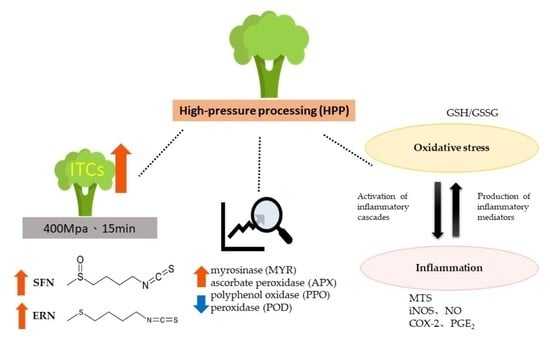
Evaluating the Anti-Inflammatory and Antioxidant Effects of Broccoli Treated with High Hydrostatic Pressure in Cell Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials/Processing
2.2. Extraction and High Performance Liquid Chromatography (HPLC) Analysis of Isothiocyanates
2.2.1. Preparation of the Extract
2.2.2. HPLC
2.3. Chemical Characterization
2.3.1. Polyphenol Determination
2.3.2. Flavonoid Determination
2.3.3. Vitamin C Determination
2.4. Enzyme Activity Assays
2.4.1. Myrosinase Activity
2.4.2. Ascorbate Peroxidase (APX) Activity
2.4.3. Peroxidase (POD) and Polyphenol Oxidase (PPO) Activities
2.5. Cell Culture
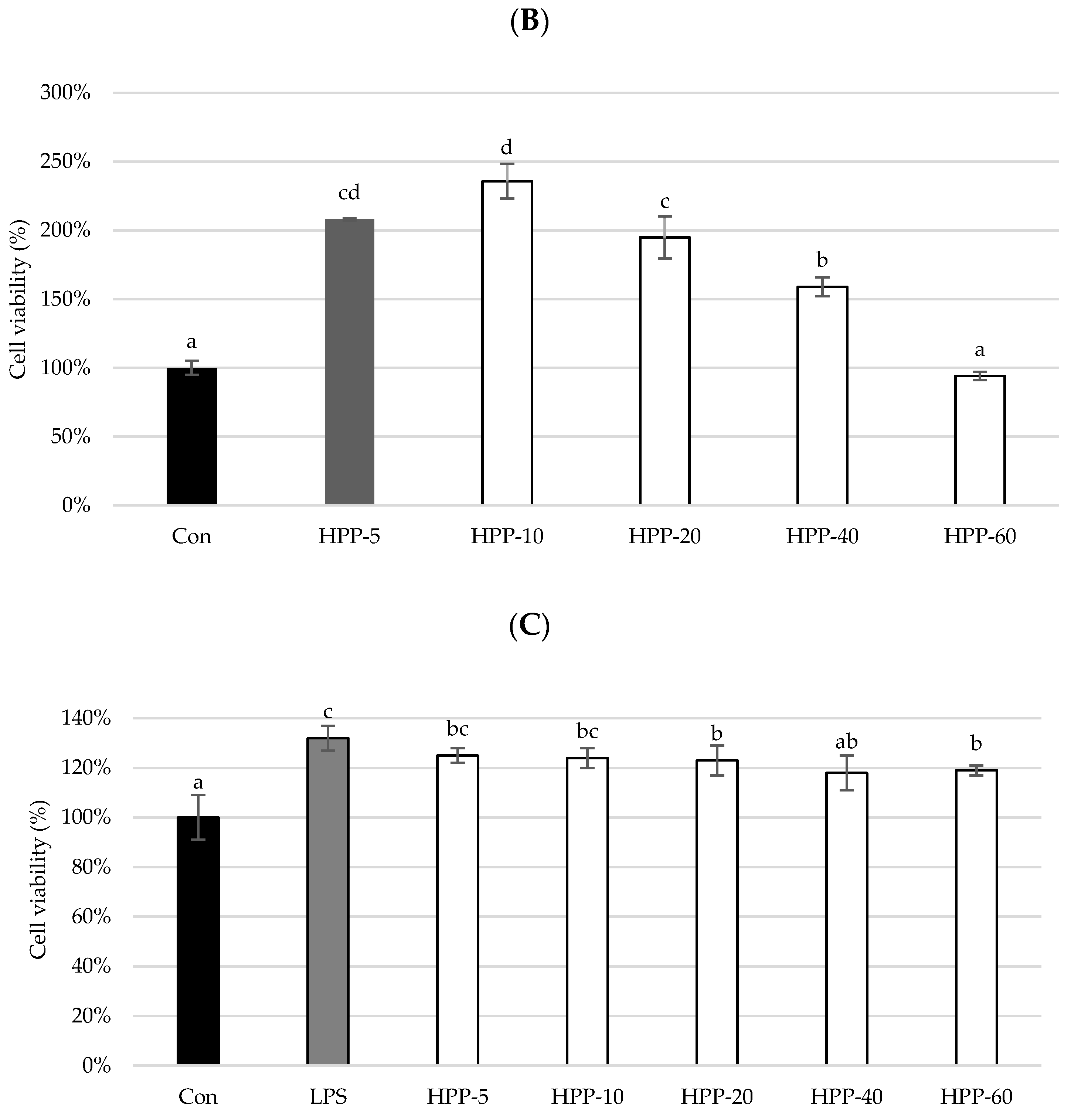
2.6. Cell Viability Assay
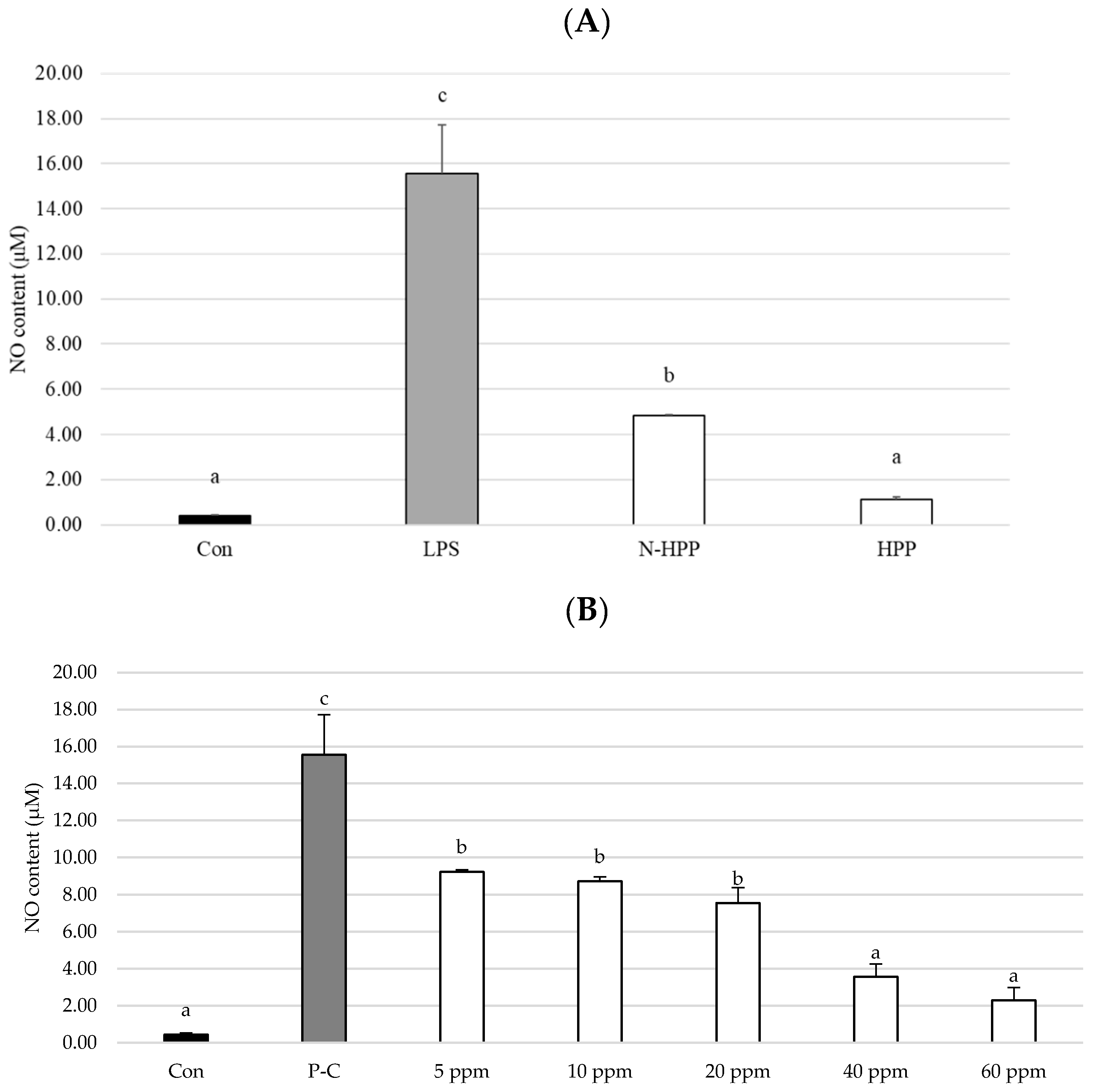
2.7. Measurement of Nitric Oxide (NO) Production
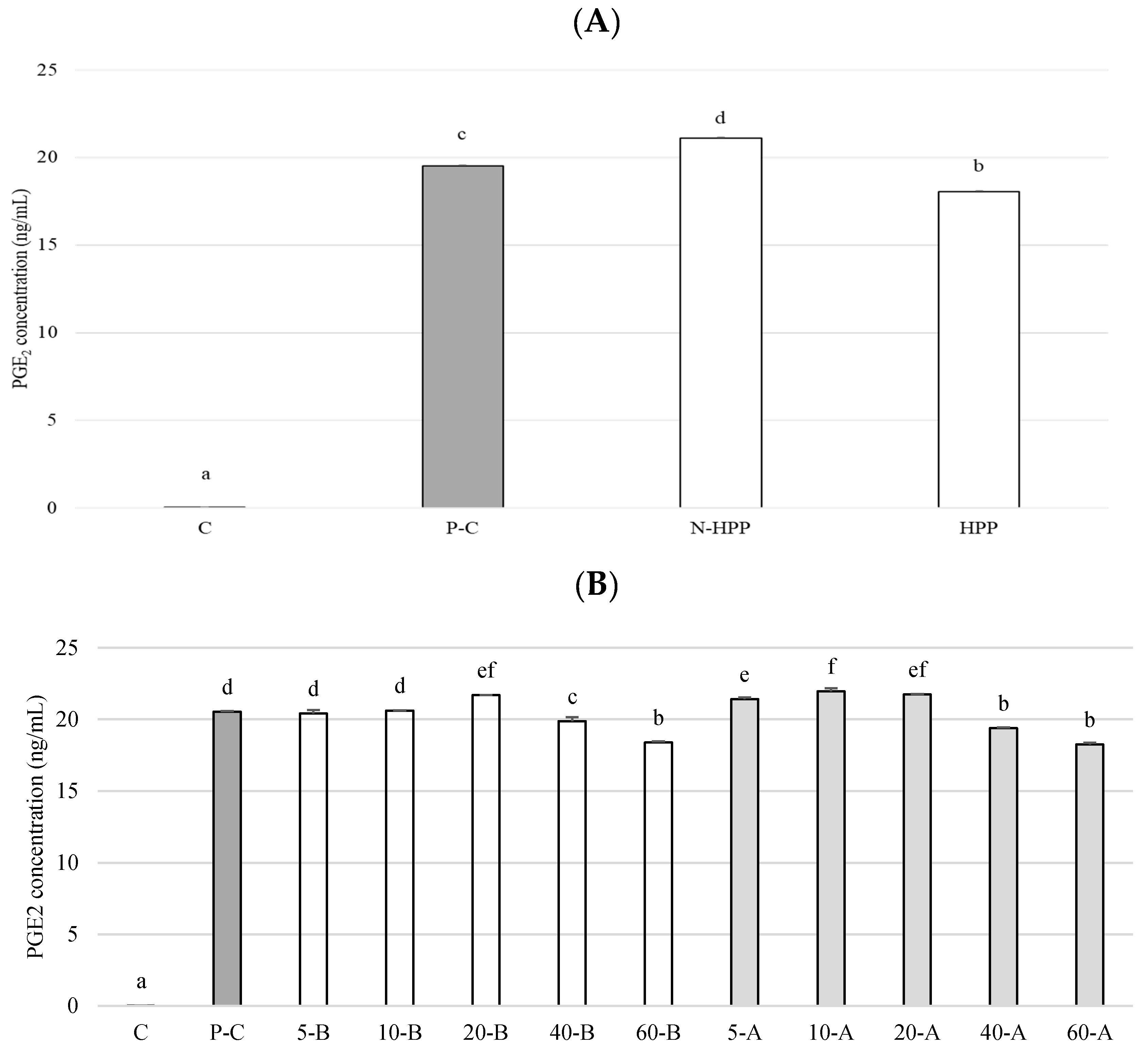
2.8. Measurement of PGE2 Content
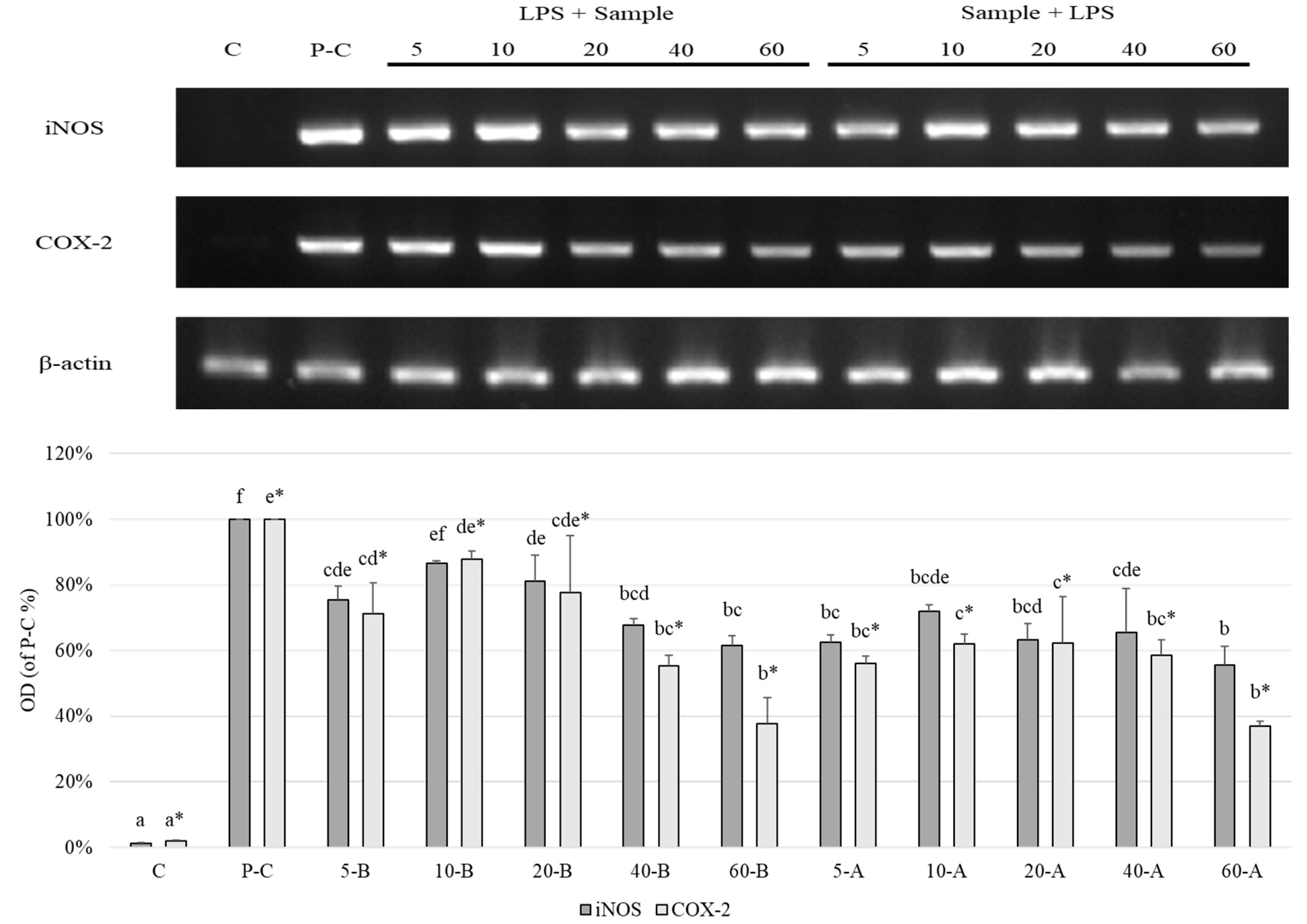
2.9. Expression Levels of iNOS and COX-2
2.9.1. Total Cellular RNA Extraction
2.9.2. RT-PCR Analysis
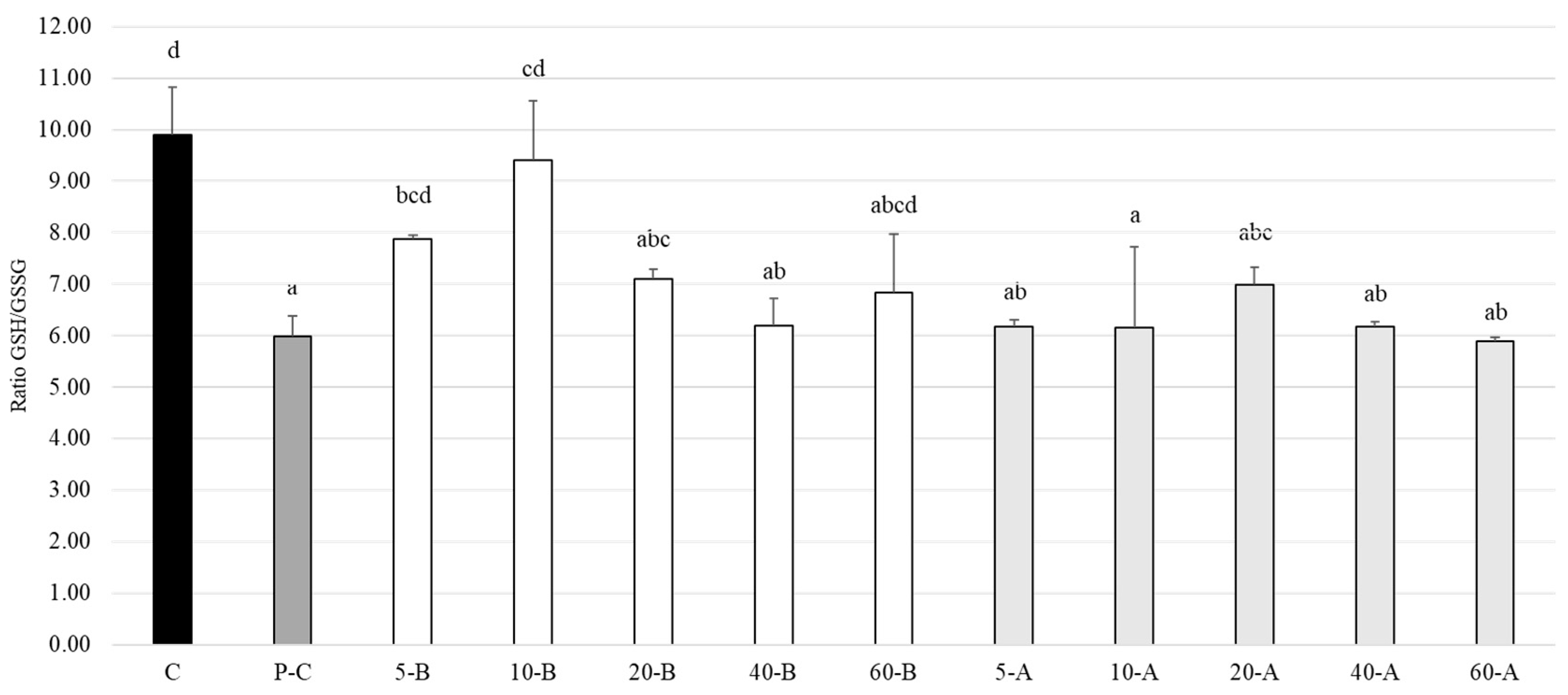
2.9.3. GSH/GSSG Ratio
2.9.4. Statistical Analyses
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lafarga, T.; Bobo, G.; Viñas, I.; Collazo, C.; Aguiló-Aguayo, I. Effects of thermal and non-thermal processing of cruciferous vegetables on glucosinolates and its derived forms. J. Food Sci. Technol. 2018, 5, 1973–1981. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.S. Construction and Transformation of Sulforaphane Biosynthetic Related Genes of Broccoli. Master’s Thesis, National Chung Hsing University, Taichung, Taiwan, 2017. [Google Scholar]
- Saw, C.L.; Huang, M.T.; Liu, Y.; Khor, T.O.; Conney, A.H.; Kong, A.N. Impact of Nrf2 on UVB-induced skin inflammation/photoprotection and photoprotective effect of sulforaphane. Mol. Carcinogen. 2010, 50, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Piao, C.S.; Gao, S.; Lee, G.H.; Kim, D.S.; Park, B.H.; Chae, S.W.; Chae, H.J.; Kim, S.H. Sulforaphane protects ischemic injury of hearts through antioxidant pathway and mitochondrial KATP channels. Pharmacol. Res. 2010, 61, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Westphal, A.; Riedl, K.M.; Cooperstone, J.L.; Kamat, S.; Balasubramaniam, V.M.; Schwartz, S.J.; Böhm, V. High-pressure processing of broccoli sprouts: Influence on bioactivation of glucosinolates to isothiocyanates. J. Agric. Food. Chem. 2017, 65, 8578–8585. [Google Scholar] [CrossRef]
- Shirakawa, M.; Ueda, H.; Shimada, T.; Hara-Nishimura, I. Myrosin cells are differentiated directly from ground meristem cells and are developmentally independent of the vasculature in Arabidopsis leaves. Plant Signal. Behav. 2016, 11, e1150403. [Google Scholar] [CrossRef]
- Halkier, B.A.; Gershenzon, J. Biology and biochemistry of glucosinolates. Annu. Rev. Plant Biol. 2006, 57, 303–333. [Google Scholar] [CrossRef]
- Eylen, D.V.; Oey, I.; Hendrickx, M.; Loey, A.V. Effects of pressure/temperature treatments on stability and activity of endogenous broccoli (Brassica oleracea L. cv. Italica) myrosinase and on cell permeability. J. Food Eng. 2008, 89, 178–186. [Google Scholar]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Tung, Y.T.; Chua, M.T.; Wang, S.Y.; Chang, S.T. Anti-inflammation activities of essential oil and its constituents from indigenous cinnamon (Cinnamomum osmophloeum) twigs. Bioresour. Technol. 2008, 99, 3908–3913. [Google Scholar] [CrossRef]
- Baluk, P.; Yao, L.C.; Feng, J.; Romano, T.; Jung, S.S.; Schreiter, J.L.; Yan, L.; Shealy, D.J.; McDonald, D.M. TNF-α drives remodeling of blood vessels and lymphatics in sustained airway inflammation in mice. J. Clin. Investig. 2009, 119, 2954–2964. [Google Scholar] [CrossRef]
- Hwang, J.H.; Lim, S.B. Antioxidant and anti-inflammatory activities of broccoli florets in LPS-stimulated RAW264.7 cells. Prev. Nutr. Food Sci. 2014, 19, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Rakariyatham, K.; Wu, X.; Tang, Z.; Han, Y.; Wang, Q.; Xiao, H. Synergism between luteolin and sulforaphane in anti-inflammation. Food Funct. 2018, 9, 5115–5123. [Google Scholar] [CrossRef] [PubMed]
- Muntean, M.V.; Marian, O.; Barbieru, V.; Cătunescu, G.M.; Ranta, O.; Drocas, I.; Terhes, S. High pressure processing in food industry-characteristics and applications. Agric. Agric. Sci. Procedia 2016, 10, 377–383. [Google Scholar] [CrossRef]
- Maitland, J.E.; Boyer, R.R.; Eifert, J.D.; Williams, R.C. High hydrostatic pressure processing reduces Salmonella enterica serovars in diced and whole tomatoes. Int. J. Food Microbiol. 2011, 149, 113–117. [Google Scholar] [CrossRef]
- Rodríguez-Roque, M.J.; de Ancos, B.; Sánchez-Moreno, C.; Cano, M.P.; Elez-Martínez, P.; Martín-Belloso, O. Impact of food matrix and processing on the in vitro bioaccessibility of vitamin C, phenolic compounds, and hydrophilic antioxidant activity from fruit juice-based beverages. J. Funct. Foods 2015, 14, 33–43. [Google Scholar] [CrossRef]
- Van Eylen, D.; Bellostas, N.; Strobel, B.W.; Oey, I.; Hendrickx, M.; Van Loey, A.; Sørensen, H.; Sørensen, J.C. Influence of pressure/temperature treatments on glucosinolate conversion in broccoli (Brassica oleraceae L. cv Italica) heads. Food Chem. 2009, 112, 646–653. [Google Scholar] [CrossRef]
- Koo, S.Y.; Cha, K.H.; Song, D.G.; Chung, D.; Pan, C.H. Amplification of sulforaphane content in red cabbage by pressure and temperature treatments. J. Korean Soc. Appl. Biol. Chem. 2011, 54, 183–187. [Google Scholar] [CrossRef]
- Alvarez-Jubete, L.; Valverde, J.; Patras, A.; Mullen, A.M.; Marcos, B. Assessing the impact of high-pressure processing on selected physical and biochemical attributes of white cabbage (Brassica oleracea L. var. capitata alba). Food Bioprocess Technol. 2014, 7, 682–692. [Google Scholar] [CrossRef]
- You, Y.; Wu, Y.; Mao, J.; Zou, L.; Liu, S. Screening of Chinese brassica species for anti-cancer sulforaphane and erucin. Afr. J. Biotechnol. 2008, 7, 147–152. [Google Scholar]
- Yu, G.; Bei, J.; Zhao, J.; Li, Q.; Cheng, C. Modification of carrot (Daucus carota Linn. var. Sativa Hoffm.) pomace insoluble dietary fiber with complex enzyme method, ultrafine comminution, and high hydrostatic pressure. Food Chem. 2018, 257, 333–340. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Yuan, G.F.; Wang, X.P.; Guo, R.F.; Wang, Q.M. Effect of salt stress on phenolic compounds, glucosinolates, myrosinase and antioxidant activity in radish sprouts. Food Chem. 2010, 121, 1014–1019. [Google Scholar] [CrossRef]
- Li, J.; Wang, Q.Z.; Zhou, Q.; Sun, Y.; Xie, B.J. Study on properties of myrosinase from radish. Food Sci. 2008, 29, 451–455. [Google Scholar]
- Zhao, K.; Xue, P.J.; Gu, G.Y. Study on determination of reducing sugar content using 3,5-dinitrosalicylic acid method. Food Sci. 2008, 29, 534–536. [Google Scholar]
- Chen, K.M.; Liu, C.P. Effects of pretreating calcium on Acacia confusa seedlings under simulated acid rain stress. Q. J. For. Res. 2012, 34, 237–250. [Google Scholar]
- Yang, H.A. Effect of High Pressure Processing on Functional Ingredient and Quality of Heat-Sensitive Juice. Master’s Thesis, National Taiwan University, Taipei, Taiwan, 2016. [Google Scholar]
- Rungapamestry, V.; Duncan, A.J.; Fuller, Z.; Ratcliffe, B. Effect of cooking brassica vegetables on the subsequent hydrolysis and metabolic fate of glucosinolates. Proc. Nutr. Soc. 2007, 66, 69–81. [Google Scholar] [CrossRef]
- Jones, R.B.; Frisina, C.L.; Winkler, S.; Imsic, M.; Tomkins, R.B. Cooking method significantly effects glucosinolate content and sulforaphane production in broccoli florets. Food Chem. 2010, 123, 237–242. [Google Scholar] [CrossRef]
- Vinicio, S.M.; Daniel, A.J.V.; Jose, A.T.; Jorge, W.C. Microstructural and physiological changes in plant cell induced by pressure: Their role on the availability and pressure-temperature stability of phytochemicals. Food Eng. Rev. 2017, 9, 314–334. [Google Scholar]
- Acosta-Estrada BA, Gutiérrez-Uribe JA, Serna-Saldívar SO (2014) Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55.
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef]
- Liu, X.W.; Bourvellec, C.L.; Renard, C.M.G.C. Interactions between cell wall polysaccharides and polyphenols: Effect of molecular internal structure. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3574–3617. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.N.; Yang, E.; Yi, C.; Zhao, M.; Jiang, Y. Effects of high pressure extraction on the extraction yield, total phenolic content and antioxidant activity of longan fruit pericarp. Innov. Food Sci. Emerg. Technol. 2009, 10, 155–159. [Google Scholar] [CrossRef]
- Landl, A.; Abadias, M.; Sárraga, C.; Viñas, I.; Picouet, P.A. Effect of high pressure processing on the quality of acidified Granny Smith apple purée product. Innov. Food Sci. Emerg. Technol. 2010, 11, 557–564. [Google Scholar] [CrossRef]
- Tola, Y.B.; Ramaswamy, H.S. Temperature and high pressure stability of lycopene and vitamin C of watermelon juice. Afr. J. Food Sci. 2015, 9, 351–358. [Google Scholar]
- Wang, J.; Barba, F.J.; Frandsen, H.B.; Sørensen, S.; Olsen, K.; Sørensen, J.C.; Orlien, V. The impact of high pressure on glucosinolate profile and myrosinase activity in seedlings from Brussels sprouts. Innov. Food Sci. Emerg. Technol. 2016, 38, 342–348. [Google Scholar] [CrossRef]
- Okunade, O.A.; Ghawi, S.K.; Methven, L.; Niranjan, K. Thermal and pressure stability of myrosinase enzymes from black mustard (Brassica nigra L. W.D.J. Koch. var. nigra), brown mustard (Brassica juncea L. Czern. var. juncea) and yellow mustard (Sinapsis alba L. subsp. maire) seeds. Food Chem. 2015, 187, 485–490. [Google Scholar] [CrossRef]
- Wang, J.; Brba, F.J.; Sorensen, J.; Frandsen, H.B.; Sorensen, S.; Olsen, K.; Orlien, V. High pressure effects on myrosinase activity and glucosinolate preservation in seedliings of brussels sprouts. Food Chem. 2018, 15, 1212–1217. [Google Scholar] [CrossRef]
- Vicente, A.R.; Martínez, G.A.; Chaves, A.R.; Civello, P.M. Effect of heat treatment on strawberry fruit damage and oxidative metabolism during storage. Postharvest Biol. Technol. 2006, 40, 116–122. [Google Scholar] [CrossRef]
- Tewari, S.; Sehrawat, R.; Nema, P.K.; Kaur, B.P. Preservation effect of high pressure processing on ascorbic acid of fruits and vegetables: A review. J. Food Biochem. 2017, 41, e12319. [Google Scholar] [CrossRef]
- Woolf, A.B.; Wibisono, R.; Farr, J.; Hallett, I.; Richter, L.; Oey, I.; Wohlers, M.; Zhou, J.; Fletcher, G.C.; Requejo-Jackman, C. Effect of high pressure processing on avocado slices. Innov. Food Sci. Emerg. Technol. 2013, 18, 65–73. [Google Scholar] [CrossRef]
- Denoya, G.I.; Vaudagna, S.R.; Polenta, G. Effect of high pressure processing and vacuum packaging on the preservation of fresh-cut peaches. LWT-Food Sci. Technol. 2015, 62, 801–806. [Google Scholar] [CrossRef]
- Fang, L.; Jiang, B.; Zhang, T. Effect of combined high pressure and thermal treatment on kiwifruit peroxidase. Food Chem. 2008, 109, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Shrestha, B.; Lim, S.Y.; Yoon, D.H.; Chang, W.C.; Shin, D.J.; Han, S.K.; Park, S.M.; Park, J.H.; Park, H.I.; et al. Cordycepin inhibits lipopolysaccharide-induced inflammation by the suppression of NF-κB through Akt and p38 inhibition in RAW 264.7 macrophage cells. Eur. J. Pharmacol. 2006, 545, 192–199. [Google Scholar] [CrossRef]
- Brandenburg, L.O.; Kipp, M.; Lucius, R.; Pufe, T.; Wruck, C.J. Sulforaphane suppresses LPS-induced inflammation in primary rat microglia. Inflamm. Res. 2010, 59, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Beltrán, C.E.; Calderón-Oliver, M.; Tapia, E.; Medina-Campos, O.N.; Sánchez-González, D.J.; Martínez-Martínez, C.M.; Ortiz-Vega, K.M.; Franco, M.; Pedraza-Chaverri, J. Sulforaphane protects against cisplatin-induced nephrotoxicity. Toxicol. Lett. 2010, 192, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Banskota, A.H.; Stefanova, R.; Sperker, S.; Lall, S.P.; Craigie, J.S.; Hafting, J.T.; Critchley, A.T. Polar lipids from the marine macroalga Palmaria palmata inhibit lipopolysaccharide-induced nitric oxide production in RAW264.7 macrophage cells. Phytochemistry 2014, 101, 101–108. [Google Scholar] [CrossRef]
- Sawle, P.; Foresti, R.; Mann, B.E.; Johnson, T.R.; Green, C.J.; Motterlini, R. Carbon monoxide-releasing molecules (CO-RMs) attenuate theinflammatory response elicited by lipopolysaccharide in RAW264.7 murine macrophages. Br. J. Pharmacol. 2009, 145, 800–810. [Google Scholar] [CrossRef] [PubMed]
- Subedi, L.; Lee, J.H.; Yumnam, S.; Ji, E.; Kim, S.Y. Anti-inflammatory effect of sulforaphane on LPS-activated microglia potentially through JNK/AP-1/NF-κB inhibition and Nrf2/HO-1 activation. Cells 2019, 8, 194. [Google Scholar] [CrossRef]
- Yang, L.P.; Zhu, X.A.; Tso, M.O.M. Minocycline and sulforaphane inhibited lipopolysaccharide-mediated retinal microglial activation. Mol. Vis. 2007, 13, 1083–1093. [Google Scholar]
- Zhou, J.; Joplin, D.G.; Cross, J.V.; Templeton, D.J. Sulforaphane inhibits prostaglandin E2 synthesis by suppressing microsomal prostaglandin E synthase 1. PLoS ONE. 2012, 7, e49744. [Google Scholar] [CrossRef]
- Park, S.J.; An, I.; Noh, G.P.; Yoo, B.H.; Lee, J.R. Inhibitory effect of broccoli leaf extract on PGE2 production by NF-κB inhibition. Korea J. Herbol. 2019, 34, 117–124. [Google Scholar]
- Qi, T.; Xu, F.; Yan, X.; Li, S.; Li, H. Sulforaphane exerts anti-inflammatory effects against lipopolysaccharide-induced acute lung injury in mice through the Nrf2/ARE pathway. Int. J. Mol. Med. 2016, 37, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Yun, C.W.; Park, W.K.; Kong, J.Y.; Kim, K.S.; Park, Y.; Lee, S.; Kim, B.K. Modulation of the activity of pro-inflammatory enzymes, COX-2 and iNOS, by chrysin derivatives. Pharmacol. Res. 2004, 49, 37–43. [Google Scholar] [CrossRef]
- Ruhee, R.T.; Ma, S.; Suzuki, K. Sulforaphane protects cells against lipopolysaccharide-stimulated inflammation in murine macrophages. Antioxidants 2019, 8, 577. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.L.; Xing, G.P.; Yu, Y.; Liang, H.; Yu, T.X.; Zheng, W.H.; Lai, T.B. Sulforaphane exerts neuroprotective effects via suppression of the inflammatory response in a rat model of focal cerebral ischemia. Int. J. Clin. Exp. Med. 2015, 8, 17811–17817. [Google Scholar]
- Guo, S.; Qiu, P.; Xu, G.; Wu, X.; Dong, P.; Yang, G.; Zheng, J.; McClements, D.J.; Xiao, H. Synergistic anti-inflammatory effects of nobiletin and sulforaphane in lipopolysaccharide-stimulated RAW 264.7 cells. J. Agric. Food Chem. 2012, 60, 2157–2164. [Google Scholar] [CrossRef]
- Lin, W.; Wu, R.T.; Wu, T.; Khor, T.O.; Wang, H.; Kong, A.N. Sulforaphane suppressed LPS-induced inflammation in mouse peritoneal macrophages through Nrf2 dependent pathway. Biochem. Pharmacol. 2008, 76, 967–973. [Google Scholar] [CrossRef]
- Choi, S.H.; Aid, S.; Bosetti, F. The distinct roles of cyclooxygenase-1 and -2 in neuroinflammation: Implications for translational research. Trends Pharmacol. Sci. 2009, 30, 174–181. [Google Scholar] [CrossRef]
- Smith, C.J.; Zhang, Y.; Koboldt, C.M.; Muhammad, J.; Zweifel, B.S.; Shaffer, A.; Talley, J.J.; Masferrer, J.L.; Seibert, K.; Isakson, P.C. Pharmacological analysis of cyclooxygenase-1 in inflammation. Proc. Natl. Acad. Sci. USA 1998, 95, 13313–13318. [Google Scholar] [CrossRef]
- Qin, W.S.; Deng, Y.H.; Cui, F.C. Sulforaphane protects against acrolein-induced oxidative stress and inflammatory responses: Modulation of Nrf-2 and COX-2 expression. Arch. Med. Sci. 2016, 12, 871–880. [Google Scholar] [CrossRef]
- Giustarini, D.; Dalle-Donne, I.; Colombo, R.; Milzani, A.; Rossi, R. An improved HPLC measurement for GSH and GSSG in human blood. Free Radic. Biol. Med. 2003, 35, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.; Afzal, A.; Jones, A.; Armstrong, D. A rapid method for the quantification of GSH and GSSG in biological samples. In Oxidative Stress Biomarkers and Antioxidant Protocols; Armstrong, D., Ed.; Humana Press: Totowa, NJ, USA, 2002; Volume 186, pp. 117–122. ISBN 978-0-89603-850-9. [Google Scholar]
- Yamada, H.; Arai, T.; Endo, N.; Yamashita, K.; Fukuda, K.; Sasada, M.; Uchiyama, T. LPS-induced ROS generation and changes in glutathione level and their relation to the maturation of human monocyte-derived dendritic cells. Life Sci. 2006, 78, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.R.; Hu, R.; Keum, Y.S.; Hebbar, V.; Shen, G.; Nair, S.S.; Kong, A.T. Effects of glutathione on antioxidant response element-mediated gene expression and apoptosis elicited by sulforaphane. Cancer Res. 2003, 63, 7520–7525. [Google Scholar] [PubMed]
- Heiss, E.; Herhaus, C.; Klimo, K.; Bartsch, H.; Gerhäuser, C. Nuclear factor κB is a molecular target for sulforaphane-mediated anti-inflammatory mechanisms. J. Biol. Chem. 2001, 276, 32008–32015. [Google Scholar] [CrossRef] [PubMed]
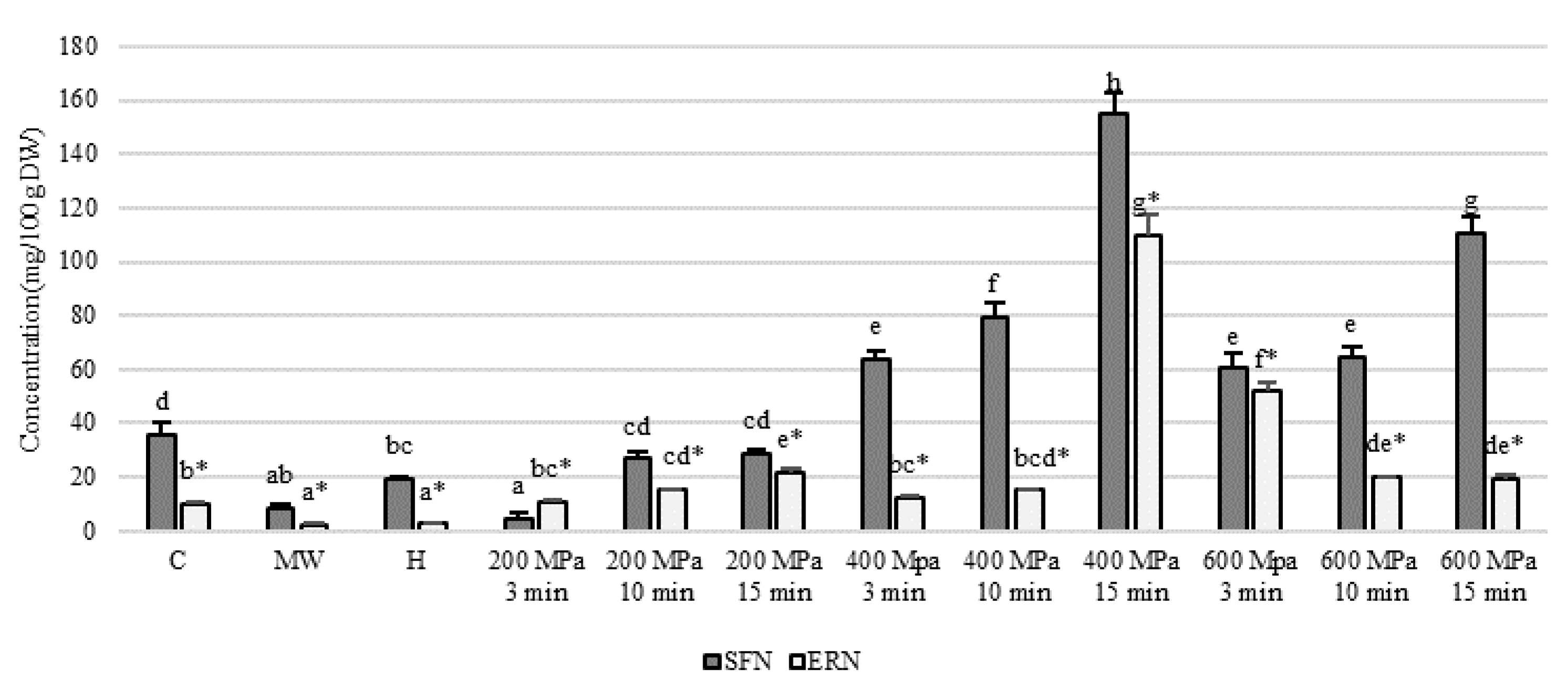
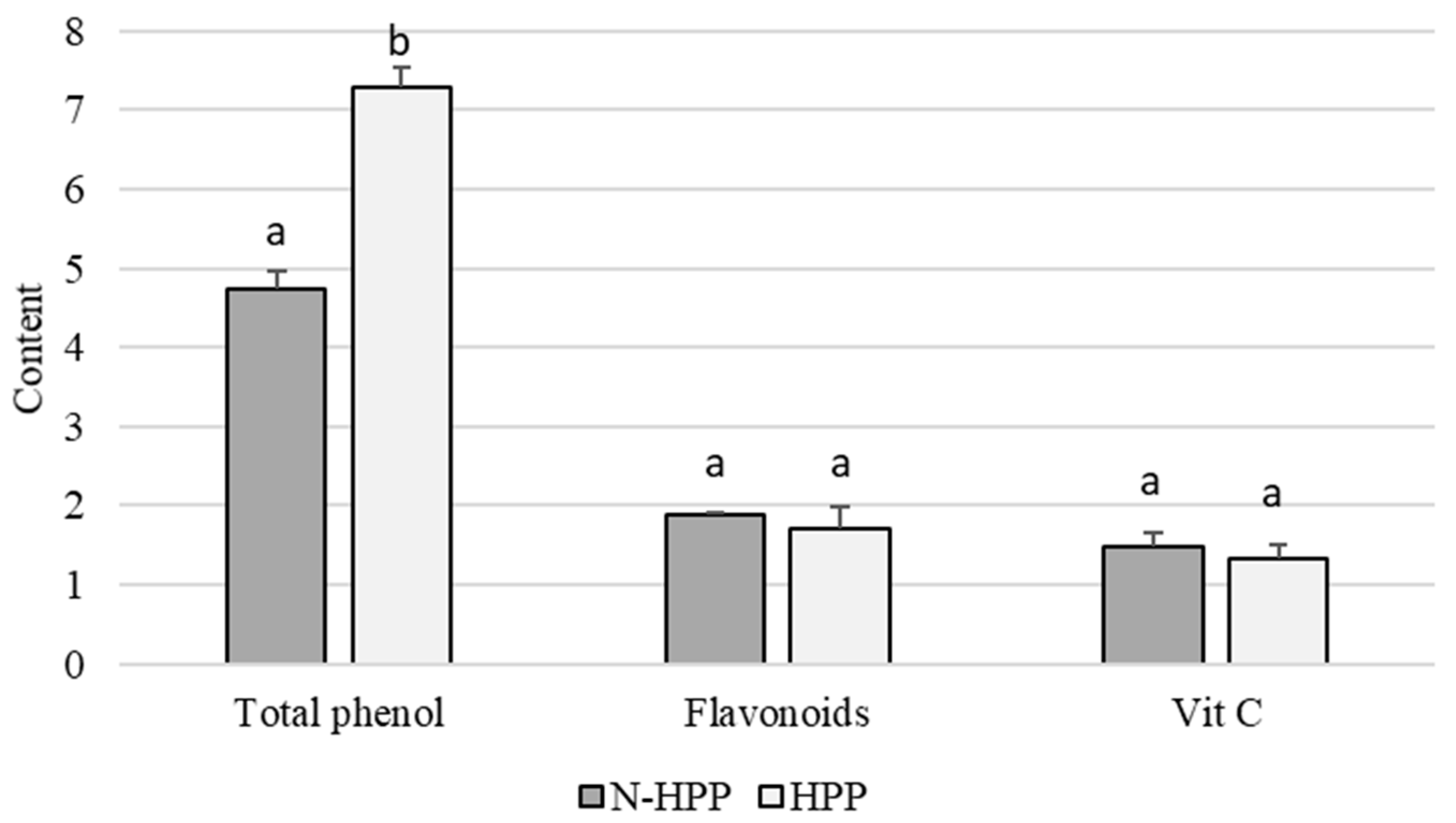
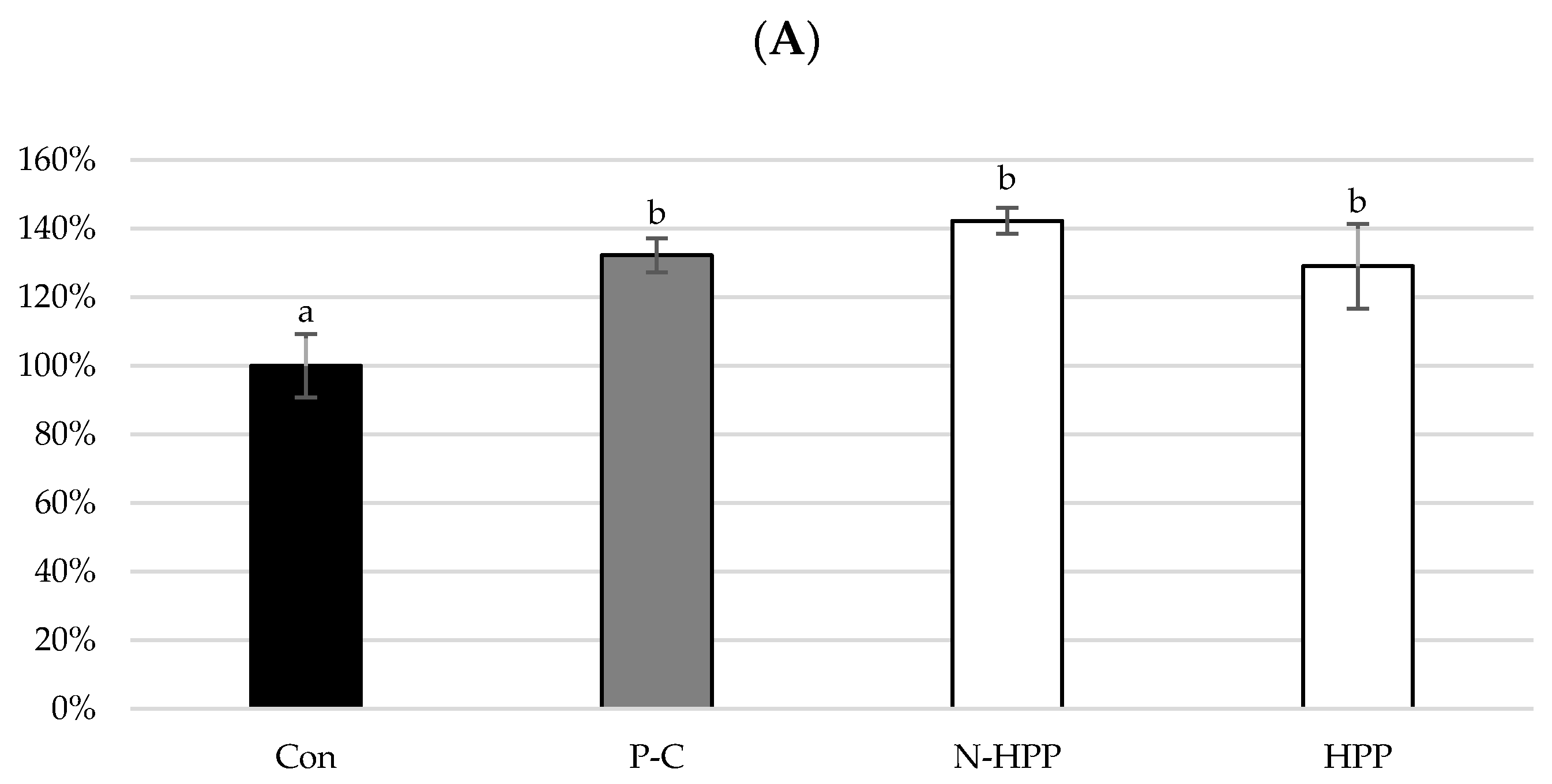
| Non-Blanched | Blanched | |||
|---|---|---|---|---|
| Non Processed | HPP Processed | Non Processed | HPP Processed | |
| MYR (μmol·g−1·min−1) | 165.75 ± 3.75 b | 267.25 ± 28.75 c | 123.50 ± 2.00 a | 250.75 ± 15.75 c |
| APX (mM·min−1·mg−1 protein) | 0.142 ± 0.002 a | 0.324 ± 0.004 b | 0.112 ± 0.011 a | 0.361 ± 0.026 b |
| PPO (∆A·min−1) | 0.0067 ± 0.0003 d | 0.0049 ± 0.000 c | 0.0041 ± 0.0001 b | 0.0025 ± 0.0001 a |
| POD (∆A·min−1) | 0.161 ± 0.007 d | 0.148 ± 0.015 c | 0.043 ± 0.002 b | 0.021 ± 0.004 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ke, Y.-Y.; Shyu, Y.-T.; Wu, S.-J. Evaluating the Anti-Inflammatory and Antioxidant Effects of Broccoli Treated with High Hydrostatic Pressure in Cell Models. Foods 2021, 10, 167. https://doi.org/10.3390/foods10010167
Ke Y-Y, Shyu Y-T, Wu S-J. Evaluating the Anti-Inflammatory and Antioxidant Effects of Broccoli Treated with High Hydrostatic Pressure in Cell Models. Foods. 2021; 10(1):167. https://doi.org/10.3390/foods10010167
Chicago/Turabian StyleKe, Yi-Yuan, Yuan-Tay Shyu, and Sz-Jie Wu. 2021. "Evaluating the Anti-Inflammatory and Antioxidant Effects of Broccoli Treated with High Hydrostatic Pressure in Cell Models" Foods 10, no. 1: 167. https://doi.org/10.3390/foods10010167
APA StyleKe, Y.-Y., Shyu, Y.-T., & Wu, S.-J. (2021). Evaluating the Anti-Inflammatory and Antioxidant Effects of Broccoli Treated with High Hydrostatic Pressure in Cell Models. Foods, 10(1), 167. https://doi.org/10.3390/foods10010167