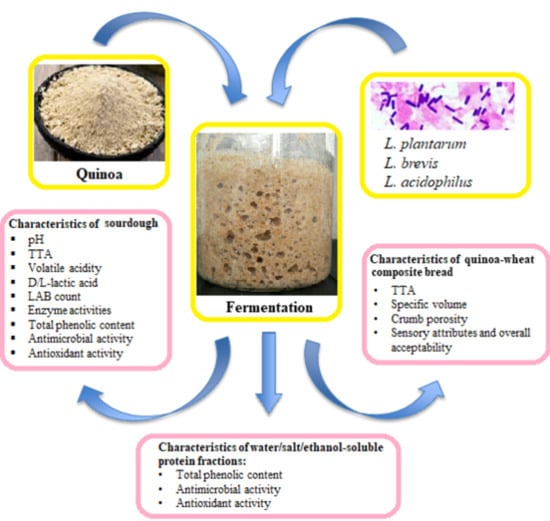
Effect of Lactic Acid Fermentation on Quinoa Characteristics and Quality of Quinoa-Wheat Composite Bread
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Fermentation of Quinoa Flour
2.3. Determination of the Characteristics of Fermented Quinoa Flour
2.4. Determination of Enzymatic Activity in Fermented Quinoa Flour
2.5. Isolation of Protein Fractions from Fermented Quinoa, Hydrolysis and Bioactive Compounds Determination
2.5.1. Defatting of Quinoa Samples for Isolation of Protein Fractions
2.5.2. Water-Soluble Protein Fraction Isolation
2.5.3. Extraction of Salt-Soluble Protein Fraction
2.5.4. Extraction of Ethanol-Soluble Protein Fraction
2.5.5. Enzymatic Hydrolysis of Protein with Pepsin
2.5.6. ABTS Radical Scavenging Assay
2.5.7. Determination of TPC in Protein Fractions
2.5.8. Determination of Antimicrobial Activity of Protein Fractions
2.6. The Preparation of Quinoa-Wheat Composite Bread
2.7. Evaluation of Quinoa-Wheat Composite Bread Characteristics
2.8. Statistical Analysis
3. Results and Discussion
3.1. Characteristics of Fermented Quinoa
3.1.1. LAB Adaptation and the Acidity Parameters of Fermented Quinoa
3.1.2. Enzymatic Activities in Fermented Quinoa
3.2. Characteristics of Protein Fractions from Fermented Quinoa
3.2.1. Antioxidant Activity and TPC in Protein Fractions from Quinoa
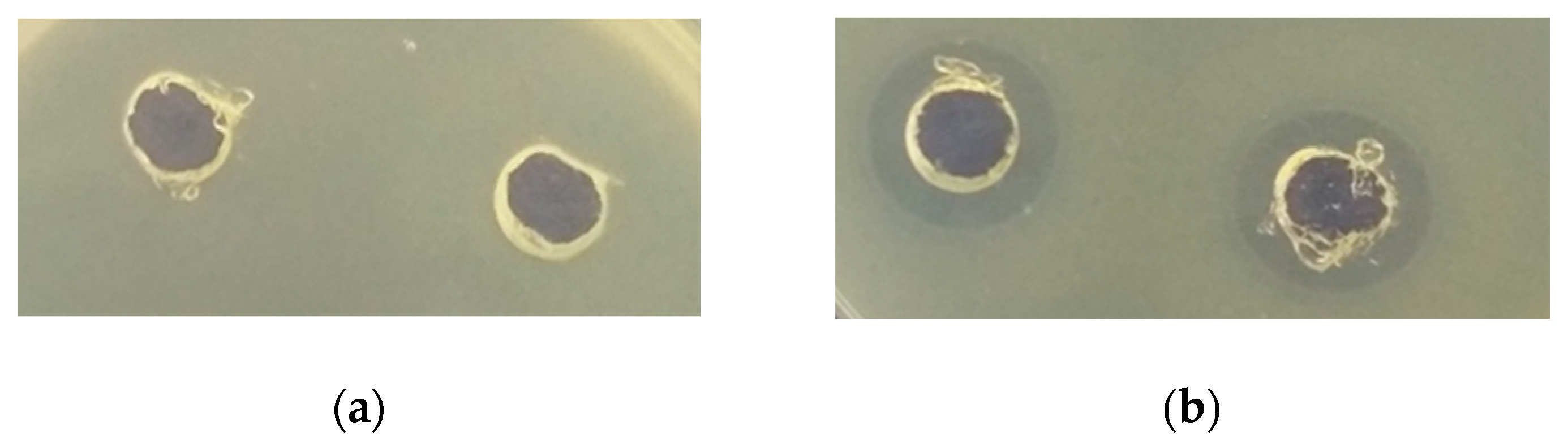
3.2.2. Antimicrobial Activity of Protein Fractions from Quinoa
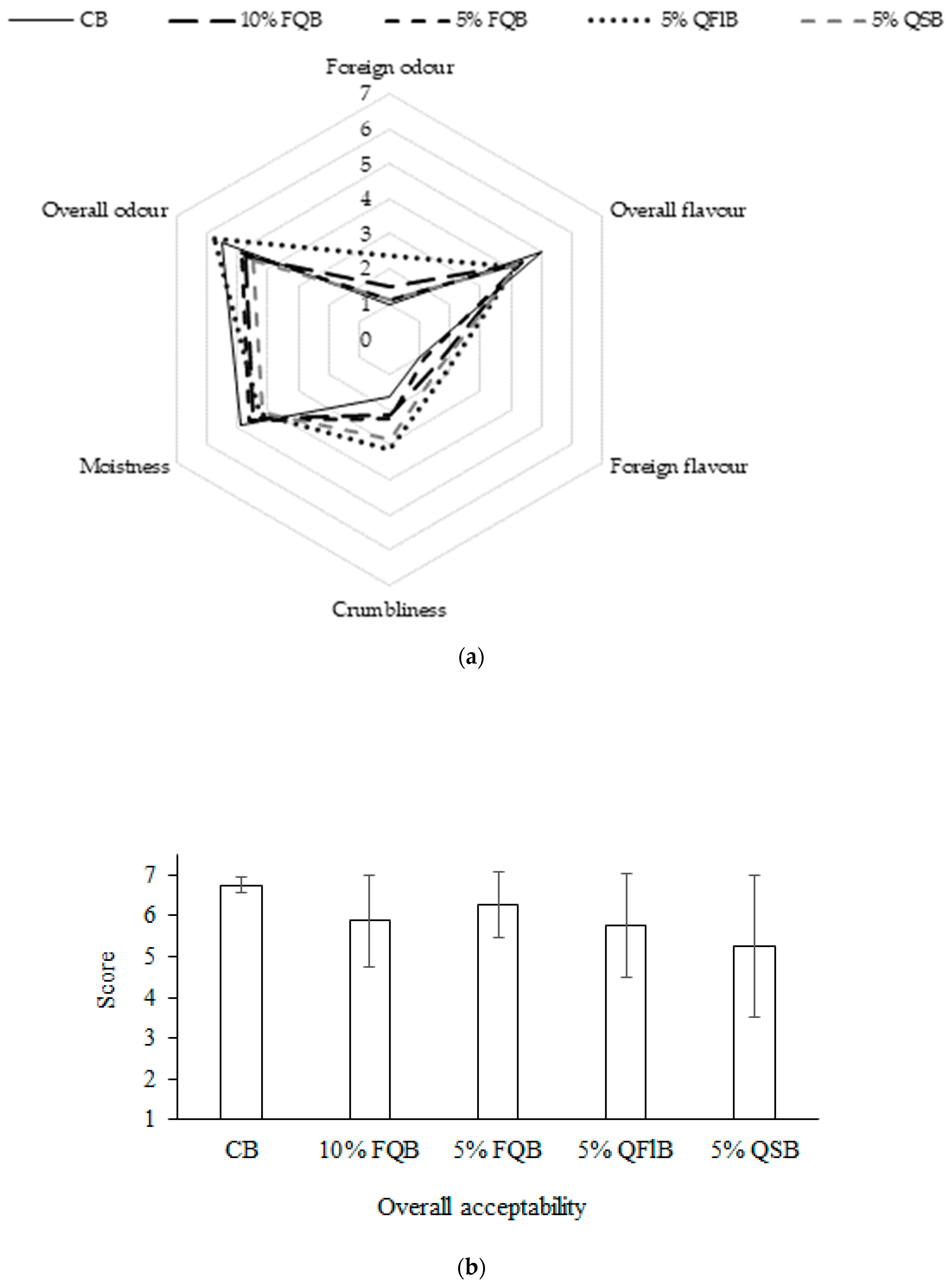
3.3. Characteristics and Sensory Evaluation of Breads Made with Quinoa Additives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Dakhili, S.; Abdolalizadeh, L.; Hosseini, S.M.; Shojaee-Aliabadi, S.; Mirmoghtadaie, L. Quinoa protein: Composition, structure and functional properties. Food Chem. 2019, 299. [Google Scholar] [CrossRef] [PubMed]
- Angeli, V.; Miguel Silva, P.; Crispim Massuela, D.; Khan, M.W.; Hamar, A.; Khajehei, F.; Graeff-Hönninger, S.; Piatti, C. Quinoa (Chenopodium quinoa Willd.): An overview of the potentials of the “Golden Grain” and socio-economic and environmental aspects of its cultivation and marketization. Foods 2020, 9, 216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agza, B.; Bekele, R.; Shiferaw, L. Quinoa (Chenopodium quinoa, Wild.): As a potential ingredient of injera in Ethiopia. J. Cereal Sci. 2018, 82, 170–174. [Google Scholar] [CrossRef]
- Jagelaviciute, J.; Cizeikiene, D. The influence of non-traditional sourdough made with quinoa, hemp and chia flour on the characteristics of gluten-free maize/rice bread. LWT Food Sci. Technol. 2021, 137. [Google Scholar] [CrossRef]
- Carrizo, S.L.; de LeBlanc, M.A.; LeBlanc, J.G.; Rollán, G.C. Quinoa pasta fermented with lactic acid bacteria prevents nutritional deficiencies in mice. Food Res. Int. 2020, 127. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Lorusso, A.; Montemurro, M.; Gobbetti, M. Use of sourdough made with quinoa (Chenopodium quinoa) flour and autochthonous selected lactic acid bacteria for enhancing the nutritional, textural and sensory features of white bread. Food Microbiol. 2016, 56, 1–13. [Google Scholar] [CrossRef]
- Arendt, E.K.; Moroni, A.; Zannini, E. Medical nutrition therapy: Use of sourdough lactic acid bacteria as a cell factory for delivering functional biomolecules and food ingredients in gluten free bread. Microb. Cell Fact. 2011, 10, S15. [Google Scholar] [CrossRef] [Green Version]
- Di Renzo, T.; Reale, A.; Boscaino, F.; Messia, M.C. Flavoring production in Kamut®, quinoa and wheat doughs fermented by Lactobacillus paracasei, Lactobacillus plantarum, and Lactobacillus brevis: A SPME-GC/MS study. Front. Microbiol. 2018, 9, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Coda, R.; Rizzello, C.G.; Pinto, D.; Gobbetti, M. Selected lactic acid bacteria synthesize antioxidant peptides during sourdough fermentation of cereal flours. Appl. Environ. Microbiol. 2012, 78, 1087–1096. [Google Scholar] [CrossRef] [Green Version]
- Rizzello, C.G.; Lorusso, A.; Russo, V.; Pinto, D.; Marzani, B.; Gobbetti, M. Improving the antioxidant properties of quinoa flour through fermentation with selected autochthonous lactic acid bacteria. Int. J. Food Microbiol. 2017, 241, 252–326. [Google Scholar] [CrossRef]
- Hernández-Ledesma, B. Quinoa (Chenopodium quinoa Willd.) as a source of nutrients and bioactive compounds: A review. BCHD 2019, 2, 27–47. [Google Scholar] [CrossRef]
- Vilcacundo, R.; Miralles, B.; Carrillo, W.; Hernández-Ledesma, B. In vitro chemopreventive properties of peptides released from quinoa (Chenopodium quinoa Willd.) protein under simulated gastrointestinal digestion. Food Res. Int. 2018, 105, 403–411. [Google Scholar] [CrossRef] [PubMed]
- El-Sohaimy, S.A.; Shehata, M.G.; Mehany, T.; Zeitoun, M.A. Nutritional, physicochemical, and sensorial evaluation of flat bread supplemented with quinoa flour. Int. J. Food Sci. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Elsohaimy, S.A.; Refaay, T.M.; Zaytoun, M.A.M. Physicochemical and functional properties of quinoa protein isolate. Ann. Agric. Sci. 2015, 60, 297–305. [Google Scholar] [CrossRef] [Green Version]
- Nongonierma, A.B.; le Maux, S.; Dubrulle, C.; Barre, C.; Geraldab, R.J.F. Quinoa (Chenopodium quinoa Willd.) protein hydrolysates with in vitro dipeptidyl peptidase IV (DPP-IV) inhibitory and antioxidant properties. J. Cereal Sci. 2015, 65, 112–118. [Google Scholar] [CrossRef] [Green Version]
- Mahdavi-Yekta, M.; Nouri, L.; Azizi, M.H. The effects of hydrolysis condition on antioxidant activity of protein hydrolyzate from quinoa. Food Sci. Nutr. 2019, 7, 930–936. [Google Scholar] [CrossRef] [Green Version]
- Chakrabarti, S.; Guha, S.; Majumder, K. Food-derived bioactive peptides in human health: Challenges and opportunities. Nutrients 2018, 10, 1738. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Martin, N.M.; Toscano, T.; Villanueva, A.; Pedroche, J.; Millan, F.; Montserrat-de la Paz, S.; Millan-Linares, M.C. Neuroprotective protein hydrolysates from hemp (Cannabis sativa L.) seeds. Food Funct. 2019, 10, 6732–6739. [Google Scholar] [CrossRef]
- Siddeeg, A.; Xu, Y.; Jiang, Q.; Xia, W. In vitro antioxidant activity of protein fractions extracted from seinat (Cucumis melo var. tibish) seeds. CYTA J. Food. 2015, 13, 472–481. [Google Scholar] [CrossRef] [Green Version]
- Ortiz-Martinez, M.; Otero-Pappatheodorou, J.T.; Serna-Saldívar, S.O.; García-Lara, S. Antioxidant activity and characterization of protein fractions and hydrolysates from normal and quality protein maize kernels. J. Cereal Sci. 2017, 76, 85–91. [Google Scholar] [CrossRef]
- Coelho, M.S.; Aquino, S.D.A.; Latorres, J.M.; Salas-Mellado, M.D.L.M. In vitro and in vivo antioxidant capacity of chia protein hydrolysates and peptides. Food Hydrocoll. 2019, 91, 19–25. [Google Scholar] [CrossRef]
- García-Cano, I.; Rocha-Mendoza, D.; Ortega-Anaya, J.; Wang, K.; Kosmerl, E.; Jiménez-Flores, R. Lactic acid bacteria isolated from dairy products as potential producers of lipolytic, proteolytic and antibacterial proteins. Appl. Microbiol. Biotechnol. 2019, 103, 5243–5257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pessione, E.; Cirrincione, S. Bioactive molecules released in food by lactic acid bacteria: Encrypted peptides and biogenic amines. Front. Microbiol. 2016, 7, 876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, L.; Pingitore, E.V.; Mozzi, F.; Saavedra, L.; Villegas, J.M.; Hebert, E.M. Lactic acid bacteria as cell factories for the generation of bioactive peptides. Protein Pept. Lett. 2017, 24, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Raveschot, C.; Cudennec, B.; Coutte, F.; Flahaut, C.; Fremont, M.; Drider, D.; Dhulster, P. Production of bioactive peptides by lactobacillus species: From gene to application. Front. Microbiol. 2018, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Sanz-Penella, J.M.; Tamayo-Ramos, J.A.; Sanz, Y.; Haros, M. Phytate reduction in bran-enriched bread by phytase-producing bifidobacteria. J. Agric. Food Chem. 2009, 57, 10239–10244. [Google Scholar] [CrossRef]
- Cizeikiene, D. Bioproducts of bacteriocins producing lactic acid bacteria, their antimicrobial and phytase activities, and applications. Ph.D. Thesis, Kaunas University of Technology, Kaunas, Lithuania, 2015. [Google Scholar]
- International Organization for Standardization. Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Microorganisms—Colony-Mount Technique at 30 °C; International Organization for Standardization: Geneva, Switzerland, 2003. [Google Scholar]
- Cereals & Grains Association. AACC International, Approved Methods of the American Association of Cereal Chemists, 10th ed.; Cereals & Grains Association: St. Paul, MN, USA, 2020. [Google Scholar]
- Xiao, Z.; Storms, R.; Tsang, A. A quantitative starch-iodine method for measuring alpha-amylase and glucoamylase activities. Anal. Biochem. 2006, 351, 146–148. [Google Scholar] [CrossRef]
- Cizeikiene, D.; Jagelaviciute, J.; Stankevicius, M.; Maruska, A. Thermophilic lactic acid bacteria affect the characteristics of sourdough and whole-grain wheat bread. Food Biosci. 2020, 38. [Google Scholar] [CrossRef]
- Quan, C.S.; Fan, S.D.; Mang, L.H.; Wang, Y.J.; Ohta, Y. Purification and properties of a phytase from Candida krusei WZ-001. J. Biosci. Bioeng. 2002, 94, 419–425. [Google Scholar]
- Olstorpe, M.; Schnurer, J.; Passoth, V. Screening of yeast strains for phytase activity. FEMS Yeast Res. 2009, 9, 478–488. [Google Scholar] [CrossRef] [Green Version]
- Cupp-Enyard, C. Sigma’s non-specific protease activity assay-casein as a substrate. J. Vis. Exp. 2008, 19, 899. [Google Scholar] [CrossRef] [PubMed]
- Hadnađev, M.; Dapčević-Hadnađev, T.; Lazaridou, A.; Moschakis, T.; Michaelidou, A.M.; Popović, S.; Biliaderis, C.G. Hempseed meal protein isolates prepared by different isolation techniques: Part I. Physicochemical properties. Food Hydrocoll. 2018, 79, 526–533. [Google Scholar] [CrossRef]
- Rajurkar, N.S.; Hande, S.M. Estimation of phytochemical content and antioxidant activity of some selected traditional Indian medicinal plants. Ind. J. Pharm. Sci. 2011, 73, 146–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphofungistic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Cizeikiene, D.; Juodeikiene, G.; Paskevicius, A.; Bartkiene, E. Antimicrobial activity of lactic acid bacteria against pathogenic and spoilage microorganism isolated from food and their control in wheat bread. Food Control. 2013, 31, 539–545. [Google Scholar] [CrossRef]
- Hansen, Å. Sourdough Bread. In Handbook of Plant-Based Fermented Food and Beverage Technology; Hui, Y.H., Özgül Evranuz, E., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 493–516. [Google Scholar]
- Lönner, C.; Preve-Åkesson, A. Acidification properties of lactic acid bacteria in rye sourdoughs. Food Microbiol. 1988, 5, 43–58. [Google Scholar] [CrossRef]
- Katina, K.; Sauri, M.; Alakomi, H.-L.; Mattila-Sandholm, T. Potential of lactic acid bacteria to inhibit rope spoilage in wheat sourdough bread. LWT Food Sci. Technol. 2002, 35, 38–45. [Google Scholar] [CrossRef]
- Nisa, Z.-U.; Rehman, S.-U.; Huma, N.; Shahid, M. Impact of mixed lactic acid bacterial (LAB) culture on flavoring profile and quality attributes of spring wheat sourdough bread. Pak. J. Agric. Sci. 2016, 53, 225–231. [Google Scholar]
- Broadbent, J.R.; Oberg, T.S.; Hughes, J.E.; Ward, R.E.; Brighton, C.; Welker, D.L.; Steele, J.L. Influence of polysorbate 80 and cyclopropane fatty acid synthase activity on lactic acid production by Lactobacillus casei ATCC 334 at low pH. J. Ind. Microbiol. Biotechnol. 2014, 41, 545–553. [Google Scholar] [CrossRef]
- Tanyıldızı, M.Ş.; Bulut, Ş.; Selen, V.; Özer, D. Optimization of lactic acid production with immobilized Rhizopus oryzae. Afr. J. Biotechnol. 2012, 11, 8546–8552. [Google Scholar]
- Kaltsa, O.; Georgopoulos, T.; Yanniotis, S.; Mandala, I. Effect of enzyme blends and dough strengthening emulsifier on extending the shelf life of sandwich bread applying response surface methodology. IJEIT 2013, 3, 149–160. [Google Scholar]
- Diaconescu, D.; Zdremtan, M.; Mester, M.; Halmagean, L.; Balint, M. A study on the influence of some biogenic effectors on bread staling: Sensory evaluation. J. Agroalim. Proc. Technol. 2013, 19, 247–252. [Google Scholar]
- Reale, A.; Konietzny, U.; Coppola, R.; Sorrentino, E.; Greiner, R. The importance of lactic acid bacteria for phytate degradation during cereal dough fermentation. J. Agric. Food Chem. 2007, 55, 2993–2997. [Google Scholar] [CrossRef] [PubMed]
- Moslehishad, M.; Mirdamadi, S.; Ehsani, M.R.; Ezzatpanah, H.; Moosavi-Movahedi, A.A. The proteolytic activity of selected lactic acid bacteria in fermenting cow’s and camel’s milk and the resultant sensory characteristics of the products. Int. J. Dairy Technol. 2013, 66, 279–285. [Google Scholar] [CrossRef]
- Donkor, O.N.; Henriksson, A.; Vasiljevic, T.; Shah, N.P. Proteolytic activity of dairy lactic acid bacteria and probiotics as determinant of growth and in vitro angiotensin-converting enzyme inhibitory activity in fermented milk. Le Lait 2007, 87, 21–38. [Google Scholar] [CrossRef]
- Llorente-Bousquets, A.; Pérez-Munguía, S.; Farrés, A. Novel extracellular proteolytic activity in Pediococcus acidilactici ATCC 8042. Can. J. Microbiol. 2008, 54, 694–699. [Google Scholar] [CrossRef]
- Fernández, M.; Martín, A.; Benito, M.J.; Casquete, R.; Recio, I.; Córdoba, M.D.G. Influence of starter cultures on the generation of antioxidant nitrogen compounds in Iberian dry-fermented sausages. Int. J. Food Sci. Technol. 2016, 51, 435–443. [Google Scholar] [CrossRef]
- Martınez-Anaya, M.A. Enzymes and bread flavor. J. Agric. Food Chem. 1996, 44, 2469–2480. [Google Scholar] [CrossRef]
- Daskaya-Dikmen, C.; Yucetepe, A.; Karbancioglu-Guler, F.; Ozcelik, B. Angiotensin-I-converting enzyme (ACE)-inhibitory peptides from plants. Nutrients 2017, 9, 316. [Google Scholar] [CrossRef]
- Liu, J.; Yong, H.; Yao, X.; Hu, H.; Yun, D.; Xiao, L. Recent advances in phenolic–protein conjugates: Synthesis, characterization, biological activities and potential applications. RSC Adv. 2019, 9, 35825–35840. [Google Scholar] [CrossRef] [Green Version]
- Nwachukwu, I.D.; Aluko, R.E. Structural and functional properties of food protein-derived antioxidant peptides. J. Food Biochem. 2019, 43, e12761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Wu, Y.; Li, L. Relationship between primary structure or spatial conformation and functional activity of antioxidant peptides from Pinctada fucata. Food Chem. 2018, 264, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Adebo, O.A.; Medina-Meza, G.I. Impact of fermentation on the phenolic compounds and antioxidant activity of whole cereal grains: A mini review. Molecules 2020, 25, 927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhidong, L.; Benheng, G.; Xuezhong, C.; Zhenmin, L.; Yun, D.; Hongliang, H.; Wen, R. Optimisation of hydrolysis conditions for antioxidant hydrolysate production from whey protein isolates using response surface methodology. Ir. J. Agric. Food Res. 2013, 52, 53–65. [Google Scholar]
- Rival, S.G.; Boeriu, C.G.; Wichers, H.J. Caseins and casein hydrolysates. 2: Antioxidative properties and relevance to lipoxygenase inhibition. J. Agric. Food Chem. 2001, 49, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Rohn, S.; Rawel, H.M.; Kroll, J. Antioxidant activity of protein-bound quercetin. J. Agric. Food Chem. 2004, 52, 4725–4729. [Google Scholar] [CrossRef]
- Xiao, J.; Mao, F.; Yang, F.; Zhao, Y.; Zhang, C.; Yamamoto, K. Interaction of dietary polyphenols with bovine milk proteins: Molecular structure–affinity relationship and influencing bioactivity aspects. Mol. Nutr. Food Res. 2011, 55, 1637–1645. [Google Scholar] [CrossRef]
- Xiao, J.; Chen, T.; Cao, H.; Chen, L.; Yang, F. Noncovalent interaction of dietary polyphenols with bovine hemoglobin in vitro: Molecular structure/property–affinity relationship aspects. Mol. Nutr. Food Res. 2011, 55, 310–317. [Google Scholar] [CrossRef]
- Mhada, M.; Metougui, M.L.; El Hazzam, K.; El Kacimi, K.; Yasri, A. Variations of saponins, minerals and total phenolic compounds due to processing and cooking of quinoa (Chenopodium quinoa Willd.) seeds. Foods 2020, 9, 660. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, Y.J.; Kim, Y.H.; Yoon, K.S. Antioxidant and antimicrobial activities of quinoa (Chenopodium quinoa Willd.) seeds cultivated in Korea. Prev. Nutr. Food Sci. 2017, 22, 195–202. [Google Scholar]
- Miranda, M.; Delatorre-Herrera, J.; Vega-Gálvez, A.; Jorquera, E.; Quispe-Fuentes, I.; Martínez, E.A. Antimicrobial potential and phytochemical content of six diverse sources of quinoa seeds (Chenopodium quinoa Willd.). Agric. Sci. 2014, 5, 1015–1024. [Google Scholar]
- Wyrwisz, J.M.K. The application of dietary fiber in bread products. J. Food Process. Technol. 2015, 6, 445–447. [Google Scholar]
| Unfermented quinoa | L. plantarum MR24 | L. brevis R26 | L. acidophilus DSM 20079 | |
|---|---|---|---|---|
| LAB count, CFU/g | - | (3.9 ± 0.3) × 109 a | (8.05 ± 0.1) × 109 b | (3.2 ± 0.1) × 1010 c |
| Acidity | ||||
| pH | 6.6 ± 0.1 c | 4.3 ± 0.05 a | 4.6 ± 0.05 b | 4.4 ± 0.04 a |
| TTA, mL of 1 M NaOH | 2.6 ± 0.02 ca | 9.2 ± 0.02 d | 5.5 ± 0.03 b | 8.1 ± 0.05 c |
| Volatile acidity, mL of 1 M NaOH | 0.02 ± 0.01 a | 3.00 ± 0.04 b | 3.00 ± 0.10 b | 2.88 ± 0.09 b |
| Lactic acid content | ||||
| L-lactic acid, g/kg | 2.3 ± 0.03 a | 13.0 ± 0.24 c | 9.2 ± 0.21 b | 42.5 ± 0.26 d |
| D-lactic acid, g/kg | 0.5 ± 0.01 a | 6.7 ± 0.15 d | 3.4 ± 0.07 c | 2.1 ± 0.04 b |
| Enzymatic activities | ||||
| Cellulase activity, CU/g | 0 | 0.577 ± 0.022 b | 0.617 ± 0.065 b | 0.450 ± 0.071 a |
| Amylase activity, AU/g | 0.259 ± 0.05 c | 0.082 ± 0.006 b | 0.333 ± 0.008 d | 0.015 ± 0.001 a |
| Phytase activity, PhU/g | 0 | 0.125 ± 0.004 b | 0.142 ± 0.003 c | 0.100 ± 0 a |
| Protease activity, PU/g | 0 | 0.038 ± 0.008 a | 0.042 ± 0.003 a | 0 |
| TPC and antioxidant activity | ||||
| TPC, GAE mg/100 g | 32.3 ± 0.2 a | 39.2 ± 0.5 b | 61.4 ± 3.0 c | 39.1 ± 1.4 b |
| Antioxidant activity, mg TE/100 g | 37.6 ± 2.8 b | 10.2 ± 0.6 a | 53.4 ± 3.7 c | 29.0 ± 3.2 b |
| LAB Used for Quinoa Fermentation | Antioxidant Activity | Total Phenolic Content | |||
|---|---|---|---|---|---|
| Non-Hydrolysed | After Hydrolysis with Pepsin | Non-Hydrolysed | After Hydrolysis with Pepsin | ||
| Water-soluble protein fraction | |||||
| Unfermented flour | 34.8 ± 5.3 b | 0 | 0.47 ± 0.02 a | 1.92 ± 0.03 b | |
| L. plantarum | 64.1 ± 6.7 d | 0 | 0.80 ± 0.02 b | 0.33 ± 0.07 a | |
| L. brevis | 22.1 ± 4.1 a | 0 | 0.27 ± 0.03 a | 0.45 ± 0.04 a | |
| L. acidophilus | 52.2 ± 12.5 c | 0 | 0.86 ± 0.02 b | 0.58 ± 0.11 a | |
| Protein fraction soluble in 0.8 M NaCl | |||||
| Unfermented flour | 27.5 ± 6.4 a | 0 | 0.88 ± 0.04 a | 0.66 ± 0.03 a | |
| L. plantarum | 21.5 ± 3.6 a | 0 | 0.90 ± 0.02 a | 0.66 ± 0.02 a | |
| L. brevis | 26.1 ± 4.5 a | 0 | 0.98 ± 0.01 a | 0.65 ± 0.02 a | |
| L. acidophilus | 35.3 ± 6.5 b | 0 | 1.37 ± 0.04 b | 0.64 ± 0.01 a | |
| Protein fraction soluble in 70% ethanol | |||||
| Unfermented flour | 1109 ± 141 c | 139.5 ± 21.6 d | 50.1 ± 0.45 b | 18.5 ± 0.38 d | |
| L. plantarum | 69.3 ± 16.5 a | 9.1 ± 1.2 a | 4.63 ± 0.21 a | 3.43 ± 0.07 a | |
| L. brevis | 881.6 ± 95.8 b | 24.8 ± 9.7 b | 68.0 ± 4.16 b | 11.2 ± 0.02 c | |
| L. acidophilus | 3261 ± 169 d | 76.2 ± 14.3 c | 186.1 ± 9.8 c | 10.5 ± 0.03 b | |
| Food Spoilage Bacteria | LAB Strain Used for Quinoa Flour Fermentation | Unfermented Flour | ||||||
|---|---|---|---|---|---|---|---|---|
| L. plantarum | L. brevis | L. acidophilus | ||||||
| Non-Hyd. | After Hyd. with Pepsin | Non-Hyd. | After Hyd. with Pepsin | Non-Hyd. | After Hyd. with Pepsin | Non-Hyd. | After Hyd. with Pepsin | |
| Water-soluble protein fraction | ||||||||
| E. coli | - | ++ | - | +/− | - | +/− | - | + |
| St. aureus | - | + | - | - | - | +/- | - | - |
| S. typhimurium | - | + | - | +/− | - | +/− | - | + |
| B. subtilis | - | ++ | - | + | - | + | - | ++ |
| B. cereus | - | + | - | - | - | +/− | - | + |
| Protein fractions soluble in 0.8 M NaCl | ||||||||
| E. coli | - | - | - | +/− | - | +/− | - | - |
| St. aureus | - | +/− | - | - | - | - | - | - |
| S. typhimurium | - | +/− | - | +/− | - | +/− | - | - |
| B. subtilis | - | +/− | - | + | +/− | + | - | + |
| B. cereus | - | - | - | - | - | +/− | +/− | +/− |
| Protein fractions soluble in 70% ethanol | ||||||||
| E. coli | - | ++ | - | + | + | ++ | - | +/− |
| St. aureus | +/− | ++ | +/− | + | + | ++ | - | + |
| S. typhimurium | ++ | ++ | + | ++ | + | ++ | - | + |
| B. subtilis | + | +++ | + | ++ | + | ++ | +/− | + |
| B. cereus | + | ++ | + | ++ | + | ++ | - | +/− |
| Bread Samples | CB | 10% FQB | 5% FQB | 5% QFlB | 5% QSB |
|---|---|---|---|---|---|
| Porosity, % | 78.1 ± 1.1 a | 77.7 ± 0.9 a | 77.3 ± 1.3 a | 80.8 ± 0 a | 80.5 ± 0.9 a |
| Specific volume, cm3/g | 3.09 ± 0.16 a | 3.32 ± 0.22 b | 3.18 ± 0.11 ab | 3.42 ± 0.17 b | 3.27 ± 0.15 b |
| TTA, mL of 1 M NaOH | 2.2 ± 0.02 b | 2.2 ± 0.01 b | 2.2 ± 0.01 b | 2.0 ± 0.01 ab | 1.8 ± 0 ac |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cizeikiene, D.; Gaide, I.; Basinskiene, L. Effect of Lactic Acid Fermentation on Quinoa Characteristics and Quality of Quinoa-Wheat Composite Bread. Foods 2021, 10, 171. https://doi.org/10.3390/foods10010171
Cizeikiene D, Gaide I, Basinskiene L. Effect of Lactic Acid Fermentation on Quinoa Characteristics and Quality of Quinoa-Wheat Composite Bread. Foods. 2021; 10(1):171. https://doi.org/10.3390/foods10010171
Chicago/Turabian StyleCizeikiene, Dalia, Ieva Gaide, and Loreta Basinskiene. 2021. "Effect of Lactic Acid Fermentation on Quinoa Characteristics and Quality of Quinoa-Wheat Composite Bread" Foods 10, no. 1: 171. https://doi.org/10.3390/foods10010171
APA StyleCizeikiene, D., Gaide, I., & Basinskiene, L. (2021). Effect of Lactic Acid Fermentation on Quinoa Characteristics and Quality of Quinoa-Wheat Composite Bread. Foods, 10(1), 171. https://doi.org/10.3390/foods10010171