0.08 μg/mL 9 | ||
| Dry needles, CHCl3/MeOH (3:1) extract, organic phase F2 | LCL 1 | 18.38 ± 0.06 μg/mL 9 | ||
| Dry needles, Me2CO extract | DPPH 2 | 10.36 ± 0.13–16.00 ± 0.26% (at 250–1000 μg/mL) 10 | [37] | |
| DMPD 3 | inactive (at 250–1000 μg/mL) 10 | |||
| PFRAP 4 | 0.316 ± 0.042–0.889 ± 0.011 (at 250–1000 μg/mL) 11 | |||
| Dry needles, EtOAc extract | DPPH 2 | 14.14 ± 0.45–28.27 ± 0.26% (at 250–1000 μg/mL) 10 | ||
| DMPD 3 | 2.15 ± 0.56–12.66 ± 2.14% (at 250–1000 μg/mL) 10 | |||
| PFRAP 4 | 0.311 ± 0.013–0.792 ± 0.033 (at 250–1000 μg/mL) 11 | |||
| Dry needles, EtOH extract | DPPH 2 | 13.41 ± 0.19–25.59 ± 0.19% (at 250–1000 μg/mL) 10 | ||
| DMPD 3 | inactive–7.72 ± 1.24% (at 250–1000 μg/mL) 10 | |||
| PFRAP 4 | 0.229 ± 0.042–0.630 ± 0.037 (at 250–1000 μg/mL) 11 | |||
| Dry needles, MeOH extract | DPPH 2 | 27.5 ± 0.4–85.0 ± 0.8% (at 0.2-1.0 mg/mL) 10 | [36] | |
| PFRAP 4 | 0.119 ± 0.009–0.438 ± 0.008 (at 0.2–0.8 mg/mL) 11 | |||
| FICA 5 | 21.5 ± 0.4% (at 1.0 mg/mL) 12 | |||
| P. halepensis | Fresh needles, CHCl3/MeOH (3:1) extract, organic phase F1 | LCL 1 | 175.0 ± 0.03 μg/mL 9 | [42] |
| Fresh needles, CHCl3/MeOH (3:1) extract, organic phase F2 | LCL 1 | inactive | ||
| Dry needles, Me2CO extract | DPPH 2 | 7.61 ± 0.20–31.18 ± 1.02% (at 250–1000 μg/mL) 10 | [37] | |
| DMPD 3 | 13.32 ± 0.98–17.66 ± 1.65% (at 250–1000 μg/mL) 10 | |||
| PFRAP 4 | 0.330 ± 0.008–0.941 ± 0.018 (at 250–1000 μg/mL) 11 | |||
| Dry needles, EtOAc extract | DPPH 2 | inactive–21.05 ± 0.71% (at 250–1000 μg/mL) 10 | ||
| DMPD 3 | 5.43 ± 1.44–9.96 ± 0.57% (at 250–1000 μg/mL) 10 | |||
| PFRAP 4 | 0.264 ± 0.012–0.849 ± 0.010 (at 250–1000 μg/mL) 11 | |||
| Dry needles, EtOH extract | DPPH 2 | 8.98 ± 0.79–18.39 ± 1.22% (at 250–1000 μg/mL) 10 | ||
| DMPD 3 | inactive (at 250–1000 μg/mL) 10 | |||
| PFRAP 4 | 0.412 ± 0.042–1.250 ± 0.022 (at 250–1000 μg/mL) 11 | |||
| Dry needles, MeOH extract | DPPH 2 | 43.1 ± 3.1–93.9 ± 0.1% (at 0.2-1.0 mg/mL) 10 | [36] | |
| PFRAP 4 | 0.236 ± 0.010–0.914 ± 0.008 (at 0.2–0.8 mg/mL) 11 | |||
| FICA 5 | 5.5 ± 0.8% (at 1.0 mg/mL) 12 | |||
| P. pinaster | Dry needles, Me2CO (80%) extract, filtrate | ORAC 6 | 478.8 ± 32.8 μM TE/g 13 | [43] |
| Dry needles, Me2CO (80%) extract, alkaline hydrolysis of the residue/EtOAc-soluble fraction | ORAC 6 | 128.0 ± 9.6 μM TE/g 13 | ||
| Dry needles, Me2CO (80%) extract, alkaline hydrolysis of the residue/H2O-soluble fraction | ORAC 6 | 60.2±7.1 μM TE/g 13 | ||
| Fresh needles, n-Hex extract | DPPH 2 | 203.28 μg/mL 9 | [39] | |
| ABTS/TEAC 7 | 170.92 μg/mL 9 | |||
| FRAP 8 | 16.28% (concentration not specified) 14 | |||
| hydroxyl radical scavenging | 158.26 μg/mL 9 | |||
| Fresh needles, Me2CO extract (sequentially) | DPPH 2 | 171.12 μg/mL 9 | ||
| ABTS/TEAC 7 | 163.45 μg/mL 9 | |||
| FRAP 8 | 19.74% (concentration not specified) 14 | |||
| hydroxyl radical scavenging | 192.35 μg/mL 9 | |||
| P. pinea | Fresh needles, CHCl3/MeOH (3:1) extract, organic phase F1 | LCL 1 | 161.8 ± 0.07 μg/mL 9 | [42] |
| Fresh needles, CHCl3/MeOH (3:1) extract, organic phase F2 | LCL 1 | 129.6 ± 0.04 μg/mL 9 | ||
| Dry needles, CHCl3/MeOH (3:1) extract, organic phase F1 | LCL 1 | 42.1 ± 0.01 μg/mL 9 | ||
| Dry needles, CHCl3/MeOH (3:1) extract, organic phase F2 | LCL 1 | 79.2 ± 0.03 μg/mL 9 | ||
| Dry needles, Me2CO (80%) extract, filtrate | ORAC 6 | 901.5 ± 35.2 μM TE/g 13 | [43] | |
| Dry needles, Me2CO (80%) extract, alkaline hydrolysis of the residue/EtOAc-soluble fraction | ORAC 6 | 70.9 ± 0.9 μM TE/g 13 | ||
| Dry needles, Me2CO (80%) extract, alkaline hydrolysis of the residue/H2O-soluble fraction | ORAC 6 | 39.7±5.5 μM TE/g 13 | ||
| Dry needles, MeOH extract | DPPH 2 | 27.9 ± 0.8–91.4 ± 0.5% (at 0.2-1.0 mg/mL) 10 | [36] | |
| PFRAP 4 | 0.154 ± 0.016–0.542 ± 0.031 (at 0.2–0.8 mg/mL) 11 | |||
| FICA 5 | 1.2 ± 0.4% (at 1.0 mg/mL) 12 | |||
| P. roxburghii | Dry needles, n-Hex fraction of MeOH extract | DPPH 2 | inactive | [44] |
| Dry needles, CH2Cl2 fraction of MeOH extract | DPPH 2 | 163.45 μg/mL 9 | ||
| Dry needles, EtOAc fraction of MeOH extract | DPPH 2 | 11.62 μg/mL 9 | ||
| Dry needles, n-BuOH fraction of MeOH extract | DPPH 2 | 3.283 μg/mL 9 | ||
| Dry needles, H2O fraction of MeOH extract | DPPH 2 | 120.0 μg/mL 9 | ||
| Dry needles, EtOH (95%) extract | ABTS/TEAC 7 | 0.57 mM (maximum TEAC content at 12.5 μg/mL) | [45] | |
| Dry needles, n-Hex fraction of EtOH (95%) extract | ABTS/TEAC 7 | inactive | ||
| Dry needles, CHCl3 fraction of EtOH (95%) extract | ABTS/TEAC 7 | 0.14 mM (maximum TEAC content at 12.5 μg/mL) | ||
| Dry needles, n-BuOH fraction of EtOH (95%) extract | ABTS/TEAC 7 | 0.38 mM (maximum TEAC content at 12.5 μg/mL) | ||
| Dry needles, n-BuOH-insoluble fraction of EtOH (95%) extract | ABTS/TEAC 7 | 0.57 mM (maximum TEAC content at 12.5 μg/mL) | ||
| P. densiflora | Dry needles, MeOH extract | DPPH 2 | 32.5 μg/mL 9 | [46] |
| nitrite radical scavenging | 80.38 ± 1.44% (at 10 μg/mL) 10 | |||
| hydroxyl radical scavenging | −29.79 ± 5.18% (at 40 μg/mL) 10 | |||
| reactive oxygen species (ROS) scavenging | −392.80 ± 21.3% (at 40 μg/mL) 10 | |||
| Dry needles, CH2Cl2 fraction of MeOH extract | DPPH 2 | 45.4 μg/mL 9 | ||
| nitrite radical scavenging | 21.36 ± 1.04% (at 10 μg/mL) 10 | |||
| hydroxyl radical scavenging | −357.45 ± 10.4% (at 40 μg/mL) 10 | |||
| reactive oxygen species (ROS) scavenging | −907.36 ± 50.0% (at 40 μg/mL) 10 | |||
| Dry needles, EtOAc fraction of MeOH extract | DPPH 2 | 13.2 μg/mL 9 | ||
| nitrite radical scavenging | 95.60 ± 0.09% (at 10 μg/mL) 10 | |||
| hydroxyl radical scavenging | 82.13 ± 5.31% (at 40 μg/mL) 10 | |||
| reactive oxygen species (ROS) scavenging | 59.15 ± 3.4% (at 40 μg/mL) 10 | |||
| Dry needles, n-BuOH fraction of MeOH extract | DPPH 2 | 24.3 μg/mL 9 | ||
| nitrite radical scavenging | 82.28 ± 1.89% (at 10 μg/mL) 10 | |||
| hydroxyl radical scavenging | 61.70 ± 4.42% (at 40 μg/mL) 10 | |||
| reactive oxygen species (ROS) scavenging | 50.55 ± 3.7% (at 40 μg/mL) 10 | |||
| Dry needles, H2O fraction of MeOH extract | DPPH 2 | 25.1 μg/mL 9 | ||
| nitrite radical scavenging | 69.02 ± 1.29% (at 10 μg/mL) 10 | |||
| hydroxyl radical scavenging | 27.66 ± 0.43% (at 40 μg/mL) 10 | |||
| reactive oxygen species (ROS) scavenging | 40.38 ±3.20% (at 40 μg/mL) 10 | |||
| Dry needles, Me2CO (80%) extract, filtrate | ORAC 6 | 466.1±27.3 μM TE/g 13 | [43] | |
| Dry needles, Me2CO (80%) extract, alkaline hydrolysis of the residue/EtOAc-soluble fraction | ORAC 6 | 61.4±3.7 μM TE/g 13 | ||
| Dry needles, Me2CO (80%) extract, alkaline hydrolysis of the residue/H2O-soluble fraction | ORAC 6 | 55.3±2.8 μM TE/g 13 | ||
| Dry needles, EtOH (95%) extract | inhibition of lipid peroxidation | 53.48 μg/mL 9 | [47] | |
| DPPH 2 | 95.12 μg/mL 9 | |||
| Dry needles, H2O extract | DPPH 2 | 176.37±29.84 μg/mL 9 | [48] | |
| ABTS/TEAC 7 | 14.90 ± 0.37 μg/mL 9 | |||
| Dry needles, EtOH (20%) extract | DPPH 2 | 83.70 ± 6.22 μg/mL 9 | ||
| ABTS/TEAC 7 | 9.02 ± 0.55 μg/mL 9 | |||
| Dry needles, EtOH (40%) extract | DPPH 2 | 75.96 ± 11.60 μg/mL 9 | ||
| ABTS/TEAC 7 | 8.56 ± 0.51 μg/mL 9 | |||
| Dry needles, EtOH (60%) extract | DPPH 2 | 78.46 ± 7.99 μg/mL 9 | ||
| ABTS/TEAC 7 | 9.12 ± 0.43 μg/mL 9 | |||
| Dry needles, EtOH (80%) extract | DPPH 2 | 126.47 ± 4.38 μg/mL 9 | ||
| ABTS/TEAC 7 | 11.80 ± 0.08 μg/mL 9 | |||
| Dry needles, EtOH (100%) extract | DPPH 2 | 373.70 ± 60.67 μg/mL 9 | ||
| ABTS/TEAC 7 | 19.76 ± 1.32 μg/mL 9 | |||
| P. nigra | Fresh needles, CHCl3/MeOH (3:1) extract, organic phase F1 | LCL 1 | inactive | [42] |
| Fresh needles, CHCl3/MeOH (3:1) extract, organic phase F2 | LCL 1 | 174.6 ± 0.15 μg/mL 9 | ||
| Dry needles, Me2CO extract | DPPH 2 | 10.14 ± 0.58–17.14 ± 1.09% (at 250–1000 μg/mL) 10 | [37] | |
| DMPD 3 | inactive (at 250–1000 μg/mL) 10 | |||
| PFRAP 4 | 0.273 ± 0.022–0.893 ± 0.078 (at 250–1000 μg/mL) 11 | |||
| Dry needles, EtOAc extract | DPPH 2 | 12.91 ± 0.26–24.36 ± 1.80% (at 250–1000 μg/mL) 10 | ||
| DMPD 3 | inactive (at 250–1000 μg/mL) 10 | |||
| PFRAP 4 | 0.346 ± 0.001–0.969 ± 0.041 (at 250–1000 μg/mL) 11 | |||
| Dry needles, EtOH extract | DPPH 2 | 14.41 ± 1.09–28.36 ± 0.77% (at 250–1000 μg/mL) 10 | ||
| DMPD 3 | inactive (at 250–1000 μg/mL) 10 | |||
| PFRAP 4 | 0.360 ± 0.024–0.965 ± 0.029 (at 250–1000 μg/mL) 11 | |||
| Dry needles, MeOH extract | DPPH 2 | 34.0 ± 2.1–92.5 ± 0.4% (at 0.2-1.0 mg/mL) 10 | [36] | |
| PFRAP 4 | 0.163 ± 0.002–0.586 ± 0.008 (at 0.2–0.8 mg/mL) 11 | |||
| FICA 5 | 21.3±2.1% (at 1.0 mg/mL) 12 | |||
| P. sylvestris | Dry needles, Me2CO (80%) extract, filtrate | ORAC 6 | 560.0±36.3 μM TE/g 13 | [43] |
| Dry needles, Me2CO (80%) extract, alkaline hydrolysis of the residue/EtOAc-soluble fraction | ORAC 6 | 91.7±3.2 μM TE/g 13 | ||
| Dry needles, Me2CO (80%) extract, alkaline hydrolysis of the residue/H2O-soluble fraction | ORAC 6 | 59.3±4.0 μM TE/g 13 | ||
| Dry needles, Me2CO extract | DPPH 2 | 15.77 ± 1.74–31.41 ± 0.84% (at 250–1000 μg/mL) 10 | [37] | |
| DMPD 3 | inactive–4.22 ± 0.11 (at 250–1000 μg/mL) 10 | |||
| PFRAP 4 | 0.327 ± 0.048–1.015 ± 0.066 (at 250–1000 μg/mL) 11 | |||
| Dry needles, EtOAc extract | DPPH 2 | 8.32 ± 0.19–13.55 ± 0.01% (at 250–1000 μg/mL) 10 | ||
| DMPD 3 | inactive (at 250–1000 μg/mL) 10 | |||
| PFRAP 4 | 0.230 ± 0.013–0.627 ± 0.011 (at 250–1000 μg/mL) 11 | |||
| Dry needles, EtOH extract | DPPH 2 | 22.64±1.41–45.86±1.35% (at 250–1000 μg/mL) 10 | ||
| DMPD 3 | 3.03 ± 0.45–14.57 ± 1.91% (at 250–1000 μg/mL) 10 | |||
| PFRAP 4 | 0.515 ± 0.005–1.343 ± 0.013 (at 250–1000 μg/mL) 11 | |||
| P. attenuata | Fresh needles, CHCl3/MeOH (3:1) extract, organic phase F1 | LCL 1 | inactive | [42] |
| Fresh needles, CHCl3/MeOH (3:1) extract, organic phase F2 | LCL 1 | 144.1 ± 0.01 μg/mL 9 | ||
| P. radiata | Fresh needles, CHCl3/MeOH (3:1) extract, organic phase F1 | LCL 1 | 228.1 ± 0.02 μg/mL 9 | [42] |
| Fresh needles, CHCl3/MeOH (3:1) extract, organic phase F2 | LCL 1 | inactive | ||
| P. cembra | Dry needles, MeOH (80%) extract | DPPH 2 | 186.1 ± 1.7 μg/mL 9 | [49] |
| ABTS/TEAC 7 | 24.0 ± 0.2 μg/mL 9 | |||
| PFRAP 4 | 104 ± 2 μg/mL 9 | |||
| FICA 5 | 1755 ± 22 μg/mL 9 | |||
| P. koraiensis | Dry needles, Me2CO (80%) extract, filtrate | ORAC 6 | 402.0±7.5 μM TE/g 13 | [43] |
| Dry needles, Me2CO (80%) extract, alkaline hydrolysis of the residue/EtOAc-soluble fraction | ORAC 6 | 111.6±6.2 μM TE/g 13 | ||
| Dry needles, Me2CO (80%) extract, alkaline hydrolysis of the residue/H2O-soluble fraction | ORAC 6 | 32.0±4.5 μM TE/g 13 | ||
| P. strobus | Dry needles, Me2CO (80%) extract, filtrate | ORAC 6 | 1223.3±12.6 μM TE/g 13 | [43] |
| Dry needles, Me2CO (80%) extract, alkaline hydrolysis of the residue/EtOAc-soluble fraction | ORAC 6 | 82.3±3.1 μM TE/g 13 | ||
| Dry needles, Me2CO (80%) extract, alkaline hydrolysis of the residue/H2O-soluble fraction | ORAC 6 | 81.3±2.4 μM TE/g 13 | ||
| P. wallichiana | Dry needles, n-Hex fraction of MeOH extract | DPPH 2 | inactive | [44] |
| Dry needles, CH2Cl2 fraction of MeOH extract | DPPH 2 | inactive | ||
| Dry needles, EtOAc fraction of MeOH extract | DPPH 2 | 8.403 μg/mL 9 | ||
| Dry needles, n-BuOH fraction of MeOH extract | DPPH 2 | 85.90 μg/mL 9 | ||
| Dry needles, H2O fraction of MeOH extract | DPPH 2 | inactive |
| Compound | IC50 (μg/mL) |
|---|---|
| 1 | 35.52 ± 0.65 |
| 2 | >100 |
| 3 | 25.91 ± 4.95 |
| 4 | >100 |
| 5 | 92.45 ± 13.19 |
| 6 | >100 |
| 7 | >100 |
| 8 | >100 |
| 9 | >100 |
| 10 | 1.95 ± 0.21 |
| 11 | 1.34 ± 0.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koutsaviti, A.; Toutoungy, S.; Saliba, R.; Loupassaki, S.; Tzakou, O.; Roussis, V.; Ioannou, E. Antioxidant Potential of Pine Needles: A Systematic Study on the Essential Oils and Extracts of 46 Species of the Genus Pinus. Foods 2021, 10, 142. https://doi.org/10.3390/foods10010142
Koutsaviti A, Toutoungy S, Saliba R, Loupassaki S, Tzakou O, Roussis V, Ioannou E. Antioxidant Potential of Pine Needles: A Systematic Study on the Essential Oils and Extracts of 46 Species of the Genus Pinus. Foods. 2021; 10(1):142. https://doi.org/10.3390/foods10010142
Chicago/Turabian StyleKoutsaviti, Aikaterini, Samer Toutoungy, Rouba Saliba, Sofia Loupassaki, Olga Tzakou, Vassilios Roussis, and Efstathia Ioannou. 2021. "Antioxidant Potential of Pine Needles: A Systematic Study on the Essential Oils and Extracts of 46 Species of the Genus Pinus" Foods 10, no. 1: 142. https://doi.org/10.3390/foods10010142








