Elemental Analysis and Phenolic Profiles of Selected Italian Wines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Analyzed Samples
2.2. Reagents and Standards
2.3. ICP Analysis of Elements
2.4. UHPLC–MS Analysis
2.4.1. Preparation of Standard Solutions
2.4.2. UHPLC–DAD-QqQ-MS Analysis of Non-Anthocyanins
2.4.3. UHPLC-LTQ OrbiTrap MS Analysis of Anthocyanins and Anthocyanin-Derived Pigments
2.5. Determination of Total Phenolic Content (TPC)
2.6. Determination of Radical-Scavenging Activity (RSA)
2.7. Data Analysis
3. Results and Discussion
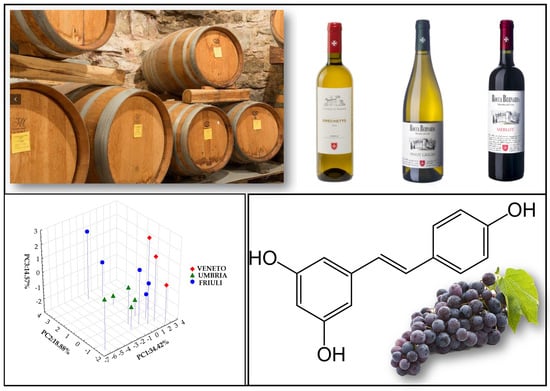
3.1. Major and Trace Elements in Italian Wines
3.2. Phenolic Profile of Italian Wines
3.2.1. Spectrophotometric Determination of TPC and RSA
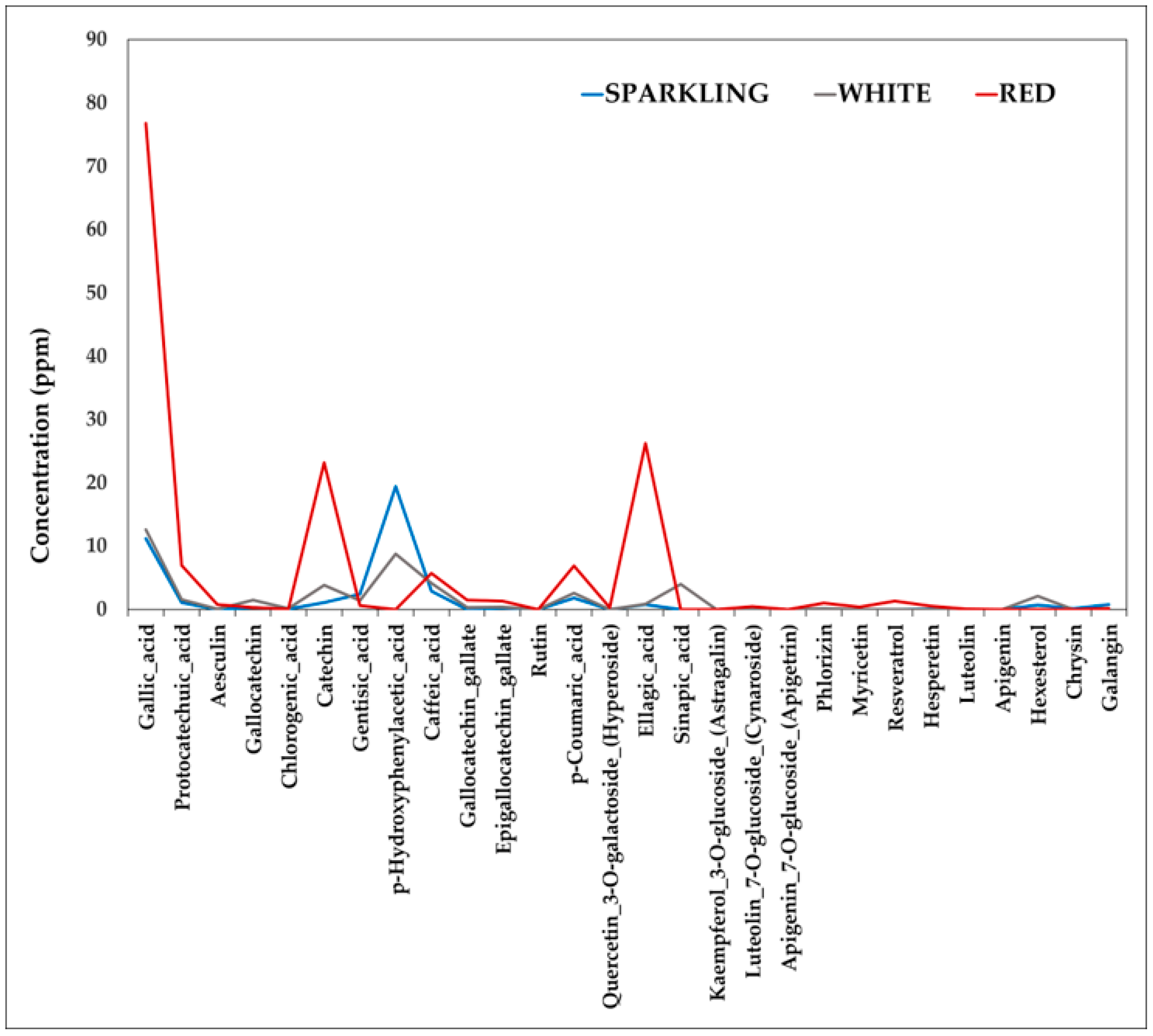
3.2.2. Quantification of Polyphenols
3.3. Identification of Anthocyanins in Red Wine Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations, FAOSTAT Data Crops. 2019. Available online: https://www.fao.org/faostat/en/#home (accessed on 27 November 2020).
- Bora, F.D.; Rîpanu, O.; Donici, A.; Bunea, C.I.; Pop, N.; Lung, M.L.; Popescu, D. Influence of micro-, macroelements and heavy metals on wine quality. Annu. Food Sci. Technol. 2016, 17, 1–10. [Google Scholar]
- Snopek, L.; Mlcek, J.; Sochorova, L.; Baron, M.; Hlavacova, I.; Jurikova, T.; Kizek, R.; Sedlackova, E.; Sochor, J. Contribution of Red Wine Consumption to Human Health Protection. Molecules 2018, 23, 1684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavlidou, E.; Mantzorou, M.; Fasoulas, A.; Tryfonos, C.; Petridis, D.; Giaginis, C. Wine: An Aspiring Agent in Promoting Longevity and Preventing Chronic Diseases. Diseases 2018, 6, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minzer, S.; Estruch, R.; Casas, R. Wine Intake in the Framework of a Mediterranean Diet and Chronic Non-Communicable Diseases: A Short Literature Review of the Last 5 Years. Molecules 2020, 25, 5045. [Google Scholar] [CrossRef] [PubMed]
- Kiviniemi, T.; Saraste, A.; Toikka, J.; Saraste, M.; Raitakari, O.; Pärkkä, J.; Lehtimäki, T.; Hartiala, J.; Viikari, J.; Koskenvuo, J. A moderate dose of red wine, but not de-alcoholized red wine increases coronary flow reserve. Atherosclerosis 2007, 195, 176–181. [Google Scholar] [CrossRef]
- Đorđević, N.; Novaković, M.; Pejin, B.; Živković, M.; Savić, A.; Mutić, J.; Tešević, V. An insight into chemical composition and biological activity of Montenegrin Vranac red wine. Sci. Hortic. 2018, 230, 142–148. [Google Scholar] [CrossRef]
- Panceri, C.P.; Gomes, T.M.; De Gois, J.S.; Borges, D.L.G.; Bordignon-Luiz, M.T. Effect of dehydration process on mineral content, phenolic compounds and antioxidant activity of Cabernet Sauvignon and Merlot grapes. Food Res. Int. 2013, 54, 1343–1350. [Google Scholar] [CrossRef]
- Šuković, D.; Knežević, B.; Gašić, U.; Sredojević, M.; Ćirić, I.; Todić, S.; Mutić, J.; Tešić, Ž. Phenolic Profiles of Leaves, Grapes and Wine of Grapevine Variety Vranac (Vitis vinifera L.) from Montenegro. Foods 2020, 9, 138. [Google Scholar] [CrossRef] [Green Version]
- Castillo-Muñoz, N.; Gómez-Alonso, S.; García-Romero, E.; Hermosín-Gutiérrez, I. Flavonol profiles of Vitis vinifera white grape cultivars. J. Food Compos. Anal. 2010, 23, 699–705. [Google Scholar] [CrossRef]
- Benmezane, F.; Cadot, Y.; Djama, R.; Djermoun, L. Determination of major anthocyanin pigments and flavonols in red grape skin of some table grape varieties (Vitis vinifera sp.) by highperformance liquid chromatography–photodiode array detection (HPLC-DAD). OENO One 2016, 50, 125–135. [Google Scholar] [CrossRef] [Green Version]
- Pohl, P. What do metals tell us about wine? Trends Anal. Chem. 2007, 26, 941–949. [Google Scholar] [CrossRef]
- Fiket, Z.; Mikac, N.; Kniewald, G. Arsenic and other trace elements in wines of eastern Croatia. Food Chem. 2011, 126, 941–947. [Google Scholar] [CrossRef]
- Filippini, T.; Tancredi, S.; Malagoli, C.; Malavolti, M.; Bargellini, A.; Vescovi, L.; Nicolini, F.; Vinceti, M. Dietary Estimated Intake of Trace Elements: Risk Assessment in an Italian Population. Expo. Health 2020, 12, 641–655. [Google Scholar] [CrossRef] [Green Version]
- Caridi, F.; D’agostino, M.; Belvedere, A.; Mottese, A.F. Multi-element analysis and geographical origin classification of italian (Calabrian) wines. Curr. Nutr. Food Sci. 2020, 16, 1259–1264. [Google Scholar] [CrossRef]
- Bronzi, B.; Brilli, C.; Beone, G.M.; Fontanella, M.C.; Ballabio, D.; Todeschini, R.; Consonni, V.; Grisoni, F.; Parri, F.; Buscema, M. Geographical identification of Chianti red wine based on ICP-MS element compositio. Food Chem. 2020, 315, 126248. [Google Scholar] [CrossRef] [PubMed]
- Pepi, S.; Vaccaro, C. Geochemical fingerprints of “Prosecco” wine based on major and trace elements. Environ. Geochem. Health 2018, 40, 833–847. [Google Scholar] [CrossRef]
- Savigrit Azienda. Available online: https://www.sagrivit.it/lazienda/ (accessed on 7 December 2020).
- Đurđić, S.; Pantelić, M.; Trifković, J.; Vukojević, V.; Natić, M.; Tešić, Ž.; Mutić, J. Elemental composition as a tool for the assessment of type, seasonal variability, and geographical origin of wine and its contribution to daily elemental intake. RSC Adv. 2017, 7, 2151–2162. [Google Scholar] [CrossRef] [Green Version]
- Pantelić, M.; Dabić Zagorac, D.; Davidović, S.; Todić, S.; Bešlić, Z.; Gašić, U.; Tešić, Ž.; Natić, M. Identification and quantification of phenolic compounds in berry skin, pulp, and seeds in 13 grapevine varieties grown in Serbia. Food Chem. 2016, 211, 243–252. [Google Scholar] [CrossRef]
- Kment, P.; Mihaljevič, M.; Ettler, V.; Šebek, O.; Strnad, L.; Rohlová, L. Differentiation of Czech wines using multielement composition: A comparison with vineyard soil. Food Chem. 2005, 91, 157–165. [Google Scholar] [CrossRef]
- Iglesias, M.; Besalú, E.; Anticó, E. Internal standardization--atomic spectrometry and geographical pattern recognition techniques for the multielement analysis and classification of Catalonian red wines. J. Agric. Food Chem. 2007, 55, 219–225. [Google Scholar] [CrossRef]
- Coetzee, P.P.; van Jaarsveld, F.P.; Vanhaecke, F. Intraregional classification of wine via ICP-MS elemental fingerprinting. Food Chem. 2014, 164, 485–492. [Google Scholar] [CrossRef]
- Geana, I.; Iordache, A.; Ionete, R.; Marinescu, A.; Ranca, A.; Culea, M. Geographical origin identification of Romanian wines by ICP-MS elemental analysis. Food Chem. 2013, 138, 1125. [Google Scholar] [CrossRef]
- Alkış, İ.M.; Arda Atakol, S.Ö.; Yılmaz, N.; Anlı, R.E.; Atakol, O. Investigation of heavy metal concentrations in some Turkish wines. J. Food Compos. Anal. 2014, 33, 105. [Google Scholar] [CrossRef]
- Vrček, I.V.; Bojić, M.; Žuntar, I.; Mendaš, G.; Medić-Šarić, M. View Correspondence (jump link) Phenol content, antioxidant activity and metal composition of Croatian wines deriving from organically and conventionally grown grapes. Food Chem. 2011, 124, 354–361. [Google Scholar] [CrossRef]
- Lara, R.; Cerutti, S.; Salonia, J.A.; Olsina, R.A.; Martinez, L.D. Trace element determination of Argentine wines using ETAAS and USN-ICP-OES. Food Chem. Toxicol. 2005, 43, 293–297. [Google Scholar] [CrossRef]
- Novikova, L.Y.; Naumova, L.G. Dependence of Fresh Grapes and Wine Taste Scores on the Origin of Varieties and Weather Conditions ofthe Harvest Year in the Northern Zone of Industrial Viticulture in Russia. Agronomy 2020, 10, 1613. [Google Scholar] [CrossRef]
- Leder, R.; Kubanović, V.; Petric, I.V.; Vahčić, N.; Banović, M. Chemometric prediction of the geographical origin of Croatian wines through their elemental profiles. J. Food Nutr. Res. 2015, 54, 229–238. [Google Scholar]
- Souza, A.S.; Fernandes, A.P.; Araujo, R.G.O.; Andrade, R.F.; Vinhas, A.C.A. Screening the geographical origin of Brazilian wines according their elemental composition. Curr. Anal. Chem. 2017, 13, 515–523. [Google Scholar] [CrossRef]
- Canuti, V.; Frost, S.; Lerno, L.A.; Tanabe, C.K.; Zweigenbaum, J.; Zanoni, B.; Ebeler, S.E. Chemical Characteristics of Sangiovese Wines from California and Italy of 2016 Vintage. J. Agric. Food Chem. 2019, 67, 2647–2659. [Google Scholar] [CrossRef] [PubMed]
- Aceto, M.; Gulino, F.; Calà, E.; Robotti, E.; Petrozziello, M.; Tsolakis, C.; Cassino, C. Authentication and traceability study on barbera d’asti and nizza docg wines: The role of trace-and ultra-trace elements. Beverages 2020, 6, 63. [Google Scholar] [CrossRef]
- Malavolti, M.; Fairweather-Tait, S.J.; Malagoli, C.; Vescovi, L.; Vinceti, M.; Filippini, T. Lead exposure in an Italian population: Food content, dietary intake and risk assessment. Food Res. Int. 2020, 137, 109370. [Google Scholar] [CrossRef] [PubMed]
- Pajović Šćepanović, R.; Wendelin, S.; Raičević, D.; Eder, R. Characterization of the phenolic profile of commercial Montenegrin red and white wines. Eur. Food Res. Technol. 2019, 245, 2233–2245. [Google Scholar] [CrossRef]
- Mitrevska, K.; Grigorakis, S.; Loupassaki, S.; Calokerinos, A.C. Antioxidant Activity and Polyphenolic Content of North Macedonian Wines. Appl. Sci. 2020, 10, 2010. [Google Scholar] [CrossRef] [Green Version]
- Tourtoglou, C.; Nenadis, N.; Paraskevopoulou, A. Phenolic composition and radical scavenging activity of commercial Greek white wines from Vitis vinifera L. cv. Malagousia. J. Food Compos. Anal. 2014, 33, 166–174. [Google Scholar] [CrossRef]
- Tuberoso, C.I.G.; Serreli, G.; Congiu, F.; Montoro, P.; Fenu, M.A. Characterization, phenolic profile, nitrogen compounds and antioxidant activity of Carignano wines. J. Food Compos. Anal. 2017, 58, 60–68. [Google Scholar] [CrossRef]
- Raičević, D.; Božinović, Z.; Petkov, M.; Ivanova-Petropulos, V.; Kodžulović, V.; Mugoša, M.; Šućur, S.; Maraš, V. Polyphenolic content and sensory profile of Montenegrin Vranac wines prodused with different oenological products and maceration. Maced. J. Chem. Chem. Eng. 2017, 36, 229–238. [Google Scholar] [CrossRef] [Green Version]
- Ivanova-Petropulos, V.; Ricci, A.; Nedelkovski, D.; Dimovska, V.; Parpinello, G.P.; Versari, A. Targeted analysis of bioactive phenolic compounds and antioxidant activity of Macedonian red wines. Food Chem. 2015, 171, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Iorizzo, M.; Macciola, V.; Testa, B.; Lombardi, S.J.; De Leonardis, A. Physicochemical and sensory characteristics of red wines from the rediscovered autochthonous Tintilia grapevine grown in the Molise region (Italy). Eur. Food Res. Technol. 2014, 238, 1037–1048. [Google Scholar] [CrossRef]
- Pajović Šćepanović, R.; Wendelin, S.; Eder, R. Phenolic composition and varietal discrimination of Montenegrin red wines (Vitis vinifera var. Vranac, Kratošija, and Cabernet Sauvignon). Eur. Food Res. Technol. 2018, 244, 2243–2254. [Google Scholar] [CrossRef]
- Majkić, T.M.; Torović, L.D.; Lesjak, M.M.; Četojević-Siminc, D.D.; Beara, I.N. Activity profiling of Serbian and some other European Merlot wines in inflammation and oxidation processes. Food Res. Int. 2019, 121, 151–160. [Google Scholar] [CrossRef]
- Chen, C. Sinapic Acid and Its Derivatives as Medicine in Oxidative Stress-Induced Diseases and Aging. Oxid. Med. Cell. Longev. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanova-Petropulos, V.; Hermosín-Gutiérrez, I.; Boros, B.; Stefova, M.; Stafilov, T.; Vojnoski, B.; Dörnyei, Á.; Kilár, F. Phenolic compounds and antioxidant activity of Macedonian red wines. J. Food Compos. Anal. 2015, 41, 1–14. [Google Scholar] [CrossRef]
- Duan, J.; Zhan, J.-C.; Wang, G.-Z.; Zhao, X.-C.; Huang, W.-D.; Zhou, G.-B. The red wine component ellagic acid induces autophagy and exhibits anti-lung cancer activity in vitro and in vivo. J. Cell. Mol. Med. 2019, 23, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Minussi, R.C.; Rossi, M.; Bologna, L.; Cordi, L.; Rotilio, D.; Pastore, G.M. Phenolic compounds and total antioxidant potential of commercial wines. Food Chem. 2003, 82, 409–416. [Google Scholar] [CrossRef]
- Mayr, C.M.; De Rosso, M.; Vedova, A.D.; Flamini, R. High-Resolution Mass Spectrometry Identification of Secondary Metabolites in Four Red Grape Varieties Potentially Useful as Traceability Markers of Wines. Beverages 2018, 4, 74. [Google Scholar] [CrossRef] [Green Version]
- Blanco-Vega, D.; López-Bellido, F.J.; Alía-Robledo, J.M.; Hermosín-Gutiérrez, I. HPLC-DAD-ESI-MS/MS characterization of pyranoanthocyanins pigments formed in model wine. J. Agric. Food Chem. 2011, 59, 9523–9531. [Google Scholar] [CrossRef] [PubMed]
- Fulcrand, H.; Cameira Dos Santos, P.-J.; Sarni-Manchado, P.; Cheynier, V.; Favre-Bonvin, J. Structure of new anthocyanin-derived wine pigments. J. Chem. Soc.-Perkin Trans. 1 1996, 7, 735–739. [Google Scholar] [CrossRef]





| Sample Sign | Wine Sample | Type of Wine | Harvested Year | Botanical Origin | Geographical Region | EtOH Vol (%) a |
|---|---|---|---|---|---|---|
| W1 | Asolo Preosecco Brut | white sparkling wine | 2014 | Prosseco | Veneto | 11.5 |
| W2 | Asolo Preosecco Extra Dry L6015 | white sparkling wine | 2014 | Prosseco | Veneto | 11.5 |
| W3 | Asolo Preosecco Extra Dry L2115 | white sparkling wine | 2014 | Prosseco | Veneto | 11.5 |
| W4 | Belfiore | rosè sparkling wine | 2014 | Gamay | Umbria | 12.0 |
| W5 | Grechetto Montenerone | white | 2013 | Grechetto | Umbria | 13.5 |
| W6 | Grechetto Montenerone | white | 2014 | Grechetto | Umbria | 13.5 |
| W7 | Merlot | red | 2013 | Merlot | Friuli | 14.0 |
| W8 | Morcinaia | red | 2008 | Merlot, Cabernet Sauvignon, Sangiovese | Umbria | 14.0 |
| W9 | Pinot Grigio | white | 2013 | Pinot Grigio | Friuli | 14.0 |
| W10 | Pinot Grigio | white | 2014 | Pinot Grigio | Friuli | 14.0 |
| W11 | Ribolla Gialla | white | 2013 | Ribolla Gialla | Friuli | 12.5 |
| W12 | Ribolla Gialla | white | 2014 | Ribolla Gialla | Friuli | 12.5 |
| W13 | Sangiovese | red | 2014 | Sangiovese | Umbria | 13.0 |
| W1 | W2 | W3 | W4 | W5 | W6 | W7 | W8 | W9 | W10 | W11 | W12 | W13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| As | 0.20 | 0.20 | 19.84 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
| Ba | 32.64 | 33.87 | 26.12 | 123.30 | 79.80 | 79.10 | 83.33 | 239.70 | 58.16 | 77.70 | 32.07 | 31.66 | 122.10 |
| Cd | 0.37 | 1.55 | 0.94 | 1.25 | 0.39 | 1.37 | 0.73 | 0.05 | 0.98 | 1.64 | 0.01 | 0.59 | 0.38 |
| Co | 3.64 | 2.09 | 0.98 | 4.40 | 2.77 | 1.83 | 2.42 | 2.68 | 7.34 | 7.77 | 2.19 | 1.61 | 2.60 |
| Cr | 0.32 | 0.32 | 0.32 | 0.32 | 4.32 | 3.03 | 0.32 | 6.42 | 15.41 | 14.82 | 11.12 | 9.16 | 3.11 |
| Pb | 0.24 | 55.97 | 0.24 | 20.79 | 79.97 | 11.68 | 2.24 | 175.04 | 45.22 | 0.24 | 0.83 | 0.54 | 14.90 |
| Sb | 32.81 | 8.59 | 19.13 | 18.57 | 67.54 | 37.91 | 9.58 | 46.50 | 44.88 | 18.50 | 7.26 | 44.26 | 29.60 |
| Se | 0.14 | 0.14 | 0.14 | 0.14 | 1.45 | 0.30 | 0.23 | 25.74 | 2.87 | 2.06 | 2.91 | 2.38 | 2.99 |
| Ni | 4.17 | 46.01 | 0.57 | 30.95 | 15.34 | 5.77 | 35.15 | 13.86 | 50.57 | 17.95 | 10.00 | 0.20 | 5.00 |
| Al | 565.70 | 260.10 | 155.30 | 719.80 | 446.90 | 290.70 | 26.55 | 155.40 | 443.60 | 329.00 | 1027.00 | 567.70 | 236.20 |
| Cu | 97.46 | 469.49 | 36.30 | 10.07 | 0.11 | 0.11 | 0.11 | 0.11 | 21.19 | 7.73 | 0.81 | 49.32 | 50.21 |
| Mn | 735.00 | 613.49 | 642.26 | 1016.26 | 761.67 | 742.74 | 838.77 | 1633.76 | 1732.28 | 1472.22 | 887.46 | 771.02 | 1299.22 |
| V | 4.18 | 1.89 | 0.57 | 0.17 | 1.53 | 0.17 | 1.95 | 3.18 | 3.24 | 2.30 | 0.81 | 0.73 | 0.17 |
| Zn | 468.40 | 1359.00 | 423.80 | 622.20 | 1356.00 | 607.50 | 298.20 | 969.60 | 1278.00 | 915.60 | 518.60 | 536.90 | 897.50 |
| Ca a | 70.73 | 76.93 | 71.71 | 111.20 | 62.51 | 83.94 | 50.73 | 67.51 | 62.77 | 98.10 | 76.45 | 99.86 | 71.11 |
| Fe a | 0.13 | 0.08 | 0.05 | 0.79 | 0.53 | 0.52 | 0.59 | 1.08 | 1.81 | 0.69 | 0.37 | 0.20 | 0.87 |
| K a | 488.70 | 706.80 | 662.70 | 1152.00 | 608.70 | 718.70 | 965.20 | 1119.00 | 567.30 | 867.40 | 700.30 | 1174.00 | 1111.00 |
| Mg a | 55.29 | 52.87 | 52.44 | 77.39 | 70.30 | 71.04 | 82.11 | 102.00 | 64.03 | 68.38 | 69.10 | 60.38 | 94.27 |
| Na a | 7.75 | 14.13 | 9.32 | 38.24 | 29.61 | 27.41 | 11.69 | 16.88 | 21.60 | 26.49 | 9.36 | 17.62 | 22.08 |
| Rb a | 2.69 | 2.96 | 3.08 | 3.83 | 3.90 | 3.85 | 5.07 | 7.38 | 4.33 | 3.96 | 4.08 | 3.59 | 5.63 |
| Non-Anthocyanins (mg/L) | W1 | W2 | W3 | W4 | W5 | W6 | W7 | W8 | W9 | W10 | W11 | W12 | W13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hydroxybenzoic acids | |||||||||||||
| Gallic acid | 1.99 | 1.09 | − | 41.73 | 38.20 | 24.22 | 74.36 | 81.35 | 4.40 | 2.53 | 2.82 | 3.24 | 74.62 |
| Protocatechuic acid | 0.53 | 0.57 | 0.57 | 2.63 | 1.33 | 1.78 | 7.27 | 5.41 | 1.70 | 1.41 | 1.57 | 1.30 | 8.23 |
| Gentisic acid | 1.45 | 3.24 | 3.36 | 1.52 | 2.90 | 1.47 | 1.01 | 0.87 | 1.47 | 0.49 | 0.71 | 1.26 | − |
| Ellagic acid | 0.71 | 0.51 | 0.65 | 1.17 | 0.81 | 1.01 | 25.24 | 24.41 | 1.36 | 0.74 | 0.49 | 0.61 | 29.02 |
| Hydroxicinnamic acids | |||||||||||||
| Sinapic acid | − | − | − | − | − | − | − | − | − | − | − | 23.67 | − |
| Caffeic acid | 0.59 | 0.67 | 1.36 | 8.79 | 9.16 | 5.47 | 4.58 | 7.18 | 5.78 | 2.58 | 1.01 | 0.95 | 5.44 |
| Chlorogenic acid | 0.04 | 0.03 | 0.04 | 0.04 | 0.07 | 0.10 | − | 0.07 | 0.03 | 0.19 | 0.12 | 0.10 | 0.20 |
| p-Coumaric acid | 1.12 | 2.03 | 1.46 | 2.64 | 3.02 | 2.99 | 10.34 | 4.63 | 1.80 | 1.65 | 1.60 | 4.30 | 5.61 |
| Flavanols | |||||||||||||
| Gallocatechin | − | − | − | 1.34 | 3.29 | 5.37 | − | − | − | − | − | − | 0.93 |
| Epigallocatechin gallate | − | − | 0.86 | − | − | − | 1.02 | 1.63 | − | 0.91 | − | 1.48 | 1.31 |
| Catechin | − | − | − | 4.23 | 5.95 | 10.40 | 25.93 | 16.98 | 3.63 | − | 1.49 | 1.69 | 26.67 |
| Gallocatechin gallate | − | − | − | − | 0.52 | − | 2.48 | − | − | − | 1.10 | 0.35 | 1.94 |
| Flavonols | |||||||||||||
| Myricetin | − | − | − | − | − | − | 0.39 | 0.41 | 0.02 | − | − | − | 0.28 |
| Rutin | − | − | − | 0.02 | − | 0.01 | − | − | − | 0.03 | − | − | 0.02 |
| Astragalin | − | − | − | 0.01 | − | − | − | 0.01 | − | 0.01 | − | − | − |
| Hyperoside | − | − | − | − | − | 0.01 | 0.20 | 0.26 | − | − | − | 0.01 | 0.47 |
| Galangin | 1.21 | 0.88 | 0.60 | 0.46 | 0.36 | 0.20 | 0.24 | 0.19 | 0.18 | − | − | − | − |
| Flavones | |||||||||||||
| Chrysin | 0.60 | − | − | − | − | − | − | − | − | − | − | − | − |
| Luteolin | − | − | − | − | − | − | − | 0.10 | − | − | − | − | − |
| Cynaroside | − | − | − | 0.67 | − | − | 0.48 | 0.53 | 0.50 | 0.57 | − | − | 0.35 |
| Apigetrin | − | − | − | 0.01 | − | − | 0.01 | 0.01 | 0.01 | 0.01 | − | − | 0.01 |
| Apigenin | 0.06 | − | 0.03 | − | − | − | − | − | − | − | − | − | − |
| Hydroxycoumarins | |||||||||||||
| Aesculin | − | − | 0.05 | − | − | − | 0.65 | 0.69 | − | − | − | 0.44 | 0.87 |
| Stilbenes | |||||||||||||
| Resveratrol | − | − | − | − | − | − | 4.00 | − | − | − | − | − | − |
| Flavanones | |||||||||||||
| Hesperetin | − | − | 0.09 | 0.05 | − | − | 0.71 | 0.44 | − | 0.10 | − | − | 0.41 |
| Dihydrochalcones | |||||||||||||
| Phlorizin | − | − | − | 0.08 | 0.13 | 0.18 | 1.03 | 0.98 | 0.13 | 0.06 | 0.05 | 0.07 | 0.98 |
| Anthocyanins (mg/L) | |||||||||||||
| Myrtillin | − | − | − | 0.02 | − | − | 0.47 | 0.05 | − | − | − | − | 0.57 |
| Malvin | − | − | − | 0.01 | − | − | 0.04 | − | − | − | − | − | 0.06 |
| Cyanidin 3-O-(2″-xylosyl)glucoside | − | − | − | − | − | − | 0.04 | 0.02 | − | − | − | − | 0.04 |
| Chrysanthemin | − | − | − | − | − | − | 0.15 | 0.02 | − | − | − | − | 0.28 |
| Peonidin 3-O-glucoside | − | − | − | 0.09 | − | − | 0.61 | 0.06 | − | − | − | − | 0.69 |
| Oenin | − | − | − | 0.55 | − | − | 3.43 | 0.64 | − | − | − | − | 3.86 |
| RSA (mmol TE/L) | 0.39 | 0.44 | 0.42 | 1.97 | 3.17 | 2.68 | 16.09 | 18.71 | 1.86 | 1.64 | 1.26 | 0.82 | 12.03 |
| TPC (g GAE/L) | 1.15 | 1.44 | 1.41 | 1.44 | 1.51 | 1.67 | 2.24 | 2.36 | 1.35 | 1.31 | 0.81 | 1.17 | 1.71 |
| Peak No. | Anthocyanins | Molecular Formula, M+ (m/z) | Calculated Mass, M+ (m/z) | Exact Mass, M+ (m/z) | Δ ppm | W4 | W7 | W8 | W13 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Delphinidin 3-O-glucoside (Myrtillin) a,b | C21H21O12+ | 465.10275 | 465.10123 | 3.27 | + | + | + | + |
| 2 | Malvidin 3,5-di-O-glucoside (Malvin) a,b | C29H35O17+ | 655.18688 | 655.18469 | 3.34 | + | + | + | + |
| 3 | Cyanidin 3-O-(2″-xylosyl)glucoside a,b | C26H29O15+ | 581.15010 | 581.14911 | 1.70 | − | + | + | + |
| 4 | Cyanidin 3-O-glucoside (Chrysanthemin) a,b | C21H21O11+ | 449.10784 | 449.10684 | 2.23 | − | + | + | + |
| 5 | Delphinidin 3-O-glucoside-pyruvate d | C24H21O14+ | 533.09258 | 533.09176 | 1.54 | − | + | + | + |
| 6 | Petunidin 3-O-glucoside isomer 1 b | C22H23O12+ | 479.11840 | 479.11710 | 2.71 | + | + | + | + |
| 7 | Petunidin 3-O-glucoside-acetaldehyde d | C24H23O12+ | 503.11840 | 503.11703 | 2.72 | − | + | − | + |
| 8 | Peonidin 3-O-glucoside a,b | C22H23O11+ | 463.12349 | 463.12231 | 2.55 | + | + | + | + |
| 9 | Malvidin 3-O-glucoside (Oenin) a,b | C23H25O12+ | 493.13405 | 493.13300 | 2.13 | + | + | + | + |
| 10 | Delphinidin 3-O-(6″-acetyl)glucoside b | C23H23O13+ | 507.11332 | 507.11215 | 2.31 | + | + | + | + |
| 11 | Malvidin 3-O-glucoside-acetaldehyde d | C25H25O12+ | 517.13405 | 517.13254 | 2.92 | − | + | − | + |
| 12 | Peonidin 3-O-glucoside-pyruvate d | C25H25O13+ | 531.11332 | 531.11145 | 3.52 | + | + | + | + |
| 13 | Malvidin 3-O-glucoside-pyruvate d | C26H25O14+ | 561.12388 | 561.12256 | 2.35 | + | + | + | + |
| 14 | Petunidin 3-O-(6″-acetyl)glucoside b | C24H25O13+ | 521.12897 | 521.12732 | 3.17 | + | + | + | + |
| 15 | Malvidin 3-O-glucoside-acetone d | C26H27O12+ | 531.14970 | 531.14862 | 2.03 | − | + | + | + |
| 16 | Malvidin 3-O-(6″-acetyl)glucoside-acetaldehyde d | C27H27O13+ | 559.14462 | 559.14362 | 1.79 | + | + | + | + |
| 17 | Malvidin 3-O-glucoside-8-ethyl-(epi)catechin d | C40H41O18+ | 809.22874 | 809.22797 | 0.95 | − | + | + | + |
| 18 | Malvidin 3-O-(6″-acetyl)glucoside-pyruvate d | C28H27O15+ | 603.13445 | 603.13293 | 2.52 | − | + | + | + |
| 19 | Peonidin 3-O-(6″-acetyl)glucoside b | C24H25O12+ | 505.13405 | 505.13358 | 0.93 | + | + | + | + |
| 20 | Malvidin 3-O-(6″-acetyl)glucoside b | C25H27O13+ | 535.14462 | 535.14325 | 2.56 | + | + | + | + |
| 21 | Delphinidin 3-O-(6″-p-coumaroyl)glucoside b | C30H27O14+ | 611.13953 | 611.13831 | 2.00 | + | + | + | + |
| 22 | Petunidin 3-O-(6″-p-coumaroyl)glucoside-8-ethyl-(epi)catechin d | C48H45O20+ | 941.24987 | 941.24832 | 1.65 | − | + | − | + |
| 23 | Malvidin 3-O-glucoside-4-vinyl-(epi)catechin d | C40H37O18+ | 805.19744 | 805.19623 | 1.50 | − | + | + | + |
| 24 | Malvidin 3-O-(6″-p-coumaroyl)glucoside-acetaldehyde d | C34H31O14+ | 663.17083 | 663.16943 | 2.11 | − | + | − | + |
| 25 | Cyanidin 3-O-(6″-p-coumaroyl)glucoside b | C30H27O13+ | 595.14462 | 595.14347 | 1.93 | + | + | + | + |
| 26 | Malvidin 3-O-(6″-p-coumaroyl)glucoside-8-ethyl-(epi)catechin d | C49H47O20+ | 955.26552 | 955.26459 | 0.97 | − | + | + | + |
| 27 | Delphinidin 3-O-glucuronide b | C21H19O13+ | 479.08202 | 479.08092 | 2.30 | + | + | + | + |
| 28 | Malvidin 3-O-(6″-p-coumaroyl)glucoside-pyruvate d | C35H31O16+ | 707.16066 | 707.15997 | 0.98 | − | + | + | + |
| 29 | Malvidin 3-O-glucoside-4-vinylcatechol d | C31H29O14+ | 625.15518 | 625.15369 | 2.38 | + | + | + | + |
| 30 | Malvidin 3-O-(6″-p-coumaroyl)glucoside b | C32H31O14+ | 639.17083 | 639.17004 | 1.24 | − | + | + | + |
| 31 | Peonidin 3-O-(6″-p-coumaroyl)glucoside b | C31H29O13+ | 609.16027 | 609.15955 | 1.18 | − | + | − | + |
| 32 | Peonidin 3-O-glucoside-4-vinylphenol d | C30H27O12+ | 579.14970 | 579.14868 | 1.76 | + | + | + | + |
| 33 | Malvidin 3-O-glucoside-4-vinylphenol d | C31H29O13+ | 609.16027 | 609.15857 | 2.79 | + | + | + | + |
| 34 | Petunidin 3-O-glucoside isomer 2 b | C22H23O12+ | 479.11840 | 479.11731 | 2.28 | + | − | − | − |
| 35 | Malvidin 3-O-glucoside-4-vinylguaiacol d | C32H31O14+ | 639.17083 | 639.17004 | 1.24 | + | + | + | + |
| 36 | Malvidin 3-O-glucoside-pyranone d | C25H25O13+ | 533.12897 | 533.12817 | 1.50 | − | + | + | + |
| 37 | Malvidin 3-O-(6″-acetyl)glucoside-4-vinylphenol d | C33H31O14+ | 651.17083 | 651.17041 | 0.64 | + | + | + | + |
| 38 | Malvidin 3-O-(6″-p-coumaroyl)glucoside-4-vinylcatechol d | C40H35O16+ | 771.19196 | 771.19006 | 2.46 | + | + | + | + |
| 39 | Malvidin-pyruvate d | C20H15O9+ | 399.07106 | 399.06982 | 3.11 | + | + | + | + |
| 40 | Malvidin 3-O-(6″-p-coumaroyl)glucoside-4-vinylphenol d | C40H35O15+ | 755.19705 | 755.19647 | 0.77 | + | + | + | + |
| 41 | Delphinidin c | C15H11O7+ | 303.04993 | 303.04916 | 2.54 | + | + | + | + |
| 42 | Petunidin c | C16H13O7+ | 317.06558 | 317.06418 | 4.42 | − | + | + | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fermo, P.; Comite, V.; Sredojević, M.; Ćirić, I.; Gašić, U.; Mutić, J.; Baošić, R.; Tešić, Ž. Elemental Analysis and Phenolic Profiles of Selected Italian Wines. Foods 2021, 10, 158. https://doi.org/10.3390/foods10010158
Fermo P, Comite V, Sredojević M, Ćirić I, Gašić U, Mutić J, Baošić R, Tešić Ž. Elemental Analysis and Phenolic Profiles of Selected Italian Wines. Foods. 2021; 10(1):158. https://doi.org/10.3390/foods10010158
Chicago/Turabian StyleFermo, Paola, Valeria Comite, Milica Sredojević, Ivanka Ćirić, Uroš Gašić, Jelena Mutić, Rada Baošić, and Živoslav Tešić. 2021. "Elemental Analysis and Phenolic Profiles of Selected Italian Wines" Foods 10, no. 1: 158. https://doi.org/10.3390/foods10010158