Responses on Must and Wine Composition of Vitis vinifera L. cvs. Riesling and Cabernet Sauvignon under a Free Air CO2 Enrichment (FACE)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Site, Experimental Design and Plant Material
2.2. Weather Conditions
2.3. Experimental Winemaking
2.4. Grape Must Sampling
2.5. High-Performance Liquid Chromatography (HPLC) Analysis of Organic Acids and Monosaccharides
2.6. FT-MIR and NMR Analysis
2.7. Preparation of Wine Samples
2.8. Quantification of Total Phenols and Trolox Equivalent Antioxidative Capacity (TEAC)
2.9. Colorimetric Parameters
2.10. High-Performance Liquid Chromatography (HPLC) Analysis of Anthocyanins
2.11. Statistical Analysis
3. Results and Discussion
3.1. Effect of eCO2 on Total Soluble Solids, FT-MIR Analysis, Organic Acids and Monosccharides in Grape Must
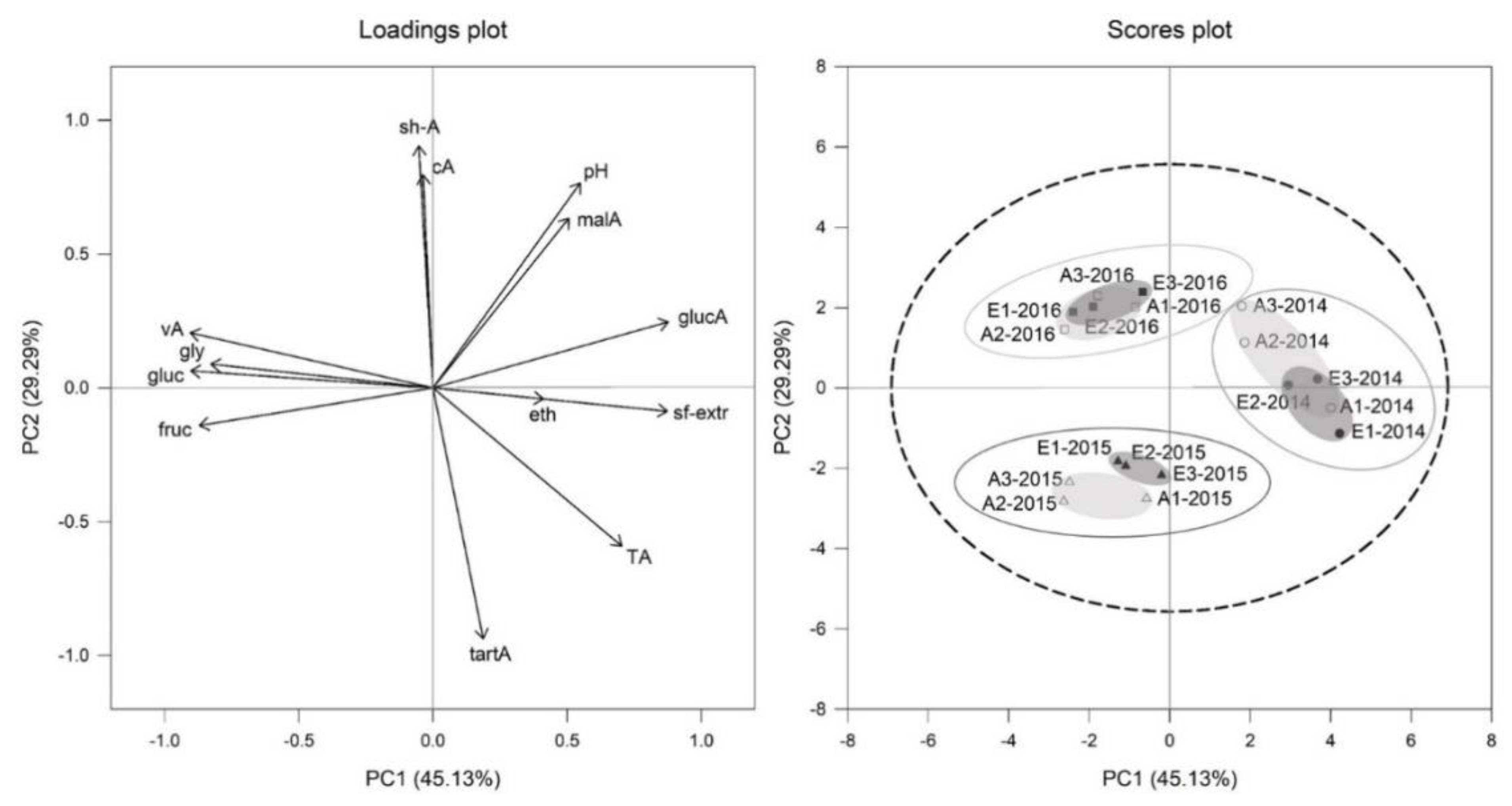
3.2. Principal Component Analysis (PCA) on Must Parameters of Two Different CO2 Regimes
3.3. Effects of eCO2 on FT-MIR and NMR Analysis, Total Phenolics and TEAC in Young Wines
3.4. Effects of eCO2 on Riesling and Cabernet Sauvignon Colorimetric Parameters in Young Wines
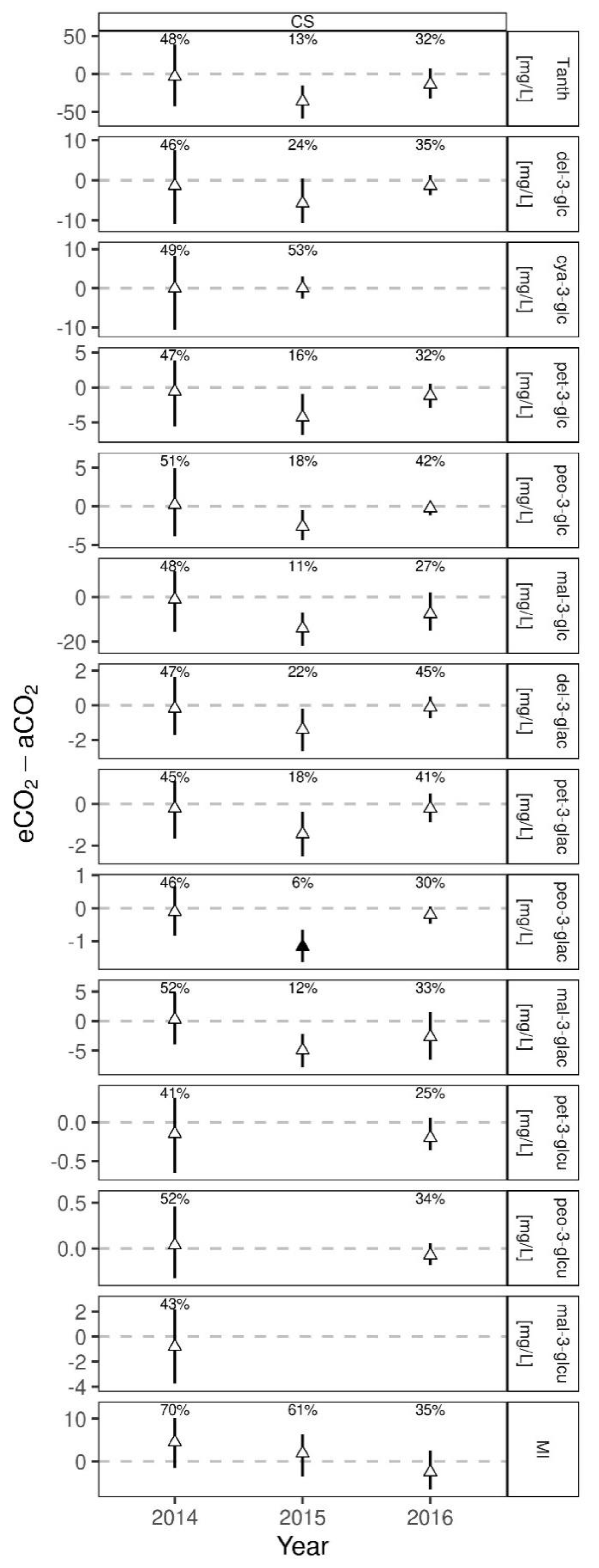
3.5. Anthocyanin and Monomeric Index Response of Cabernet Sauvignon Young Wines to eCO2 Treatment
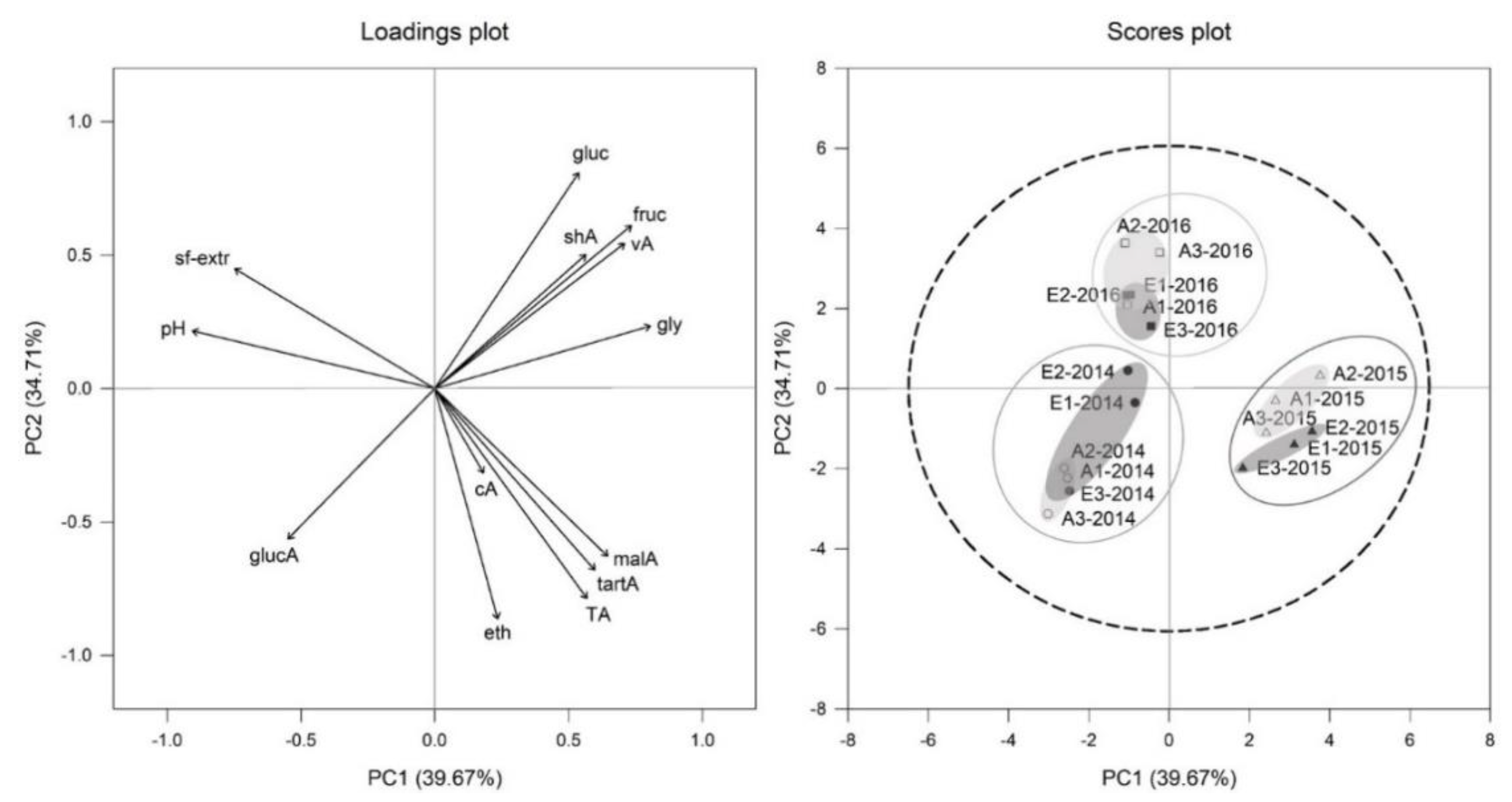
3.6. Principal Component Analysis (PCA) on Young Wines of Two Different CO2 Regimes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ciais, P.; Sabine, C.; Bala, G.; Bopp, L.; Brovkin, V.; Canadell, J.; Chhabra, A.A.; Defries, R.; Galloway, J.; Heimann, M.; et al. Carbon and other biogeochemical cycles. In Climate Change 2013: The Physical Science Basis; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; pp. 465–570. [Google Scholar]
- Coombe, B.G. Influence of temperature on composition and quality of grapes. Acta Hortic. 1987, 206, 23–35. [Google Scholar] [CrossRef]
- Bindi, M.; Fibbi, L.; Gozzini, B.; Orlandini, S.; Miglietta, F. Modelling the impact of future climate scenarios on yield and yield variability of grapevine. Clim. Res. 1996, 7, 213–224. [Google Scholar] [CrossRef]
- Jones, G.V.; Davis, R.E. Climate influences on grapevine phenology, grape composition, and wine production and quality for Bordeaux, France. Am. J. Enol. Vitic. 2000, 51, 249–261. [Google Scholar]
- Schultz, H.R. Climate change and viticulture: A European perspective on climatology, carbon dioxide and UV-B effects. Aust. J. Grape Wine Res. 2000, 6, 2–12. [Google Scholar] [CrossRef]
- Tate, A.B. Global warming’s impact on wine. J. Wine Res. 2001, 12, 95–109. [Google Scholar] [CrossRef]
- Jones, G.V.; White, M.A.; Cooper, O.R.; Storchmann, K. Climate change and global wine quality. Clim. Chang. 2005, 73, 319–343. [Google Scholar] [CrossRef]
- Duchêne, E.; Huard, F.; Dumas, V.; Schneider, C.; Merdinoglu, D. The challenge of adapting grapevine varieties to climate change. Clim. Res. 2010, 41, 193–204. [Google Scholar] [CrossRef] [Green Version]
- Mira de Orduna, R. Climate Change associated effects on grape and wine quality and production. Food Res. Int. 2010, 43, 1844–1855. [Google Scholar] [CrossRef]
- Hannah, L.; Roehrdanz, P.R.; Ikegami, M.; Shepard, A.V.; Shaw, M.R.; Tabor, G.; Zhi, L.; Marquet, P.A.; Hijmans, R.J. Climate change, wine, and conservation. Proc. Natl. Acad. Sci. USA 2013, 110, 6907–6912. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Lüscher, J.; Kizildeniz, T.; Vucetic, V.; Dai, Z.; Luedeling, E.; Van Leeuwen, C.; Gomes, E.; Pascual, I.; Irigoyen, J.J.; Morales, F.; et al. Sensitivity of grapevine phenology to water availability, temperature and CO2 concentration. Front. Environ. Sci. 2016, 4, 48. [Google Scholar] [CrossRef]
- Gonçalves, B.; Falco, V.; Moutinho-Pereira, J.M.; Bacelar, E.; Peixoto, F.; Correia, C. Effects of elevated CO2 on grapevine (Vitis vinifera L.): Volatile composition, phenolic content and in vitro antioxidant activity of red wine. J. Agric. Food Chem. 2009, 57, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Moutinho-Pereira, J.M.; Gonçalves, B.; Bacelar, E.; Boaventura, C.; Coutinho, J.; Correia, C.M. Effects of elevated CO2 on grapevine (Vitis vinifera L.): Physiological and yield attributes. Vitis 2009, 48, 159–165. [Google Scholar]
- Edwards, E.J.; Unwin, D.J.; Sommer, K.J.; Downey, M.O.; Mollah, M. The response of commercially managed, field grown, grapevines (Vitis vinifera L.) to a simulated future climate consisting of elevated CO2 in combination with elevated air temperature. Acta Hortic. 2016, 1115, 103–110. [Google Scholar] [CrossRef]
- Edwards, E.J.; Unwin, D.; Kilmister, R.; Treeby, M. Multi-seasonal effects of warming and elevated CO2 on the physiology, growth and production of mature, field grown, Shiraz grapevines. OENO One 2017, 51, 127–132. [Google Scholar] [CrossRef] [Green Version]
- Bindi, M.; Fibbi, L.; Gozzini, B.; Orlandini, S.; Seghi, L. The effect of elevated CO2 concentration on grapevine growth under field conditions. Acta Hortic. 1996, 427, 325–330. [Google Scholar] [CrossRef]
- Bindi, M.; Fibbi, L.; Miglieta, F. Free air CO2 enrichment (FACE) of grapevine (Vitis vinifera L.): II. Growth and quality of grape and wine in response to elevated CO2 concentrations. Eur. J. Agron. 2001, 14, 145–155. [Google Scholar] [CrossRef]
- Bindi, M.; Raschi, A.; Lanini, M.; Miglietta, F.; Tognetti, R. Physiological and yield responses of grapevine (Vitis vinifera L.) exposed to elevated CO2 concentrations in a Free Air CO2 Enrichment (FACE). J. Crop Improv. 2005, 13, 345–359. [Google Scholar] [CrossRef]
- Wohlfahrt, Y.; Smith, J.; Tittmann, S.; Honermeier, B.; Stoll, M. Primary productivity and physiological responses of Vitis vinifera L. cvs. under Free Air Carbon dioxide Enrichment (FACE). Eur. J. Agron. 2018, 101, 149–162. [Google Scholar] [CrossRef]
- Wohlfahrt, Y.; Collins, C.; Stoll, M. Grapevine bud fertility under conditions of elevated carbon dioxide. OENO One 2019, 2, 303–314. [Google Scholar] [CrossRef]
- Wohlfahrt, Y.; Tittmann, S.; Schmidt, D.; Rauhut, D.; Honermeier, B.; Stoll, M. The effect of elevated CO2 on berry development and bunch structure of Vitis vinifera L. cvs. Riesling and Cabernet Sauvignon. Appl. Sci. 2020, 10, 2486. [Google Scholar] [CrossRef] [Green Version]
- Singleton, V.L. Effects on red wine quality of removing juice before fermentation to simulate variation in berry size. Am. J. Enol. Vitic. 1972, 23, 106–113. [Google Scholar]
- Roby, G.; Matthews, M. Relative proportions of seed, skin and flesh, in ripe berries from Cabernet Sauvignon grapevines grown in a vineyard either well irrigated or under water deficit. Aust. J. Grape Wine Res. 2004, 10, 74–82. [Google Scholar] [CrossRef]
- Roby, G.; Harbertson, J.F.; Adams, D.A.; Matthews, M.A. Berry size and vine water deficits as factors in winegrape composition: Anthocyanins and tannins. Aust. J. Grape Wine Res. 2004, 10, 100–107. [Google Scholar] [CrossRef]
- Šuklje, K.; Lisjak, K.; Ĉesnik, H.B.; Janeš, L.; Du Toit, W.; Coetzee, Z.; Vanzo, A.; Deloire, A. Classification of grape berries according to diameter and total soluble solids to study the effect of light and temperature on methoxypyrazine, glutathione, and hydroxycinnamate evolution during ripening of Sauvignon blanc (Vitis vinifera L.). J. Agric. Food Chem. 2012, 60, 9454–9461. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.S.; Schultz, H.R.; Volschenk, C.G.; Hunter, J.J. Berry size variation of Vitis vinifera L. cv. Syrah: Morphological dimensions, berry composition and wine quality. S. Afr. J. Enol. Vitic. 2015, 36, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Friedel, M.; Sorrentino, V.; Blank, M.; Schuettler, A. Influence of berry diameter and colour on some determinants of wine composition of Vitis vinifera L. cv. Riesling. Aust. J. Grape Wine Res. 2016, 22, 215–225. [Google Scholar] [CrossRef]
- Chen, W.-K.; He, F.; Wang, Y.-X.; Liu, X.; Duan, C.-Q.; Wang, J. Influences of berry size on fruit composition and wine quality of Vitis vinifera L. cv. Cabernet Sauvignon grapes. S. Afr. J. Enol. Vitic. 2018, 39, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, M.; Wegmann-Herr, P.; Schmarr, H.-G.; Gök, R.; Winterhalter, P.; Fischer, U. Impact of rootstock, clonal selection and berry size of Vitis vinifera sp. Riesling on the formation of TDN, vitispiranes and other volatile compounds. J. Agric. Food Chem. 2020, 68, 3834–3849. [Google Scholar] [CrossRef] [PubMed]
- Salazar Parra, C.; Aguirreolea, J.; Sánchez-Díaz, M.; Irigoyen, J.J.; Morales, F. Effects of climate change scenarios on Tempranillo grapevine (Vitis vinifera L.) ripening: Response to a combination of elevated CO2 and temperature, and moderate drought. Plant Soil 2010, 337, 179–191. [Google Scholar] [CrossRef]
- Cozzolino, D.; Cynkar, W.U.; Dambergs, R.G.; Gishen, M.; Smith, P. Grape (Vitis vinifera) compositional data spanning ten successive vintages in the context of abiotic growing parameters. Agric. Ecosyst. Environ. 2010, 139, 565–570. [Google Scholar] [CrossRef]
- Schneider, A.; Gerbi, V.; Redoglia, M. A rapid HPLC method for separation and determination of major organic acids in grape musts and wines. Am. J. Enol. Vitic. 1987, 38, 151–155. [Google Scholar]
- Knoll, C.; Fritsch, S.; Schnell, S.; Grossmann, M.; Rauhut, D.; du Toit, M. Influence of pH and ethanol on malolactic fermentation and volatile aroma compound composition in white wines. LWT Food Sci. Technol. 2011, 44, 2077–2086. [Google Scholar] [CrossRef]
- Patz, C.D.; Blieke, A.; Ristow, R.; Dietrich, H. Application of FT-MIR spectrometry in wine analysis. Anal. Chim. Acta 2004, 513, 81–89. [Google Scholar] [CrossRef]
- Godelmann, R.; Kost, C.; Patz, C.D.; Ristow, R.; Wachter, H. Quantitation of compounds in wine using H NMR spectroscopy: Description of the method and collaborative study. J. AOAC Int. 2016, 99, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- OIV (The International Organisation of Vine and Wine). Determination of chromatic characteristics according to CIELab. Compendium of International Methods of Analysis of Wines and Musts (2 vol.). 2006. Available online: http://www.oiv.int/public/medias/2478/oiv-ma-as2-11.pdf (accessed on 6 September 2020).
- Giusti, M.M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. In Current Protocols in Food Analytical Chemistry; Wrolstad, R.E., Acree, T.E., An, H., Decker, E.A., Penner, M.H., Reid, D.S., Schwartz, S.J., Shoemaker, C.F., Sporns, P., Eds.; John Wiley & Sons, Inc.: New York, NY, USA, 2001. [Google Scholar]
- Bonerz, D.; Würth, K.; Patz, C.-D.; Dietrich, H. Der Monomerindex: Eine schnelle und kostengünstige Methode zur Bestimmung von Anthocyanen und Anthocyanaddukten in Buntsäften, Nektaren, Konzentraten und Rotweinen. Deutsche Lebensmittel-Rundschau 2006, 102, 195–201. [Google Scholar]
- Würth, K.; Bonerz, D.; Will, F.; Patz, C.-D.; Quast, P.; Hillebrand, S.; Winterhalter, P.; Dietrich, H. Anthocyanalterung in Säften und Konzentraten der schwarzen Johannisbeere–Teil 1: Kinetik der Abnahme von Anthocyanen bei der Lagerung. Deutsche Lebensmittel-Rundschau 2009, 105, 176–182. [Google Scholar]
- Hey, M.; Patz, C.-D.; Kürbel, P.; Dietrich, H. Primäre und sekundäre Inhaltsstoffe in Schwarzen Möhren (Daucus carota ssp. sativus) und daraus hergestellten Säften mit Jahrgangseinfluss und Bewässerung. Deutsche Lebensmittel-Rundschau 2013, 109, 209–216. [Google Scholar]
- The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 8 September 2020).
- Bürkner, P.-C. brms: An R Package for Bayesian Multilevel Models Using Stan. J. Stat. Softw. 2017, 80, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Bürkner, P.-C. Advanced Bayesian Multilevel Modeling with the R Package brms. R J. 2018, 10, 395–411. [Google Scholar] [CrossRef]
- Carpenter, B.; Gelman, A.; Hoffman, M.D.; Lee, D.; Goodrich, B.; Betancourt, M.; Brubaker, M.; Guo, J.; Li, P.; Riddell, A. Stan: A Probabilistic Programming Language. J. Stat. Softw. 2017, 76, 1–32. [Google Scholar] [CrossRef] [Green Version]
- Kaur, A.; Gregori, D.; Patil, G.P.; Taillie, C. Ecological applications of generalized linear models and quasi-likelihood methods–an overview. Stat. Appl. 1996, 8, 59–82. [Google Scholar]
- Vehtari, A.; Gelman, A.; Simpson, D.; Carpenter, B.; Bürkner, P.-C. Rank-normalization, folding, and localization: An improved $\widehat{R}$ for assessing convergence of MCMC. Bayesian Anal. Adv. Publ. 2020. Available online: https://projecteuclid.org/euclid.ba/1593828229 (accessed on 8 September 2020). [CrossRef]
- Flegal, J.M.; Hughes, J.; Vats, D.; Dai, N. mcmcse: Monte Carlo Standard Errors for MCMC. 2017. Available online: https://rdrr.io/cran/mcmcse/ (accessed on 4 December 2019).
- Vats, D.; Flegal, J.M.; Jones, G.L. Multivariate output analysis for Markov chain Monte Carlo. Biometrika 2019, 106, 321–337. [Google Scholar] [CrossRef] [Green Version]
- Peynaud, E.; Maurié, A. Synthesis of tartaric and malic acids by grape vines. Am. J. Enol. Vitic. 1958, 9, 32–36. [Google Scholar]
- Kliewer, W.M.; Howarth, L.; Omori, M. Concentrations of tartaric acid and malic acids and their salts in Vitis vinifera grapes. Am. J. Enol. Vitic. 1967, 18, 42–54. [Google Scholar]
- Kliewer, W.M. Concentration of tartrates, malates, glucose and fructose in the fruits of the genus Vitis. Am. J. Enol. Vitic. 1967, 18, 87–96. [Google Scholar]
- Liu, H.-F.; Wu, B.H.; Fan, P.-G.; Li, S.-H.; Li, L.-S. Sugar and acid concentrations in 98 grape cultivars analyzed by principal component analysis. J. Sci. Food Agric. 2006, 86, 1526–1536. [Google Scholar] [CrossRef]
- Sponholz, W.R. Eigenschaften und chemische Zusammensetzung des Traubenmostes. In Handbuch der Lebensmitteltechnologie, Chemie des Weines; Würdig, G., Woller, R., Eds.; Eugen GmbH & Co.: Stuttgart, Germany, 1989; pp. 45–122. [Google Scholar]
- Biasi, R.; Brunori, E.; Ferrara, C.; Salvati, L. Assessing impacts of climate change on phenology and quality traits of Vitis vinifera L.: The contribution of local knowledge. Plants 2019, 8, 121. [Google Scholar] [CrossRef] [Green Version]
- Sponholz, W.R.; Dittrich, H.H. Über das Vorkommen von Galacturon- und Glucuronsäure sowie von 2- und 5-0xo-Gluconsäure in Weinen, Sherries, Obst- und Dessertweinen. Vitis 1984, 23, 214–224. [Google Scholar]
- Jackowetz, J.N.; Mira de Orduña, R. Survey of SO2 binding carbonyls in 237 red and white table wines. Food Control 2013, 32, 687–692. [Google Scholar] [CrossRef]
- Lao, C.; Lopez-Tamames, E.; Buxaderas, S.; De la Torre-Boronat, M.C. Grape pectic enzyme treatment effect on white musts & wines composition. J. Food Sci. 1996, 61, 553–556. [Google Scholar]
- Dittrich, H.H.; Sponholz, W.R.; Kast, W. Comparative investigations on musts and wines from healthy and Botrytis-infested grape-berries. I. Metabolism of organic acids, metabolites of sugars, and leuco-anthocyanin contents. Vitis 1974, 13, 36–49. [Google Scholar]
- Voss, D.H. Relating colourimeter measurement of plant colour to the Royal Horticultural Society colour chart. HortScience 1992, 27, 1256–1260. [Google Scholar] [CrossRef]
- Larrauri, J.A.; Sánchez-Moreno, C.; Rupérez, P.; Saura-Calixto, F. Free radical scavenging capacity in the aging of selected red Spanish wines. J. Agric. Food Chem. 1999, 47, 1603–1606. [Google Scholar] [CrossRef]
- Somers, T.C.; Evans, M.E. Evolution of red wines I. Ambient influences on colour composition during early maturation. Vitis 1986, 25, 31–39. [Google Scholar]
- Somers, T.C.; Wescombe, L.G. Evolution of red wines. II. An assessment of the role of acetaldehyde. Vitis 1987, 26, 27–36. [Google Scholar]
- Dallas, C.; Laureano, O. Effects of pH, sulphur dioxide, alcohol content, temperature and storage time on colour composition of a young Portuguese red table wine. J. Sci. Food Agric. 1994, 65, 477–485. [Google Scholar] [CrossRef]
- Blesic, M.; Zele, M.; Bukvic, A.; Viles, A.; Smajic, M.; Spaho, N. Influence of filtration on colour characteristics of young Herzegovinian white wines. J. Agric. Fac. Ege Univ. 2013, 1, 191–194. [Google Scholar]
- Recamales, A.F.; Sayago, A.; González-Miret, M.L.; Hernanz, D. The effect of time and storage conditions on the phenolic composition and colour of white wine. Food Res. Int. 2006, 39, 220–229. [Google Scholar] [CrossRef]
- Somers, T.C.; Vérette, E. Phenolic Composition of Natural Wine Types. In Wine Analysis. Modern Methods of Plant Analysis; Linskens, H.F., Jackson, J.F., Eds.; New Series; Springer: Berlin/Heidelberg, Germany, 1988; Volume 6, pp. 219–257. [Google Scholar]
- Zoecklein, B.W.; Fugelsang, K.C.; Gump, B.H.; Nury, F.S. (Eds.) Phenolic Compounds and Wine Color. In Wine Analysis and Production; Chapman and Hall: New York, NY, USA, 1999; pp. 115–151. [Google Scholar]
- Mazza, G.; Francis, F.J. Anthocyanins in grapes and grape products. Crit. Rev. Food Sci. Nutr. 1995, 35, 341–371. [Google Scholar] [CrossRef] [PubMed]
- Wulf, L.W.; Nagel, C.W. High-pressure liquid chromatographic separation of anthocyanins of Vitis vinifera. Am. J. Enol. Vitic. 1978, 29, 42–49. [Google Scholar]
- Dallas, C.; Ricardo da Silva, J.M.; Laureano, O. Products formed in model wine solutions involving anthocyanins, procyanidins B2 and acetaldehyde. J. Agric. Food Chem. 1996, 44, 2402–2407. [Google Scholar] [CrossRef]
- Cheynier, V.; Souquet, J.-M.; Kontek, A.; Moutounet, M. Anthocyanin degradation in oxidizing grape musts. J. Sci. Food Agric. 1994, 66, 283–288. [Google Scholar] [CrossRef]
- Eder, R.; Wendelin, S.; Barna, J. Klassifizierung von Rotweinsorten mittels Anthocyananalyse. 1. Mitteilung multivariater statistischer Methoden zur Differenzierung von Traubenproben. Mitt. Klosterneubg. 1994, 44, 201–212. [Google Scholar]
- Buttrose, M.S.; Hale, C.R.; Kliewer, W.M. Effect of temperature on the composition of ‘Cabernet Sauvignon’ berries. Am. J. Enol. Vitic. 1971, 22, 71–75. [Google Scholar]
- Kliewer, W.M.; Torres, R.E. Effect of controlled day and night temperatures on grape coloration. Am. J. Enol. Vitic. 1972, 23, 71–77. [Google Scholar]
- Kliewer, W.M. Influence of temperature, solar radiation, and nitrogen on coloration and composition of ‘Emperor’ grapes. Am. J. Enol. Vitic. 1977, 28, 96–103. [Google Scholar]
- Keller, M. Managing grapevines to optimise fruit development in a challenging environment: A climate change primer for viticulturists. Aust. J. Grape Wine Res. 2010, 16, 56–69. [Google Scholar] [CrossRef]
- Han, F.-L.; Zhang, W.-N.; Pan, Q.-H.; Zheng, C.-R.; Chen, H.-Y.; Duan, C.-Q. Principal Component Regression Analysis of the relation between CIELab color and monomeric anthocyanins in young Cabernet Sauvignon wines. Molecules 2008, 13, 2859–2870. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Analyzed Compound | Abbreviation | Analytical Method | Medium |
|---|---|---|---|
| total soluble solids | TSS | refractometry | must |
| density 20/20 | FT-MIR | must | |
| sugar-free extract | sf-extr | must, wine | |
| total acidity | TA | must, wine | |
| tartaric acid | tartA | wine | |
| malic acid | malA | wine | |
| pH | must, wine | ||
| glycerol | gly | must, wine | |
| ethanol | must | ||
| gluconic acid | glucA | must | |
| volatile acid | vA | must, wine | |
| actual alcohol | alc | wine | |
| residual sugar | sugar | wine | |
| glucose | gluc | HPLC | must |
| fructose | fruc | must | |
| tartaric acid | tartA | must | |
| malic acid | malA | must | |
| citric acid | cA | must | |
| shikimic acid | shA | must | |
| 2,3-butanediol | NMR | wine | |
| 2-methyl-propanol | wine 1 | ||
| 2-phenylethanol | wine | ||
| 3-methyl-butanol | wine | ||
| caftaric acid | cafA | wine | |
| citric acid | cA | wine 1 | |
| epicatechin | wine 1 | ||
| galacturonic acid | galA | wine 1 | |
| methanol | wine | ||
| shikimic acid | shA | wine | |
| succinic acid | sucA | wine | |
| trigonelline | wine 1 | ||
| total phenolics | TP | spectrophotometry | wine |
| trolox equivalent antioxidative capacity | TEAC | spectrophotometry | wine 1 |
| CIELab coordinates | L*, a*, b* | spectrophotometry | wine |
| monomeric index | MI | spectrophotometry | wine 1 |
| total anthocyanins | Tanth | HPLC | wine 1 |
| delphinidin-3-O-glucoside | del-3-glc | wine 1 | |
| cyanidin-3-O-glucoside | cya-3-glc | wine 1 | |
| petunidin-3-O-glucoside | pet-3-glc | wine 1 | |
| peonidin-3-O-glucoside | peo-3-glc | wine 1 | |
| malvidin-3-O-glucoside | mal-3-glc | wine 1 | |
| delphinidin-3-O-(6″-acetyl)-glucoside | del-3-glac | wine 1 | |
| petunidin-3-O-(6″-acetyl)-glucoside | pet-3-glac | wine 1 | |
| peonidin-3-O-(6″-acetyl)-glucoside | peo-3-glac | wine 1 | |
| malvidin-3-O-(6″-acetyl)-glucoside | mal-3-glac | wine 1 | |
| petunidin-3-O-(6″-p-coumaroyl)-glucoside | pet-3-glcu | wine 1 | |
| peonidin-3-O-(6″-p-coumaroyl)-glucoside | peo-3-glcu | wine 1 | |
| malvidin-3-O-(6″-p-coumaroyl)-glucoside | mal-3-glcu | wine 1 |
| Cabernet Sauvignon | Riesling | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| year | 2014 | 2015 | 2016 | 2014 | 2015 | 2016 | ||||||
| treatment | aCO2 | eCO2 | aCO2 | eCO2 | aCO2 | eCO2 | aCO2 | eCO2 | aCO2 | eCO2 | aCO2 | eCO2 |
| TSS [°Brix] | 19.1 ± 0.9 | 19.1 ± 0.8 | 20.4 ± 0.8 | 19.8 ± 0.5 | 19.8 ± 0.5 | 19.5 ± 0.5 | 19.6 ± 0.4 | 19.6 ± 0.5 | 21.0 ± 0.5 | 20.5 ± 0.8 | 20.5 ± 0.4 | 20.2 ± 0.0 |
| density 20/20 | 1.082 ± 0.004 | 1.081 ± 0.004 | 1.089 ± 0.003 | 1.086 ± 0.002 | 1.083 ± 0.002 | 1.083 ± 0.002 | 1.082 ± 0.001 | 1.082 ± 0.002 | 1.091 ± 0.002 | 1.090 ± 0.002 | 1.087 ± 0.001 | 1.086 ± 0.000 |
| sf-extract [g/L] | 27.2 ± 0.5 | 27.2 ± 0.9 | 22.7 ± 0.1 | 22.5 ± 0.2 | 21.0 ± 0.7 | 21.3 ± 0.1 | 25.1 ± 2.2 | 25.8 ± 3.5 | 21.0 ± 0.9 | 20.0 ± 0.9 | 26.2 ± 1.1 | 26.9 ± 0.4 |
| TA [g/L] | 13.20 ± 0.56 | 13.98 ± 0.57 | 12.63 ± 0.49 | 13.77 ± 0.21 | 10.92 ± 0.83 | 11.57 ± 0.60 | 11.52 ± 0.38 | 11.37 ± 0.06 | 12.50 ± 0.53 | 12.87 ± 0.15 | 9.37 ± 0.55 | 9.73 ± 0.42 |
| pH | 3.00 ± 0.02 | 2.98 ± 0.03 | 2.77 ± 0.06 | 2.77 ± 0.06 | 2.95 ± 0.02 | 2.93 ± 0.02 | 2.93 ± 0.02 | 2.94 ± 0.01 | 2.67 ± 0.06 | 2.73 ± 0.06 | 2.93 ± 0.06 | 2.90 ± 0.00 |
| gly [g/L] | 0.13 ± 0.01 | 0.12 ± 0.00 | 0.97 ± 0.23 | 1.10 ± 0.10 | 1.16 ± 0.19 | 1.35 ± 0.22 | 0.12 ± 0.01 | 0.12 ± 0.01 | 0.67 ± 0.15 | 0.67 ± 0.12 | 0.44 ± 0.22 | 0.52 ± 0.10 |
| ethanol [g/L] | 0.12 ± 0.00 | 0.28 ± 0.27 | 0.10 ± 0.00 | 0.10 ± 0.00 | 0.08 ± 0.01 | 0.08 ± 0.00 | 0.10 ± 0.01 | 0.10 ± 0.00 | 0.10 ± 0.00 | 0.10 ± 0.00 | 0.07 ± 0.00 | 0.07 ± 0.01 |
| glucA [g/L] | 0.53 ± 0.01 | 0.45 ± 0.13 | 0.10 ± 0.00 | 0.10 ± 0.00 | 0.16 ± 0.01 | 0.16 ± 0.01 | 0.57 ± 0.02 | 0.19 ± 0.33 | 0.17 ± 0.06 | 0.13 ± 0.06 | 0.16 ± 0.01 | 0.16 ± 0.01 |
| vA [g/L] | 0.30 ± 0.07 | 0.23 ± 0.07 | 0.40 ± 0.10 | 0.37 ± 0.06 | 0.41 ± 0.05 | 0.44 ± 0.05 | 0.23 ± 0.06 | 0.26 ± 0.02 | 0.43 ± 0.06 | 0.43 ± 0.06 | 0.41 ± 0.04 | 0.43 ± 0.01 |
| gluc [g/L] | 91.1 ± 6.8 | 87.5 ± 4.2 | 99.0 ± 4.6 | 95.6 ± 3.7 | 97.8 ± 2.7 | 97.3 ± 3.5 | 91.4 ± 1.2 | 95.0 ± 3.5 | 100.2 ± 2.3 | 97.9 ± 2.6 | 101.9 ± 1.8 | 99.9 ± 0.2 |
| fruc [g/L] | 90.1 ± 7.7 | 85.3 ± 3.7 | 100.5 ± 5.4 | 96.2 ± 3.6 | 95.8 ± 4.4 | 94.7 ± 3.8 | 93.1 ± 1.1 | 97.6 ± 3.6 | 104.7 ± 3.0 | 101.3 ± 3.2 | 102.9 ± 1.8 | 100.6 ± 0.3 |
| tartA [g/L] | 7.26 ± 0.79 | 7.74 ± 0.21 | 8.55 ± 0.52 | 9.03 ± 0.20 | 5.95 ± 0.40 | 6.11 ± 0.13 | 7.78 ± 0.20 | 7.39 ± 0.18 | 8.24 ± 0.29 | 9.05 ± 0.55 | 6.73 ± 0.48 | 6.97 ± 0.08 |
| malA [g/L] | 6.30 ± 0.30 | 6.43 ± 0.24 | 4.85 ± 0.32 | 5.82 ± 0.25 | 6.04 ± 0.87 | 6.45 ± 0.80 | 3.91 ± 0.21 | 4.06 ± 0.26 | 4.47 ± 0.22 | 4.51 ± 0.21 | 3.49 ± 0.35 | 3.62 ± 0.38 |
| cA [g/L] | 0.22 ± 0.02 | 0.22 ± 0.01 | 0.20 ± 0.01 | 0.22 ± 0.00 | 0.23 ± 0.02 | 0.25 ± 0.01 | 0.17 ± 0.00 | 0.17 ± 0.01 | 0.16 ± 0.01 | 0.19 ± 0.05 | 0.15 ± 0.03 | 0.17 ± 0.03 |
| shA [mg/L] | 69.1 ± 7.1 | 61.9 ± 4.9 | 54.4 ± 2.2 | 57.8 ± 2.0 | 71.3 ± 3.8 | 68.7 ± 2.2 | 42.7 ± 1.8 | 46.3 ± 2.7 | 47.9 ± 1.7 | 46.7 ± 3.4 | 48.0 ± 3.3 | 45.0 ± 0.9 |
| Cabernet Sauvignon | Riesling | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| year | 2014 | 2015 | 2016 | 2014 | 2015 | 2016 | ||||||
| treatment | aCO2 | eCO2 | aCO2 | eCO2 | aCO2 | eCO2 | aCO2 | eCO2 | aCO2 | eCO2 | aCO2 | eCO2 |
| total alcohol [g/L] | 105.83 ± 5.02 | 106.06 ± 4.88 | 99.96 ± 5.94 | 95.87 ± 4.37 | 93.73 ± 2.65 | 92.85 ± 2.48 | 91.96 ± 1.55 | 91.99 ± 2.14 | 102.25 ± 3.49 | 99.80 ± 2.56 | 101.33 ± 1.58 | 98.52 ± 0.18 |
| sf-extract [g/L] | 24.9 ± 0.6 | 25.6 ± 0.4 | 24.7 ± 0.7 | 24.7 ± 0.8 | 21.9 ± 0.7 | 21.7 ± 0.5 | 19.0 ± 3.4 | 21.5 ± 0.3 | 23.4 ± 1.2 | 23.5 ± 0.4 | 19.9 ± 0.5 | 20.1 ± 0.5 |
| TA [g/L] | 6.93 ± 0.25 | 7.03 ± 0.13 | 7.13 ± 0.47 | 7.33 ± 0.15 | 6.67 ± 0.29 | 6.87 ± 0.12 | 10.90 ± 0.41 | 11.08 ± 0.10 | 12.66 ± 0.35 | 12.88 ± 0.11 | 9.17 ± 0.38 | 9.57 ± 0.21 |
| tartA [g/L] | 3.20 ± 0.48 | 3.22 ± 0.10 | 3.67 ± 0.50 | 3.90 ± 0.10 | 2.73 ± 0.40 | 2.87 ± 0.15 | 5.94 ± 0.92 | 5.28 ± 0.07 | 5.92 ± 0.37 | 5.94 ± 0.08 | 4.27 ± 0.35 | 4.43 ± 0.06 |
| malA [g/L] | 2.34 ± 0.25 | 2.43 ± 0.05 | 2.27 ± 0.06 | 2.43 ± 0.32 | 2.63 ± 0.15 | 2.70 ± 0.20 | 3.72 ± 0.21 | 3.62 ± 0.14 | 4.33 ± 0.12 | 4.57 ± 0.10 | 2.87 ± 0.25 | 3.03 ± 0.32 |
| pH | 3.25 ± 0.11 | 3.24 ± 0.03 | 3.23 ± 0.06 | 3.20 ± 0.00 | 3.47 ± 0.06 | 3.40 ± 0.00 | 2.41 ± 0.27 | 2.65 ± 0.00 | 2.46 ± 0.07 | 2.46 ± 0.05 | 3.03 ± 0.06 | 3.00 ± 0.00 |
| gly [g/L] | 8.80 ± 0.37 | 8.53 ± 0.17 | 7.83 ± 0.47 | 7.37 ± 0.25 | 7.07 ± 0.21 | 7.10 ± 0.17 | 6.09 ± 0.72 | 5.33 ± 0.17 | 6.09 ± 0.33 | 6.14 ± 0.27 | 5.40 ± 0.30 | 5.30 ± 0.26 |
| vA [g/L] | 0.38 ± 0.01 | 0.37 ± 0.01 | 0.33 ± 0.06 | 0.30 ± 0.00 | 0.33 ± 0.06 | 0.33 ± 0.06 | 0.44 ± 0.01 | 0.46 ± 0.01 | 0.62 ± 0.02 | 0.66 ± 0.02 | 0.50 ± 0.00 | 0.50 ± 0.00 |
| 2,3-butanediol [mg/L] | 294.50 ± 37.03 | 269.67 ± 23.22 | 303.44 ± 45.35 | 233.75 ± 30.06 | 219.20 ± 52.45 | 217.84 ± 23.32 | 290.42 ± 31.70 | 267.98 ± 39.77 | 240.70 ± 37.71 | 257.47 ± 55.66 | 281.43 ± 3.14 | 267.91 ± 6.27 |
| 2-methyl-propanol [mg/L] | 149.17 ± 9.83 | 162.17 ± 17.21 | 110.26 ± 15.30 | 133.15 ± 6.08 | 103.77 ± 1.95 | 94.79 ± 18.07 | - | - | - | - | - | - |
| 2-phenylethanol [mg/L] | 71.67 ± 3.75 | 69.50 ± 4.09 | 71.28 ± 12.94 | 69.74 ± 16.86 | 79.53 ± 8.14 | 84.02 ± 1.72 | 39.00 ± 1.67 | 39.41 ± 2.08 | 38.87 ± 2.05 | 40.40 ± 3.31 | 37.74 ± 3.10 | 35.01 ± 4.89 |
| 3-methyl-butanol [mg/L] | 474.67 ± 12.11 | 498.50 ± 21.47 | 354.49 ± 38.83 | 394.54 ± 38.42 | 372.53 ± 57.90 | 383.49 ± 44.14 | 154.37 ± 6.39 | 157.22 ± 2.49 | 145.26 ± 21.96 | 143.69 ± 4.32 | 141.78 ± 12.75 | 135.55 ± 21.03 |
| cafA [mg/L] | 27.83 ± 1.61 | 31.17 ± 1.89 | 23.83 ± 0.52 | 25.71 ± 1.26 | 17.48 ± 0.99 | 19.11 ± 0.71 | 61.58 ± 3.92 | 65.96 ± 9.35 | 39.83 ± 18.58 | 29.97 ± 10.33 | 48.45 ± 13.31 | 44.67 ± 3.61 |
| cA [g/L] | 0.30 ± 0.02 | 0.32 ± 0.02 | 0.32 ± 0.02 | 0.35 ± 0.1 | 0.33 ± 0.04 | 0.34 ± 0.04 | - | - | - | - | - | - |
| epicatechin [mg/L] | 41.7 ± 19.7 | 43.2 ± 17.9 | 46.3 ± 3.7 | 51.4 ± 10.4 | - | - | - | - | - | - | - | - |
| galA [mg/L] | 504.50 ± 36.34 | 425.33 ± 25.27 | 237.39 ± 37.07 | 166.26 ± 3.10 | 268.59 ± 48.92 | 264.97 ± 43.52 | - | - | - | - | - | - |
| methanol [mg/L] | 97.83 ± 4.54 | 92.83 ± 1.26 | 77.39 ± 5.34 | 70.27 ± 3.77 | 76.25 ± 24.48 | 81.15 ± 5.71 | 37.95 ± 3.60 | 36.12 ± 2.26 | 42.24 ± 2.92 | 40.24 ± 5.08 | 44.29 ± 3.20 | 40.49 ± 3.68 |
| shA [mg/L] | 135.83 ± 6.53 | 135.17 ± 5.35 | 111.77 ± 13.16 | 106.04 ± 1.25 | 129.43 ± 16.67 | 127.62 ± 8.70 | 44.12 ± 2.70 | 47.95 ± 1.52 | 43.96 ± 0.93 | 45.38 ± 1.42 | 44.90 ± 4.37 | 42.03 ± 0.88 |
| sucA [g/L] | 0.99 ± 0.19 | 0.88 ± 0.11 | 0.92 ± 0.32 | 0.69 ± 0.12 | 1.04 ± 0.23 | 1.04 ± 0.22 | 0.60 ± 0.04 | 0.63 ± 0.07 | 0.83 ± 0.05 | 0.80 ± 0.12 | 0.81 ± 0.07 | 0.79 ± 0.01 |
| trigonelline [mg/L] | 13.5 ± 0.9 | 14.0 ± 0.5 | 13.4 ± 1.3 | 13.9 ± 1.2 | 11.4 ± 0.5 | 11.1 ± 1.0 | - | - | - | - | - | - |
| TP [mg/L] | 1853.3 ± 206.4 | 1870.0 ± 179.0 | 1859.2 ± 320.9 | 1749.3 ± 109.0 | 1177.7 ± 45.3 | 1164.3 ± 31.1 | 211.3 ± 6.7 | 215.3 ± 9.8 | 180.0 ± 25.9 | 164.7 ± 15.8 | 165.0 ± 9.3 | 162.5 ± 4.1 |
| TEAC | 21.8 ± 1.9 | 21.2 ± 1.2 | 21.4 ± 3.5 | 19.0 ± 1.0 | 13.4 ± 0.2 | 13.3 ± 0.3 | - | - | - | - | - | - |
| Cabernet Sauvignon | Riesling | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| year | 2014 | 2015 | 2016 | 2014 | 2015 | 2016 | ||||||
| treatment | aCO2 | eCO2 | aCO2 | eCO2 | aCO2 | eCO2 | aCO2 | eCO2 | aCO2 | eCO2 | aCO2 | eCO2 |
| L* | 10.94 ± 2.43 | 12.98 ± 1.97 | 17.35 ± 3.93 | 21.21 ± 2.24 | 23.77 ± 2.06 | 24.05 ± 2.30 | 99.47 ± 0.18 | 99.00 ± 0.72 | 97.51 ± 0.73 | 96.93 ± 0.59 | 97.47 ± 0.57 | 98.00 ± 0.41 |
| a* | 41.39 ± 3.28 | 44.14 ± 2.30 | 49.31 ± 4.67 | 53.85 ± 2.17 | 56.59 ± 1.97 | 56.93 ± 2.05 | −0.91 ± 0.10 | −0.90 ± 0.05 | −0.32 ± 0.73 | −0.66 ± 0.12 | −0.66 ± 0.04 | −0.57 ± 0.10 |
| b* | 41.82 ± 3.30 | 44.30 ± 1.10 | 45.72 ± 3.39 | 46.90 ± 0.98 | 42.13 ± 2.43 | 42.62 ± 1.48 | 4.84 ± 0.32 | 4.69 ± 0.08 | 6.40 ± 0.43 | 5.50 ± 0.12 | 5.79 ± 0.41 | 5.38 ± 0.19 |
| Cabernet Sauvignon | ||||||
|---|---|---|---|---|---|---|
| year | 2014 | 2015 | 2016 | |||
| treatment | aCO2 | eCO2 | aCO2 | eCO2 | aCO2 | eCO2 |
| Tanth [mg/L] | 403.73 ± 28.67 | 400.89 ± 32.88 | 228.62 ± 25.74 | 191.47 ± 4.71 | 200.38 ± 10.20 | 186.70 ± 13.10 |
| del-3-glc [mg/L] | 53.31 ± 7.84 | 52.13 ± 9.31 | 34.95 ± 8.71 | 28.60 ± 2.30 | 14.92 ± 2.13 | 13.54 ± 1.58 |
| cya-3-glc [mg/L] | 7.30 ± 1.33 | 7.37 ± 2.59 | 2.47 ± 2.36 | 2.43 ± 0.65 | - | - |
| pet-3-glc [mg/L] | 36.92 ± 3.63 | 36.58 ± 4.02 | 24.15 ± 3.86 | 19.72 ± 0.44 | 13.91 ± 1.50 | 12.75 ± 1.20 |
| peo-3-glc [mg/L] | 29.37 ± 2.68 | 29.79 ± 4.93 | 14.05 ± 1.82 | 11.37 ± 1.23 | 5.85 ± 0.81 | 5.59 ± 0.06 |
| mal-3-glc [mg/L] | 178.27 ± 7.64 | 177.30 ± 6.22 | 103.43 ± 2.86 | 89.05 ± 4.92 | 113.30 ± 5.19 | 105.72 ± 5.10 |
| del-3-glac [mg/L] | 11.01 ± 1.44 | 10.85 ± 1.64 | 8.53 ± 1.65 | 7.03 ± 0.49 | 4.18 ± 0.44 | 4.07 ± 0.15 |
| pet-3-glac [mg/L] | 8.98 ± 1.04 | 8.81 ± 1.11 | 7.50 ± 1.40 | 5.97 ± 0.64 | 4.68 ± 0.56 | 4.45 ± 0.53 |
| peo-3-glac [mg/L] | 5.63 ± 0.34 | 5.51 ± 0.52 | 3.98 ± 0.60 | 2.78 ± 0.45 | 2.03 ± 0.06 | 1.83 ± 0.21 |
| mal-3-glac [mg/L] | 41.87 ± 1.51 | 42.33 ± 1.11 | 29.55 ± 3.25 | 24.52 ± 1.14 | 40.11 ± 1.56 | 37.62 ± 4.32 |
| pet-3-glcu [mg/L] | 1.36 ± 0.21 | 1.19 ± 0.20 | - | - | 0.66 ± 0.14 | 0.47 ± 0.27 |
| peo-3-glcu [mg/L] | 2.32 ± 0.18 | 2.37 ± 0.25 | - | - | 0.74 ± 0.16 | 0.66 ± 0.04 |
| mal-3-glcu [mg/L] | 27.39 ± 1.10 | 26.67 ± 1.12 | - | - | - | - |
| MI | 33.50 ± 4.01 | 38.07 ± 3.41 | 28.87 ± 7.15 | 30.34 ± 4.16 | 28.77 ± 5.73 | 26.00 ± 2.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wohlfahrt, Y.; Patz, C.-D.; Schmidt, D.; Rauhut, D.; Honermeier, B.; Stoll, M. Responses on Must and Wine Composition of Vitis vinifera L. cvs. Riesling and Cabernet Sauvignon under a Free Air CO2 Enrichment (FACE). Foods 2021, 10, 145. https://doi.org/10.3390/foods10010145
Wohlfahrt Y, Patz C-D, Schmidt D, Rauhut D, Honermeier B, Stoll M. Responses on Must and Wine Composition of Vitis vinifera L. cvs. Riesling and Cabernet Sauvignon under a Free Air CO2 Enrichment (FACE). Foods. 2021; 10(1):145. https://doi.org/10.3390/foods10010145
Chicago/Turabian StyleWohlfahrt, Yvette, Claus-Dieter Patz, Dominik Schmidt, Doris Rauhut, Bernd Honermeier, and Manfred Stoll. 2021. "Responses on Must and Wine Composition of Vitis vinifera L. cvs. Riesling and Cabernet Sauvignon under a Free Air CO2 Enrichment (FACE)" Foods 10, no. 1: 145. https://doi.org/10.3390/foods10010145
APA StyleWohlfahrt, Y., Patz, C. -D., Schmidt, D., Rauhut, D., Honermeier, B., & Stoll, M. (2021). Responses on Must and Wine Composition of Vitis vinifera L. cvs. Riesling and Cabernet Sauvignon under a Free Air CO2 Enrichment (FACE). Foods, 10(1), 145. https://doi.org/10.3390/foods10010145






