Development of Immunohistochemical Methods for Casein Detection in Meat Products
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
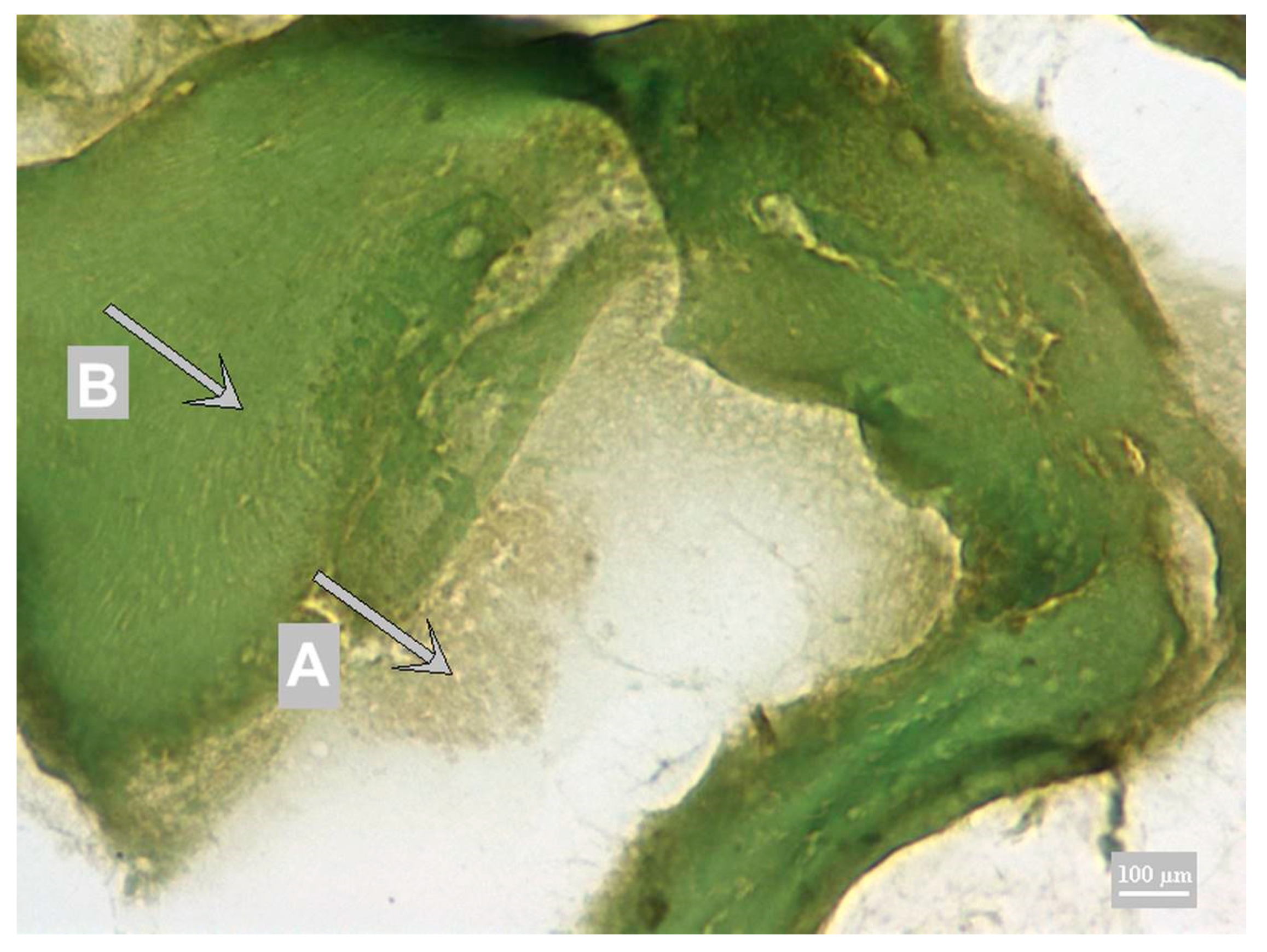
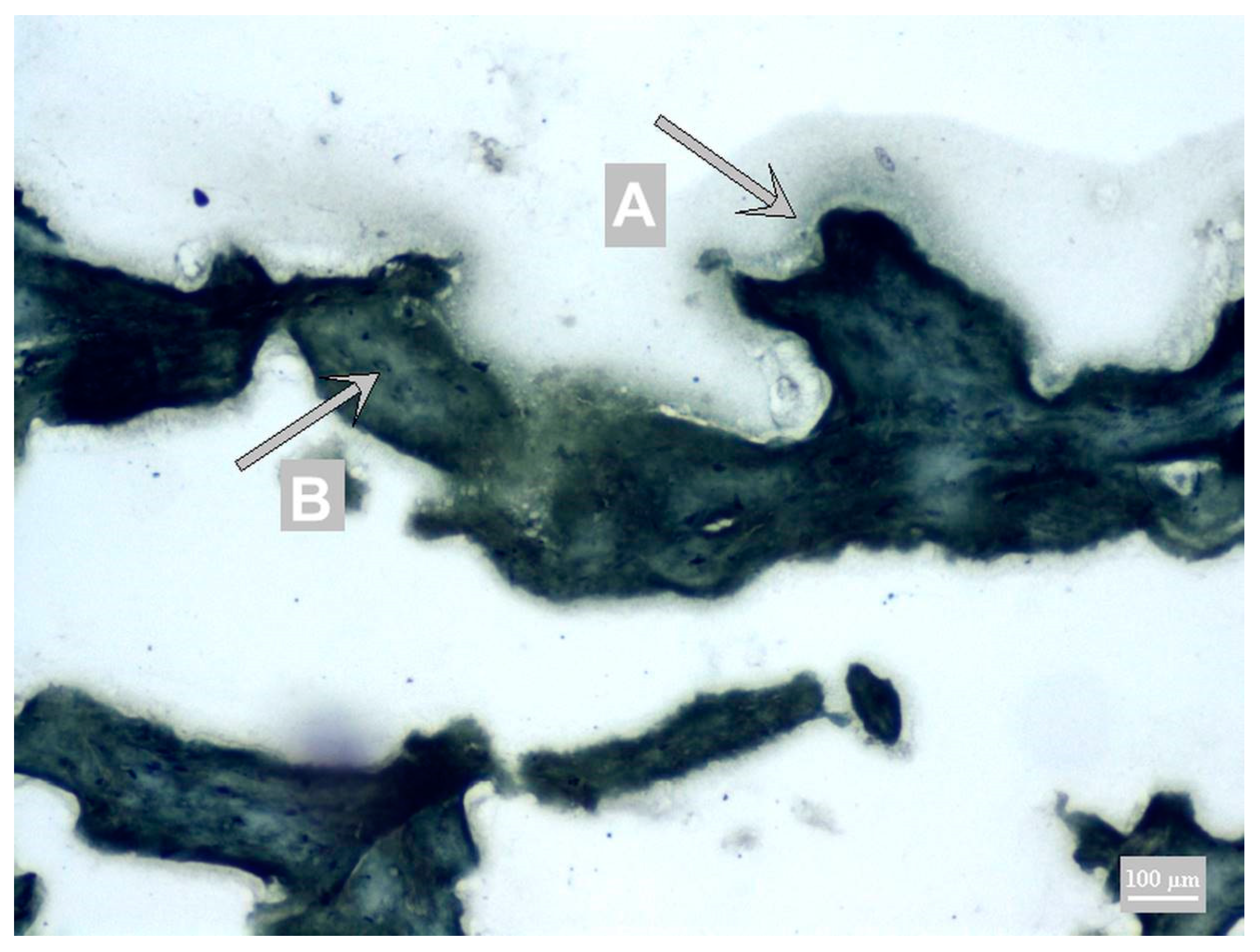
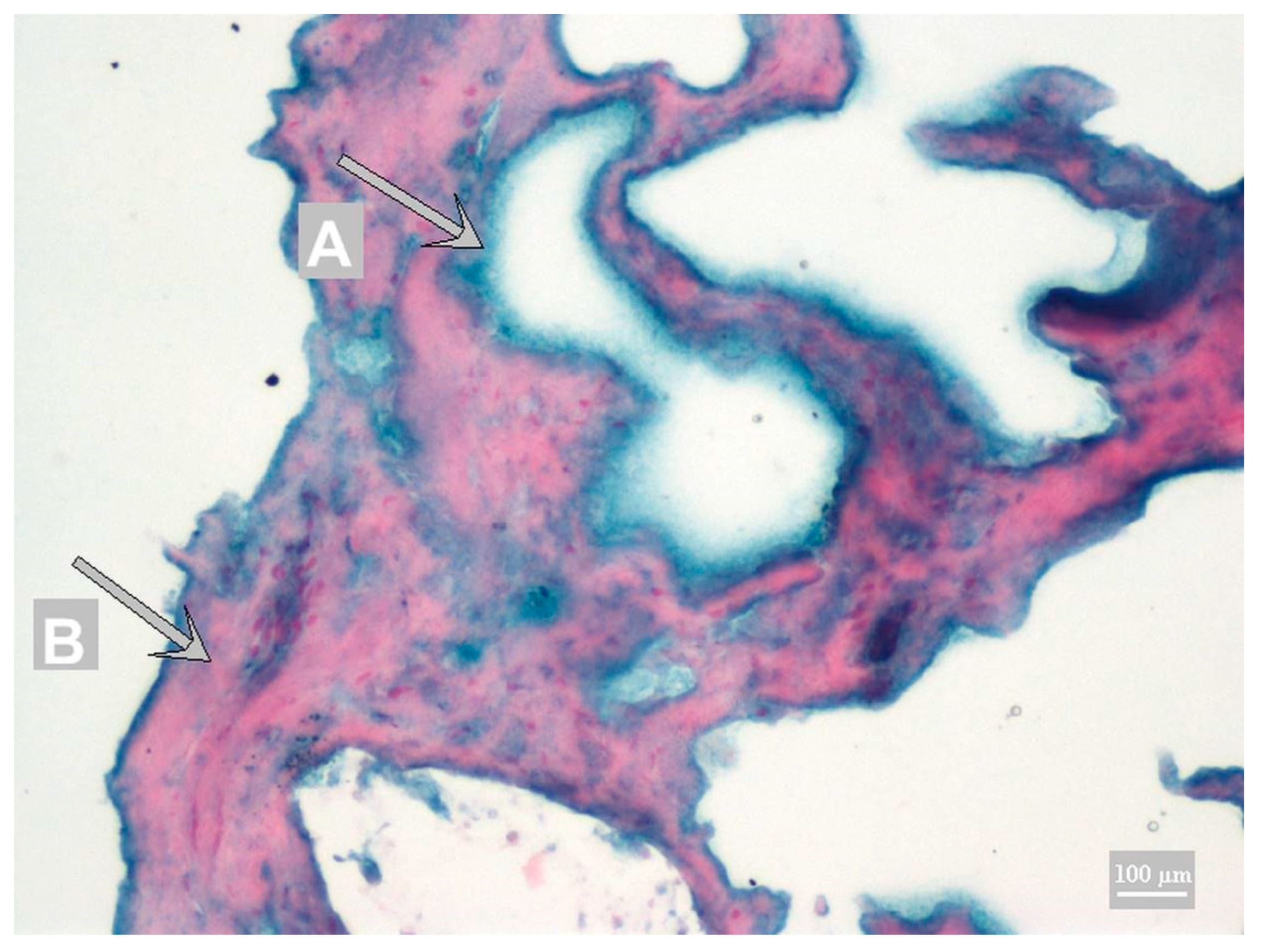
3.1. Method Development
3.2. Method Validation
3.3. Limit of Detection (LOD)
3.4. Repeatability and Reproducibility
3.5. Sensitivity and Specificity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- O’Regan, J.; Ennis, M.P.; Mulvihill, D.M. Milk proteins. In Handbook of Hydrocolloids; Woodhead Publishing: Cambridge, UK, 2009; pp. 298–358. [Google Scholar] [CrossRef]
- Kumar, S.; Zanzad, P.N.; Ambadkar, R.K.; Rindhe, S.N.; Kumar, P.; Karle, S.D. Storage stability of chicken sausage incorporated with selected levels of sodium caseinate. J. Vet. Public Health 2011, 9, 33–37. [Google Scholar]
- Tarté, R. Ingredients in Meat Products: Properties, Functionality and Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2009; ISBN 978-0-387-71327-4. [Google Scholar]
- Mora, L.; Escudero, E.; Aristoy, M.C.; Toldrá, F. A peptidomic approach to study the contribution of added casein proteins to the peptide profile in Spanish dry-fermented sausages. Int. J. Food Microbiol. 2015, 212, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Mallika, E.N.; Prabhakar, K.; Reddy, P.M. Low Fat Meat Products-An Overview. Vet. World 2009, 2, 364–366. [Google Scholar]
- Jalal, H.; Mir, S.; Wani, S.A.; Sofi, A.H.; Pal, M.A.; Rather, F. Development of low fat meat products. Int. J. Food Nutr. Saf. 2013, 4, 98–107. [Google Scholar]
- European Commission. Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the provision of food information to consumers, amending Regulations (EC) No 1924/2006 and (EC) No 1925/2006 of the European Parliament and of the Council, and repealing Commission Directive 87/250/EEC, Council Directive 90/496/EEC, Commission Directive 1999/10/EC, Directive 2000/13/EC of the European Parliament and of the Council, Commission Directives 2002/67/EC and 2008/5/EC and Commission Regulation (EC). Off. J. Eur. Union L 2011, 304, 18–63. [Google Scholar]
- D’Auria, E.; Mameli, C.; Piras, C.; Cococcioni, L.; Urbani, A.; Zuccotti, G.V.; Roncada, P. Precision medicine in cow’s milk allergy: Proteomics perspectives from allergens to patients. J. Proteom. 2018, 188, 173–180. [Google Scholar] [CrossRef]
- Hochwallner, H.; Schulmeister, U.; Swoboda, I.; Spitzauer, S.; Valenta, R. Cow’s milk allergy: From allergens to new forms of diagnosis, therapy and prevention. Methods 2014, 66, 22–33. [Google Scholar] [CrossRef]
- Miciński, J.; Kowalski, I.M.; Zwierzchowski, G.; Szarek, J.; Pierożyński, B.; Zabłocka, E. Characteristics of cow’s milk proteins including allergenic properties and methods for its reduction. Pol. Ann. Med. 2013, 20, 69–76. [Google Scholar] [CrossRef]
- Villa, C.; Costa, J.; Oliveira, M.B.P.; Mafra, I. Bovine milk allergens: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 137–164. [Google Scholar] [CrossRef]
- Downs, M.L.; Taylor, S.L. Effects of thermal processing on the enzyme-linked immunosorbent assay (ELISA) detection of milk residues in a model food matrix. J. Agric. Food Chem. 2010, 58, 10085–10091. [Google Scholar] [CrossRef]
- Pospiech, M.; Tremlova, B.; Renčová, E.; Randulová, Z. Immunohistochemical detection of soya protein–optimisation and verification of the method. Czech J. Food Sci. 2009, 27, 11–19. [Google Scholar] [CrossRef]
- Řezáčová-Lukášková, Z.; Tremlová, B.; Pospiech, M.; Renčová, E.; Randulová, Z.; Steinhauser, L.; Reichová, A.; Bednář, J. Comparison of immunohistochemical, histochemical and immunochemical methods for the detection of wheat protein allergens in meat samples and cooked, dry, raw and fermented sausage samples. Food Addit. Contam. Part A 2011, 28, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Bednářová, M.; Pospiech, M.; Tremlová, B.; Řezáčová-Lukášková, Z.; Bednář, J. Antigen retrieval and fixation of sections on slides for immunohistochemical detection of soya protein in meat products. J. Food Nutr. Res. 2015, 54, 1–8. [Google Scholar]
- Hendl, J. Review of Statistical Methods of Data Processing; Portál: Prague, Czech Republic, 2004; ISBN 80-7178-820-1. [Google Scholar]
- Petracci, M.; Bianchi, M.; Mudalal, S.; Cavani, C. Functional ingredients for poultry meat products. Trends Food Sci. Technol. 2013, 33, 27–39. [Google Scholar] [CrossRef]
- Barbut, S. Effects of caseinate, whey and milk powders on the texture and microstructure of emulsified chicken meat batters. Lwt-Food Sci. Technol. 2006, 39, 660–664. [Google Scholar] [CrossRef]
- Thomas, M.A.; Lemmer, B. HistoGreen: A new alternative to 3,3′-diaminobenzidine-tetrahydrochloride-dihydrate (DAB) as a peroxidase substrate in immunohistochemistry? Brain Res. Protoc. 2005, 14, 107–118. [Google Scholar] [CrossRef]
- Petrášová, M.; Pospiech, M.; Tremlová, B.; Javůrková, Z. Immunofluorescence detection of pea protein in meat products. Food Addit. Contam. Part A 2016, 33, 1283–1289. [Google Scholar] [CrossRef]
- Trullols, E.; Ruisanchez, I.; Rius, F.X. Validation of qualitative analytical methods. Trac. Trends Anal. Chem. 2004, 23, 137–145. [Google Scholar] [CrossRef]
- European Commission. Commission Decision 2002/657/EC of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off. J. Eur. Union 2002, 221, 8–36. [Google Scholar]
- Magnusson, B. The Fitness for Purpose of Analytical Methods: A Laboratory Guide to Method Validation and Related Topics; Eurachem: Teddington, UK, 2014; ISBN 978-91-87461-59-0. Available online: www.eurachem.org (accessed on 18 November 2020).
- Borková, M.; Snášelova, J. Possibilities of different animal milk detection in milk and dairy products–a review. Czech J. Food Sci. 2005, 23, 41–50. [Google Scholar] [CrossRef]
- Hurley, I.P.; Coleman, R.C.; Ireland, H.E.; Williams, J.H. Use of sandwich IgG ELISA for the detection and quantification of adulteration of milk and soft cheese. Int. Dairy J. 2006, 16, 805–812. [Google Scholar] [CrossRef]
- Schubert-Ullrich, P.; Rudolf, J.; Ansari, P.; Galler, B.; Führer, M.; Molinelli, A.; Baumgartner, S. Commercialized rapid immunoanalytical tests for determination of allergenic food proteins: An overview. Anal. Bioanal. Chem. 2009, 395, 69–81. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the evaluation of allergenic foods and food ingredients for labelling purposes. EFSA J. 2014, 12, 3894.
- Spizzirri, U.G.; Cirillo, G. (Eds.) Food Safety: Innovative Analytical Tools for Safety Assessment; Scrivener Publishing: Salem, MA, USA, 2017. [Google Scholar] [CrossRef]
- Gern, J.E.; Yang, E.; Evrard, H.M.; Sampson, H.A. Allergic reactions to milk-contaminated nondairy products. N. Engl. J. Med. 1991, 324, 976–979. [Google Scholar] [CrossRef]
- Taylor, C.R. Quality assurance and standardization in immunohistochemistry. A proposal for the annual meeting of the Biological Stain Commission, June, 1991. Biotech. Histochem. 1992, 67, 110–117. [Google Scholar] [CrossRef]
- Taylor, C.; Shi, S.R.; Cote, R. Antigen retrieval for immunohistochemistry. Appl. Immunohistochem. 1996, 4, 144–166. [Google Scholar]
- Fuchs, M. Potravinové Alergie; Food Allergies; Maxdorf: Praha, Czech Republic, 2013; p. 43. ISBN 978-80-7345-335-0. [Google Scholar]
- Wal, J.M. Cow’s milk allergens. Allergy 1998, 53, 1013–1022. [Google Scholar] [CrossRef]
- Bu, G.; Luo, Y.; Zheng, Z.; Zheng, H. Effect of heat treatment on the antigenicity of bovine α-lactalbumin and β-lactoglobulin in whey protein isolate. Food Agric. Immunol. 2009, 20, 195–206. [Google Scholar] [CrossRef]
- Diaz-Amigo, C. Towards a comprehensive validation of ELISA kits for food allergens. Case 2-milk. Food Anal. Methods 2010, 3, 351–356. [Google Scholar] [CrossRef]
- Nishanthi, M.; Chandrapala, J.; Vasiljevic, T. Properties of whey protein concentrate powders obtained by spray drying of sweet, salty and acid whey under varying storage conditions. J. Food Eng. 2017, 214, 137–146. [Google Scholar] [CrossRef]
- Matsuda, R.; Yoshioka, Y.; Akiyama, H.; Aburatani, K.; Watanabe, Y.; Matsumoto, T.; Morishita, N.; Sato, H.; Mishima, T.; Gamo, R.; et al. Interlaboratory evaluation of two enzyme-linked immunosorbent assay kits for the detection of egg, milk, wheat, buckwheat, and peanut in foods. J. AOAC Int. 2006, 89, 1600–1608. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Yagi, T.; Kato, A.; Yamamoto, S.; Akimoto, M.; Arihara, K. Interlaboratory study of ELISA kits for the detection of egg and milk protein in processed foods. J. AOAC Int. 2015, 98, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Spychaj, A.; Pospiech, E.; Iwańska, E.; Montowska, M. Detection of allergenic additives in processed meat products. J. Sci. Food Agric. 2018, 98, 4807–4815. [Google Scholar] [CrossRef] [PubMed]
- González-Fernández, C.; Santos, E.M.; Jaime, I.; Rovira, J. Influence of starter cultures and sugar concentrations on biogenic amine contents in chorizo dry sausage. Food Microbiol. 2003, 20, 275–284. [Google Scholar] [CrossRef]
- Nowak-Wegrzyn, A.; Shapiro, G.G.; Beyer, K.; Bardina, L.; Sampson, H.A. Contamination of dry powder inhalers for asthma with milk proteins containing lactose. J. Allergy Clin. Immunol. 2004, 113, 558–560. [Google Scholar] [CrossRef]
- Youssef, M.K.; Barbut, S. Physicochemical effects of the lipid phase and protein level on meat emulsion stability, texture, and microstructure. J. Food Sci. 2010, 75, S108–S114. [Google Scholar] [CrossRef]
- Zaffran, V.D.; Sathe, S.K. Immunoreactivity of Biochemically Purified Amandin from Thermally Processed Almonds (Prunus dulcis L.). J. Food Sci. 2018, 83, 1805–1809. [Google Scholar] [CrossRef]
- Dupont, C.; Chouraqui, J.P.; Linglart, A.; Bocquet, A.; Darmaun, D.; Feillet, F.; Frelut, M.L.; Girardet, J.P.; Hankard, R.; Rozé, J.C.; et al. Nutritional management of cow’s milk allergy in children: An update. Arch. Pédiatrie 2018, 25, 236–243. [Google Scholar] [CrossRef]
- Sletten, G.B.; Holden, L.; Egaas, E.; Faeste, C.K. Differential influence of the degree of processing on immunogenicity following proteolysis of casein and β-lactoglobulin. Food Agric. Immunol. 2008, 19, 213–228. [Google Scholar] [CrossRef]
| Model Sample | Caseinate Addition | Primary Antibody Dilution | ||||
|---|---|---|---|---|---|---|
| 1:10 | 1:100 | 1:500 | 1:1000 | 1:1500 | ||
| C5 | 1% | +++ | +++ | +++ | +++ | +++ |
| C6 | 2.5% | +++ | +++ | +++ | ++ | + |
| Chromogens | Background Staining | ||
|---|---|---|---|
| Calleja | Toluidine Blue | Fast Nuclei Red | |
| DAB | + | + | - |
| HistoGreen | - | - | +++ |
| Caseinate Addition (%) | Quantity of Casein (mg kg−1) | No. of Repetitions | Positive/Negative |
|---|---|---|---|
| 0 | 0 | 8 | 0/8 |
| 0.0001 | 9.4 | 8 | 8/0 |
| 0.001 | 94 | 8 | 8/0 |
| 0.01 | 940 | 8 | 8/0 |
| 0.1 | 9400 | 8 | 8/0 |
| 1 | 94,000 | 8 | 8/0 |
| 10 | 940,000 | 8 | 8/0 |
| Repetition | R1 | R2 |
|---|---|---|
| 1 | Yes | No |
| 2 | Yes | No |
| 3 | Yes | No |
| 4 | Yes | No |
| 5 | Yes | No |
| Correspondence | 100% | 100% |
| Examiner | R1 | R2 |
|---|---|---|
| A | Yes | No |
| A | Yes | No |
| A | Yes | No |
| A | Yes | No |
| A | Yes | No |
| B | Yes | No |
| B | Yes | No |
| B | Yes | No |
| B | Yes | No |
| B | Yes | No |
| Correspondence | 100% | 100% |
| Sample | C1 | C2 | C3 | C4 | C5 | C6 | C7 | W1 | W2 | W3 | W4 | W5 | M1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IHC | + | + | + | + | + | + | + | + | + | + | + | + | + |
| ELISA | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Meat Product | ||||||||||||||||||||||||||
| Type of Additives and Identification | M2 | M3 | M4 | M5 | M6 | M7 | M8 | M9 | M10 | M11 | M12 | M13 | M14 | M15 | M16 | M17 | M18 | M19 | M20 | Co1 | Co2 | Ch1 | Ch2 | Ch3 | L1 | |
| IHC | + | + | + | + | + | + | + | + | + | − * | +/− * | + | + | + | − * | − | − * | + | + | − | − | + | + | + | + | |
| ELISA | + | + | + | + | − * | + | + | + | + | + | + | − * | + | − * | + | − | + | + | + | − | + ** | + | + | + | + | |
| Meat Product | FR | CP | HM | CP | FR | SA | ML | FR | ML | SA | ||||||||||||||||
| Correspondence of IHC and ELISA methods | ||||||||||||||||||||||||||
| Meat Product | Total (%) | |||||||||||||||||||||||||
| FR | 90 | 5/6 (83%) | 3/3 (100%) | 1/1 (100%) | ||||||||||||||||||||||
| CP | 44 | 1/1 (100%) | 3/8 (37.5%) | |||||||||||||||||||||||
| HM | 100 | 2/2 (100%) | ||||||||||||||||||||||||
| SA | 50 | 0/1 (0%) | 1/1 (100%) | |||||||||||||||||||||||
| ML | 100 | 1/1 (100%) | 1/1 (100%) | |||||||||||||||||||||||
| All Types | 72 | 18/25 (72) | ||||||||||||||||||||||||
| IHC+ | IHC− | Sum | |
|---|---|---|---|
| ELISA+ | 29 | 5 | 34 |
| ELISA− | 3 | 2 | 5 |
| Sum | 33 | 7 | 40 |
| Chst | 0.5 | ||
| p = | 0.4795 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalčáková, L.; Pospiech, M.; Tremlová, B.; Javůrková, Z.; Chernukha, I. Development of Immunohistochemical Methods for Casein Detection in Meat Products. Foods 2021, 10, 28. https://doi.org/10.3390/foods10010028
Kalčáková L, Pospiech M, Tremlová B, Javůrková Z, Chernukha I. Development of Immunohistochemical Methods for Casein Detection in Meat Products. Foods. 2021; 10(1):28. https://doi.org/10.3390/foods10010028
Chicago/Turabian StyleKalčáková, Ludmila, Matej Pospiech, Bohuslava Tremlová, Zdeňka Javůrková, and Irina Chernukha. 2021. "Development of Immunohistochemical Methods for Casein Detection in Meat Products" Foods 10, no. 1: 28. https://doi.org/10.3390/foods10010028
APA StyleKalčáková, L., Pospiech, M., Tremlová, B., Javůrková, Z., & Chernukha, I. (2021). Development of Immunohistochemical Methods for Casein Detection in Meat Products. Foods, 10(1), 28. https://doi.org/10.3390/foods10010028






