Food Bioactive Compounds and Emerging Techniques for Their Extraction: Polyphenols as a Case Study
Abstract
1. Introduction
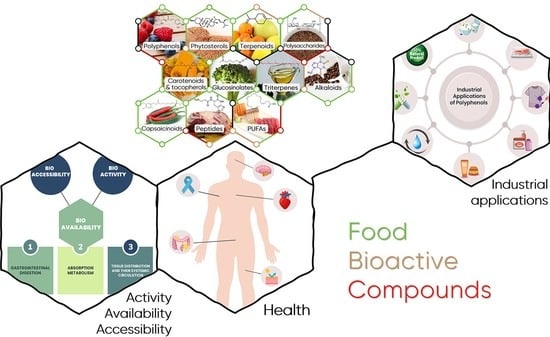
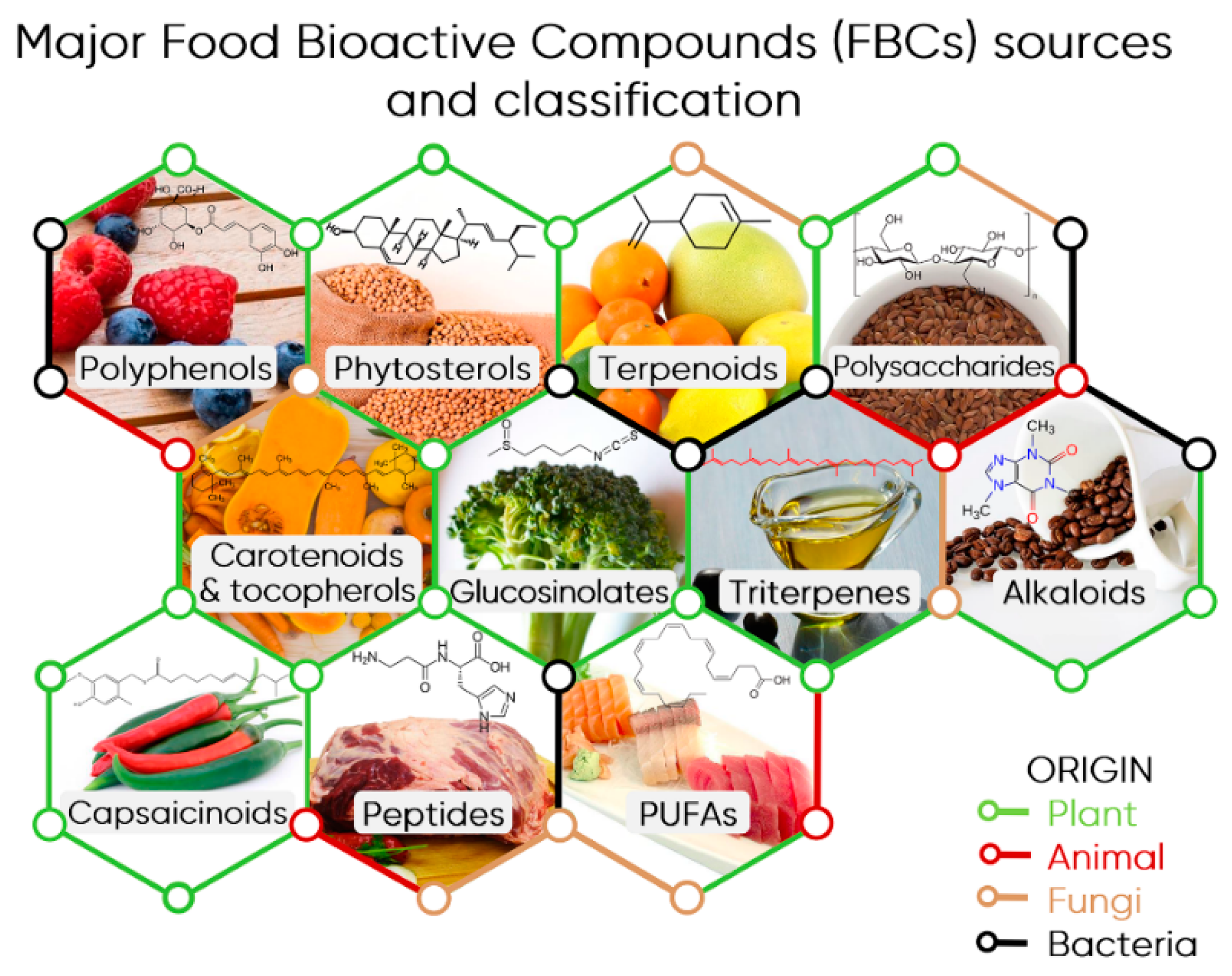
2. Food Bioactive Compounds from Triterpenes to Polyphenols
2.1. FBCs Classification
2.2. Overview of Main FBC Classes
3. Structure, Classification, and Biosynthesis of Polyphenols
Structure and Classification
4. Polyphenols and Health
4.1. Cancer
4.2. Cardiovascular Diseases (CVDs)
4.3. Neurodegenerative Diseases (NDDs)
5. Bioaccessibility and Bioavailability of Polyphenols
6. Potential Applications—Food and Cosmetic Industries and Environment
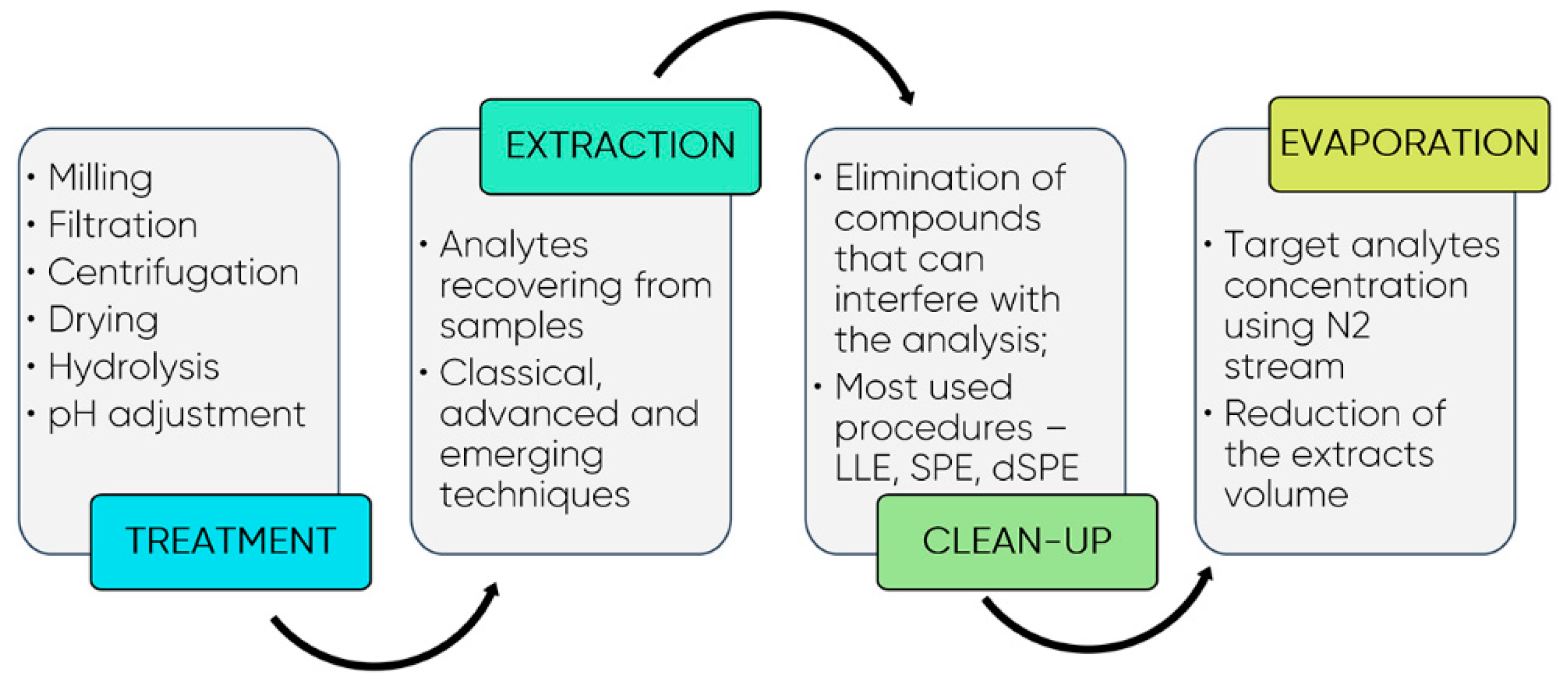
7. Emerging Extraction Techniques and Methodologies for Polyphenols Quantification
Analytical Methods for Quantification
8. Remarks and Future Trends
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 4CL | 4-coumaryl:CoA ligase |
| AD | Alzheimer’s disease |
| ANOVA | analysis of variance |
| ANS | anthocyanidin synhase |
| At1R | Angiotensin II type 1 receptor |
| C4H | cinnamic acid-4-hydroxylase |
| CETs | classical extraction techniques |
| CHR | chalcone reductase |
| CHS | chalcone synthase |
| CK | creatine kinase |
| CNS | central nervous system |
| cTnT | cardiac troponin |
| CVDs | Cardiovascular diseases |
| eBASIS | Bioactive Substances in Food Information Systems |
| DFR | dihydroflavanone reductase |
| DHA | docosahexaenoic acid |
| eNOS | Endothelial nitric oxide synthase |
| dSPE | dispersive solid-phase extraction |
| EPA | eicosapentaenoic acid |
| FBCs | Food bioactive compounds |
| FHT | flavanone hydroxytransferase |
| FLS | flavonol synthase |
| FOS | fructooligosaccharides |
| FSI | flanone synthase |
| GCB | graphitized carbon black |
| GC-MS | gas chromatography mass/spectrometry |
| GI | gastrointestinal tract |
| GSLs | Glucosinolates |
| HDL-C | high-density lipoprotein cholesterol |
| HILIC | Hydrophilic interaction liquid chromatography |
| HVED | high voltage electric discharge |
| IFS | isoflavanone synthase |
| LAR | leucoanthocyanidin synthase |
| LC-MS | liquid chromatography/mass spectrometry |
| LDH | lactate dehydrogenase |
| LDL | low-density lipoprotein |
| LLE | liquid-liquid extraction |
| LVEF | ventricular ejection fraction |
| LVIDs | systolic internal diameter |
| MAE | microwave-assisted extraction |
| NCDs | non-communicable diseases |
| NDDs | Neurodegenerative diseases |
| NF-kB | Nuclear factor kappa B |
| NMR | nuclear magnetic resonance |
| NPS | Novel Psychoactive Substances |
| Nrf2 | Nuclear factor (erythroid-derived 2)-like 2 |
| PAL | phenylalanine ammonia lyase |
| PAHs | polycyclic aromatic hydrocarbon |
| PCA | principal components analysis |
| PCBs | Polychlorinated Biphenyls |
| PD | Parkinson’s disease |
| PEF | pulsed electric fields |
| PHWE | pressurized hot water extraction |
| PLE | pressurized liquid extraction |
| PSA | Primary secondary amine |
| PUFAs | Polyunsaturated fatty acids |
| ROS | reactive oxygen species |
| SPE | solid-phase extraction |
| SbCE | Subcritical extraction |
| SFE | supercritical fluid extraction |
| StSy | stilbene synthase |
| TAL | tyrosine ammonia lyase |
| TC | total cholesterol |
| UAE | ultrasound-assisted extraction |
| USDA | United States Department of Agriculture |
| µETs | microextraction techniques |
References
- NCD Countdown. NCD Countdown 2030: Worldwide trends in non-communicable disease mortality and progress towards Sustainable Development Goal target 3.4. Lancet 2018, 392, 1072–1088. [Google Scholar] [CrossRef]
- Afshin, A.; Sur, P.J.; Fay, K.A.; Cornaby, L.; Ferrara, G.; Salama, J.S.; Mullany, E.C.; Abate, K.H.; Abbafati, C.; Abebe, Z.; et al. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef]
- Mentella, M.C.; Scaldaferri, F.; Ricci, C.; Gasbarrini, A.; Miggiano, G.A.D. Cancer and Mediterranean Diet: A Review. Nutrients 2019, 11, 2059. [Google Scholar] [CrossRef]
- Sofi, F.; Cesari, F.; Abbate, R.; Gensini, G.F.; Casini, A. Adherence to Mediterranean diet and health status: Meta-analysis. BMJ 2008, 337, a1344. [Google Scholar] [CrossRef] [PubMed]
- Biesalski, H.K.; Dragsted, L.O.; Elmadfa, I.; Grossklaus, R.; Muller, M.; Schrenk, D.; Walter, P.; Weber, P. Bioactive compounds: Definition and assessment of activity. Nutrition 2009, 25, 1202–1205. [Google Scholar] [CrossRef] [PubMed]
- Bernhoft, A. A brief review on bioactive compounds in plants. Bioact. Compd. Plants-Benefits Risks Man Anim. 2010, 50, 11–17. [Google Scholar]
- Plumb, J.; Pigat, S.; Bompola, F.; Cushen, M.; Pinchen, H.; Norby, E.; Astley, S.; Lyons, J.; Kiely, M.; Finglas, P. eBASIS (Bioactive Substances in Food Information Systems) and Bioactive Intakes: Major Updates of the Bioactive Compound Composition and Beneficial Bioeffects Database and the Development of a Probabilistic Model to Assess Intakes in Europe. Nutrients 2017, 9, 320. [Google Scholar] [CrossRef]
- Dudareva, N.; Pichersky, E. Biochemical and molecular genetic aspects of floral scents. Plant Physiol. 2000, 122, 627–633. [Google Scholar] [CrossRef]
- Astley, S.; Finglas, P. Nutrition and Health. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Bhagwat, S.; Haytowitz, D.B. USDA Database for the Flavonoid Content of Selected Foods. Release 3.2 (November 2015). Nutrient Data Laboratory, Beltsville Human Nutrition Research Center. Agric. Res. Serv. US Dep. Agric. 2016. [Google Scholar] [CrossRef]
- Neveu, V.; Perez-Jiménez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010. [Google Scholar] [CrossRef]
- Albenzio, M.; Santillo, A.; Caroprese, M.; Della Malva, A.; Marino, R. Bioactive Peptides in Animal Food Products. Foods 2017, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Stadnik, J.; Kęska, P. Meat and fermented meat products as a source of bioactive peptides. Acta Sci. Pol. Technol. Aliment. 2015, 14, 181–190. [Google Scholar] [CrossRef]
- Ferreira, I.C.; Barros, L.; Abreu, R.M. Antioxidants in wild mushrooms. Curr. Med. Chem. 2009, 16, 1543–1560. [Google Scholar] [CrossRef] [PubMed]
- Mamo, G. Anaerobes as Sources of Bioactive Compounds and Health Promoting Tools. Adv. Biochem. Eng. Biotechnol. 2016, 156, 433–464. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.A.; Connolly, J.D. Triterpenoids. Nat. Prod. Rep. 2018, 35, 1294–1329. [Google Scholar] [CrossRef] [PubMed]
- Moreau, R.A.; Nyström, L.; Whitaker, B.D.; Winkler-Moser, J.K.; Baer, D.J.; Gebauer, S.K.; Hicks, K.B. Phytosterols and their derivatives: Structural diversity, distribution, metabolism, analysis, and health-promoting uses. Prog. Lipid Res. 2018, 70, 35–61. [Google Scholar] [CrossRef] [PubMed]
- Tetali, S.D. Terpenes and isoprenoids: A wealth of compounds for global use. Planta 2019, 249, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.L.; Hefle, S.L. Chapter 16—Naturally Occurring Toxicants in Foods. In Foodborne Diseases, 3rd ed.; Dodd, C.E.R., Aldsworth, T., Stein, R.A., Cliver, D.O., Riemann, H.P., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 327–344. [Google Scholar] [CrossRef]
- Prieto, M.A.; López, C.J.; Simal-Gandara, J. Glucosinolates: Molecular structure, breakdown, genetic, bioavailability, properties and healthy and adverse effects. Adv. Food Nutr. Res. 2019, 90, 305–350. [Google Scholar] [CrossRef]
- Porter, N.T.; Martens, E.C. The Critical Roles of Polysaccharides in Gut Microbial Ecology and Physiology. Annu. Rev. Microbiol. 2017, 71, 349–369. [Google Scholar] [CrossRef]
- Marion-Letellier, R.; Savoye, G.; Ghosh, S. Polyunsaturated fatty acids and inflammation. IUBMB Life 2015, 67, 659–667. [Google Scholar] [CrossRef]
- Shanab, S.M.M.; Hafez, R.M.; Fouad, A.S. A review on algae and plants as potential source of arachidonic acid. J. Adv. Res. 2018, 11, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef] [PubMed]
- Kondratyuk, T.P.; Pezzuto, J.M. Natural Product Polyphenols of Relevance to Human Health. Pharm. Biol. 2004, 42, 46–63. [Google Scholar] [CrossRef]
- Belščak-Cvitanović, A.; Durgo, K.; Huđek, A.; Bačun-Družina, V.; Komes, D. Overview of polyphenols and their properties. In Polyphenols: Properties, Recovery, and Applications; Galanakis, C.M., Ed.; Woodhead Publishing: Cambridge, UK, 2018; pp. 3–44. [Google Scholar] [CrossRef]
- Amawi, H.; Ashby, C.R.; Tiwari, A.K. Cancer chemoprevention through dietary flavonoids: What’s limiting? Chin. J. Cancer 2017, 36, 50. [Google Scholar] [CrossRef]
- Gonçalves, J.; Mendes, B.; Silva, C.L.; Câmara, J.S. Development of a novel microextraction by packed sorbent-based approach followed by ultrahigh pressure liquid chromatography as a powerful technique for quantification phenolic constituents of biological interest in wines. J. Chromatogr. A 2012, 1229, 13–23. [Google Scholar] [CrossRef]
- Aguiar, J.; Gonçalves, J.L.; Alves, V.L.; Câmara, J.S. Chemical Fingerprint of Free Polyphenols and Antioxidant Activity in Dietary Fruits and Vegetables Using a Non-Targeted Approach Based on QuEChERS Ultrasound-Assisted Extraction Combined with UHPLC-PDA. Antioxidants (Basel) 2020, 9, 305. [Google Scholar] [CrossRef]
- Pietta, P.; Minoggio, M.; Bramati, L. Plant Polyphenols: Structure, Occurrence and Bioactivity. In Studies in Natural Products Chemistry; Rahman, A.-U., Ed.; Elsevier: Amsterdam, The Netherlands, 2003; Volume 28, pp. 257–312. [Google Scholar]
- Oliveira, L.D.L.D.; Carvalho, M.V.D.; Melo, L. Health promoting and sensory properties of phenolic compounds in food. Revista Ceres 2014, 61, 764–779. [Google Scholar] [CrossRef]
- Alihosseini, F.; Sun, G. Antibacterial colorants for textiles. In Functional Textiles for Improved Performance, Protection and Health; Pan, N., Sun, G., Eds.; Woodhead Publishing: Duxford, UK, 2011; pp. 376–403. [Google Scholar]
- Arts, I.C.; van de Putte, B.; Hollman, P.C. Catechin contents of foods commonly consumed in The Netherlands. 1. Fruits, vegetables, staple foods, and processed foods. J. Agric. Food. Chem. 2000, 48, 1746–1751. [Google Scholar] [CrossRef]
- Arts, I.C.; van De Putte, B.; Hollman, P.C. Catechin contents of foods commonly consumed in The Netherlands. 2. Tea, wine, fruit juices, and chocolate milk. J. Agric. Food. Chem. 2000, 48, 1752–1757. [Google Scholar] [CrossRef]
- Pérez-Chabela, M.L.; Hernández-Alcántara, A.M. Agroindustrial Coproducts as Sources of Novel Functional Ingredients. In Food Processing for Increased Quality and Consumption; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 219–250. [Google Scholar]
- Klejdus, B.; Vacek, J.; Benešová, L.; Kopecký, J.; Lapčík, O.; Kubáň, V. Rapid-resolution HPLC with spectrometric detection for the determination and identification of isoflavones in soy preparations and plant extracts. Anal. Bioanal. Chem. 2007, 389, 2277–2285. [Google Scholar] [CrossRef] [PubMed]
- Krizova, L.; Dadakova, K.; Kasparovska, J.; Kasparovsky, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef] [PubMed]
- Francavilla, A.; Joye, I.J. Anthocyanins in Whole Grain Cereals and Their Potential Effect on Health. Nutrients 2020, 12, 2922. [Google Scholar] [CrossRef] [PubMed]
- Celli, G.B.; Tan, C.; Selig, M.J. Anthocyanidins and Anthocyanins. In Encyclopedia of Food Chemistry; Melton, L., Varelis, P., Shahidi, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 3, pp. 218–223. [Google Scholar]
- Bureau, S.; Renard, C.M.G.C.; Reich, M.; Ginies, C.; Audergon, J.-M. Change in anthocyanin concentrations in red apricot fruits during ripening. LWT—Food Sci. Technol. 2009, 42, 372–377. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Bento, C.; Jesus, F.; Alves, G.; Silva, L.R. Chapter 2—Sweet Cherry Phenolic Compounds: Identification, Characterization, and Health Benefits. In Studies in Natural Products Chemistry; Attaur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 59, pp. 31–78. [Google Scholar]
- Saibabu, V.; Fatima, Z.; Khan, L.A.; Hameed, S. Therapeutic Potential of Dietary Phenolic Acids. Adv. Pharmacol. Sci. 2015, 2015, 823539. [Google Scholar] [CrossRef]
- Teixeira, J.; Gaspar, A.; Garrido, E.M.; Garrido, J.; Borges, F. Hydroxycinnamic acid antioxidants: An electrochemical overview. BioMed Res. Int. 2013, 2013, 251754. [Google Scholar] [CrossRef]
- Chong, J.; Poutaraud, A.; Hugueney, P. Metabolism and roles of stilbenes in plants. Plant Sci. 2009, 177, 143–155. [Google Scholar] [CrossRef]
- Sirerol, J.A.; Rodríguez, M.L.; Mena, S.; Asensi, M.A.; Estrela, J.M.; Ortega, A.L. Role of Natural Stilbenes in the Prevention of Cancer. Oxidative Med. Cell. Longev. 2016, 2016, 3128951. [Google Scholar] [CrossRef]
- Nikolić, I.; Savić-Gajić, I.; Tačić, A.; Savić, I. Classification and biological activity of phytoestrogens: A review. Adv. Technol. 2017, 6, 96–106. [Google Scholar] [CrossRef]
- Fraga-Corral, M.; García-Oliveira, P.; Pereira, A.G.; Lourenço-Lopes, C.; Jimenez-Lopez, C.; Prieto, M.A.; Simal-Gandara, J. Technological Application of Tannin-Based Extracts. Molecules 2020, 25, 614. [Google Scholar] [CrossRef]
- Khanbabaee, K.; van Ree, T. Tannins: Classification and Definition. Nat. Prod. Rep. 2001, 18, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Koleckar, V.; Kubikova, K.; Rehakova, Z.; Kuca, K.; Jun, D.; Jahodar, L.; Opletal, L. Condensed and hydrolysable tannins as antioxidants influencing the health. Mini Rev. Med. Chem. 2008, 8, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-X.; Wijesekara, I.; Li, Y.; Kim, S.-K. Phlorotannins as bioactive agents from brown algae. Process Biochem. 2011, 46, 2219–2224. [Google Scholar] [CrossRef]
- Saltveit, M.E. Synthesis and Metabolism of Phenolic Compounds. Fruit Veg. Phytochem. 2017, 115–124. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Plant Physiology and Development, 6th ed.; Sinauer Associates: Sunderland, MA, USA, 2014; p. 700. [Google Scholar]
- Fowler, Z.L.; Koffas, M.A.G. Biosynthesis and biotechnological production of flavanones: Current state and perspectives. Appl. Microbiol. Biotechnol. 2009, 83, 799–808. [Google Scholar] [CrossRef]
- de Araujo, F.F.; de Paulo Farias, D.; Neri-Numa, I.A.; Pastore, G.M. Polyphenols and their applications: An approach in food chemistry and innovation potential. Food Chem. 2021, 338, 127535. [Google Scholar] [CrossRef]
- Corrêa, R.C.G.; Peralta, R.M.; Haminiuk, C.W.I.; Maciel, G.M.; Bracht, A.; Ferreira, I.C.F.R. New phytochemicals as potential human anti-aging compounds: Reality, promise, and challenges. Crit. Rev. Food Sci. Nutr. 2018, 58, 942–957. [Google Scholar] [CrossRef]
- Daglia, M. New advances in biotechnology of phytochemicals. Biotechnol. Adv. 2020, 38, 107445. [Google Scholar] [CrossRef]
- Estrela, J.M.; Mena, S.; Obrador, E.; Benlloch, M.; Castellano, G.; Salvador, R.; Dellinger, R.W. Polyphenolic Phytochemicals in Cancer Prevention and Therapy: Bioavailability versus Bioefficacy. J. Med. Chem. 2017, 60, 9413–9436. [Google Scholar] [CrossRef]
- Thyagarajan, A.; Forino, A.S.; Konger, R.L.; Sahu, R.P. Dietary Polyphenols in Cancer Chemoprevention: Implications in Pancreatic Cancer. Antioxidants 2020, 9, 651. [Google Scholar] [CrossRef]
- Khan, H.; Reale, M.; Ullah, H.; Sureda, A.; Tejada, S.; Wang, Y.; Zhang, Z.-J.; Xiao, J. Anti-cancer effects of polyphenols via targeting p53 signaling pathway: Updates and future directions. Biotechnol. Adv. 2020, 38, 107385. [Google Scholar] [CrossRef] [PubMed]
- Caleja, C.; Ribeiro, A.; Barreiro, M.F.; Ferreira, I.C.F.R. Phenolic Compounds as Nutraceuticals or Functional Food Ingredients. Curr. Pharm. Des. 2017, 23, 2787–2806. [Google Scholar] [CrossRef]
- Chen, L.C.; Huang, H.L.; Huangfu, W.C.; Yen, S.C.; Ngo, S.T.; Wu, Y.W.; Lin, T.E.; Sung, T.Y.; Lien, S.T.; Tseng, H.J.; et al. Biological Evaluation of Selected Flavonoids as Inhibitors of MNKs Targeting Acute Myeloid Leukemia. J. Nat. Prod. 2020, 83, 2967–2975. [Google Scholar] [CrossRef]
- Davoodvandi, A.; Darvish, M.; Borran, S.; Nejati, M.; Mazaheri, S.; Reza Tamtaji, O.; Hamblin, M.R.; Masoudian, N.; Mirzaei, H. The therapeutic potential of resveratrol in a mouse model of melanoma lung metastasis. Int. Immunopharmacol. 2020, 88, 106905. [Google Scholar] [CrossRef]
- Su, C.-C.; Wang, C.-J.; Huang, K.-H.; Lee, Y.-J.; Chan, W.-M.; Chang, Y.-C. Anthocyanins from Hibiscus sabdariffa calyx attenuate in vitro and in vivo melanoma cancer metastasis. J. Funct. Foods 2018, 48, 614–631. [Google Scholar] [CrossRef]
- Kuete, V.; Mbaveng, A.T.; Nono, E.C.; Simo, C.C.; Zeino, M.; Nkengfack, A.E.; Efferth, T. Cytotoxicity of seven naturally occurring phenolic compounds towards multi-factorial drug-resistant cancer cells. Phytomedicine 2016, 23, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, H.; Wang, S.; Gai, C.; Cui, X.; Xu, Z.; Li, W.; Zhang, W. Targeted delivery of quercetin by nanoparticles based on chitosan sensitizing paclitaxel-resistant lung cancer cells to paclitaxel. Mater. Sci. Eng. C 2021, 119, 111442. [Google Scholar] [CrossRef]
- Yan, J.; Wang, Q.; Zheng, X.; Sun, H.; Zhou, Y.; Li, D.; Lin, Y.; Wang, X. Luteolin enhances TNF-related apoptosis-inducing ligand’s anticancer activity in a lung cancer xenograft mouse model. Biochem. Biophys. Res. Commun. 2012, 417, 842–846. [Google Scholar] [CrossRef] [PubMed]
- Tajaldini, M.; Samadi, F.; Khosravi, A.; Ghasemnejad, A.; Asadi, J. Protective and anticancer effects of orange peel extract and naringin in doxorubicin treated esophageal cancer stem cell xenograft tumor mouse model. Biomed. Pharmacother. 2020, 121, 109594. [Google Scholar] [CrossRef]
- Li, H.; Sureda, A.; Devkota, H.P.; Pittala, V.; Barreca, D.; Silva, A.S.; Tewari, D.; Xu, S.; Nabavi, S.M. Curcumin, the golden spice in treating cardiovascular diseases. Biotechnol. Adv. 2020, 38, 107343. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, Y.; Song, F.; Yao, Y.; Ya, F.; Li, D.; Ling, W.; Yang, Y. Effects of purified anthocyanin supplementation on platelet chemokines in hypocholesterolemic individuals: A randomized controlled trial. Nutr. Metab. (Lond.) 2016, 13, 86. [Google Scholar] [CrossRef] [PubMed]
- Samavat, H.; Newman, A.R.; Wang, R.; Yuan, J.M.; Wu, A.H.; Kurzer, M.S. Effects of green tea catechin extract on serum lipids in postmenopausal women: A randomized, placebo-controlled clinical trial. Am. J. Clin. Nutr. 2016, 104, 1671–1682. [Google Scholar] [CrossRef] [PubMed]
- Lippert, E.; Ruemmele, P.; Obermeier, F.; Goelder, S.; Kunst, C.; Rogler, G.; Dunger, N.; Messmann, H.; Hartmann, A.; Endlicher, E. Anthocyanins Prevent Colorectal Cancer Development in a Mouse Model. Digestion 2017, 95, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Chai, K.; Li, X.; Zhu, Y.; Huang, W. Luteolin exerts pro-apoptotic effect and anti-migration effects on A549 lung adenocarcinoma cells through the activation of MEK/ERK signaling pathway. Chem. Biol. Interact. 2016, 257, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Ting, Y.; Chiou, Y.-S.; Pan, M.-H.; Ho, C.-T.; Huang, Q. In vitro and in vivo anti-cancer activity of tangeretin against colorectal cancer was enhanced by emulsion-based delivery system. J. Funct. Foods 2015, 15, 264–273. [Google Scholar] [CrossRef]
- Kim, S.H.; Hwang, K.A.; Choi, K.C. Treatment with kaempferol suppresses breast cancer cell growth caused by estrogen and triclosan in cellular and xenograft breast cancer models. J. Nutr. Biochem. 2016, 28, 70–82. [Google Scholar] [CrossRef]
- Chatterjee, K.; Mukherjee, S.; Vanmanen, J.; Banerjee, P.; Fata, J.E. Dietary Polyphenols, Resveratrol and Pterostilbene Exhibit Antitumor Activity on an HPV E6-Positive Cervical Cancer Model: An in vitro and in vivo Analysis. Front. Oncol. 2019, 9, 352. [Google Scholar] [CrossRef]
- McCormack, D.E.; Mannal, P.; McDonald, D.; Tighe, S.; Hanson, J.; McFadden, D. Genomic Analysis of Pterostilbene Predicts Its Antiproliferative Effects Against Pancreatic Cancer In Vitro and In Vivo. J. Gastrointest. Surg. 2012, 16, 1136–1143. [Google Scholar] [CrossRef]
- Hassabou, N.F.; Farag, A.F. Anticancer effects induced by artichoke extract in oral squamous carcinoma cell lines. J. Egypt. Natl. Cancer Inst. 2020, 32, 17. [Google Scholar] [CrossRef]
- Wang, F.; Fan, K.; Zhao, Y.; Xie, M.-L. Apigenin attenuates TGF-β1-stimulated cardiac fibroblast differentiation and extracellular matrix production by targeting miR-155-5p/c-Ski/Smad pathway. J. Ethnopharmacol. 2021, 265, 113195. [Google Scholar] [CrossRef]
- Seyyedebrahimi, S.; Khodabandehloo, H.; Nasli Esfahani, E.; Meshkani, R. The effects of resveratrol on markers of oxidative stress in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled clinical trial. Acta Diabetol. 2018, 55, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Sun, X.; Xu, C. Protective effects of resveratrol improve cardiovascular function in rats with diabetes. Exp. Ther. Med. 2017. [Google Scholar] [CrossRef]
- Abdel-Moneim, A.; Yousef, A.I.; Abd El-Twab, S.M.; Abdel Reheim, E.S.; Ashour, M.B. Gallic acid and p-coumaric acid attenuate type 2 diabetes-induced neurodegeneration in rats. Metab. Brain Dis. 2017, 32, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Tu, S.; Xiao, F.; Min, X.; Chen, H.; Fan, X.; Cao, K. Catechin Attenuates Coronary Heart Disease in a Rat Model by Inhibiting Inflammation. Cardiovasc. Toxicol. 2018, 18, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Saad, S.; Ahmad, I.; Kawish, S.M.; Khan, U.A.; Ahmad, F.J.; Ali, A.; Jain, G.K. Improved cardioprotective effects of hesperidin solid lipid nanoparticles prepared by supercritical antisolvent technology. Colloids Surf. B Biointerfaces 2020, 187, 110628. [Google Scholar] [CrossRef]
- Rolnik, A.; Żuchowski, J.; Stochmal, A.; Olas, B. Quercetin and kaempferol derivatives isolated from aerial parts of Lens culinaris Medik as modulators of blood platelet functions. Ind. Crop. Prod. 2020, 152, 112536. [Google Scholar] [CrossRef]
- Perez, A.; Gonzalez-Manzano, S.; Jimenez, R.; Perez-Abud, R.; Haro, J.M.; Osuna, A.; Santos-Buelga, C.; Duarte, J.; Perez-Vizcaino, F. The flavonoid quercetin induces acute vasodilator effects in healthy volunteers: Correlation with beta-glucuronidase activity. Pharmacol. Res. 2014, 89, 11–18. [Google Scholar] [CrossRef]
- Magyar, K.; Halmosi, R.; Palfi, A.; Feher, G.; Czopf, L.; Fulop, A.; Battyany, I.; Sumegi, B.; Toth, K.; Szabados, E. Cardioprotection by resveratrol: A human clinical trial in patients with stable coronary artery disease. Clin. Hemorheol. Microcirc. 2012, 50, 179–187. [Google Scholar] [CrossRef]
- Liu, X.X.; Li, S.H.; Chen, J.Z.; Sun, K.; Wang, X.J.; Wang, X.G.; Hui, R.T. Effect of soy isoflavones on blood pressure: A meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 463–470. [Google Scholar] [CrossRef]
- Huang, H.; Chen, G.; Liao, D.; Zhu, Y.; Pu, R.; Xue, X. The effects of resveratrol intervention on risk markers of cardiovascular health in overweight and obese subjects: A pooled analysis of randomized controlled trials. Obes. Rev. 2016, 17, 1329–1340. [Google Scholar] [CrossRef]
- Alves-Santos, A.M.; Sugizaki, C.S.A.; Lima, G.C.; Naves, M.M.V. Prebiotic effect of dietary polyphenols: A systematic review. J. Funct. Foods 2020, 74, 104169. [Google Scholar] [CrossRef]
- Giacoppo, S.; Galuppo, M.; Lombardo, G.E.; Ulaszewska, M.M.; Mattivi, F.; Bramanti, P.; Mazzon, E.; Navarra, M. Neuroprotective effects of a polyphenolic white grape juice extract in a mouse model of experimental autoimmune encephalomyelitis. Fitoterapia 2015, 103, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Kent, K.; Charlton, K.; Roodenrys, S.; Batterham, M.; Potter, J.; Traynor, V.; Gilbert, H.; Morgan, O.; Richards, R. Consumption of anthocyanin-rich cherry juice for 12 weeks improves memory and cognition in older adults with mild-to-moderate dementia. Eur. J. Nutr. 2017, 56, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, R.; Francioso, A.; d’Erme, M.; Trovato, M.; Mancini, P.; Piacentini, L.; Casale, A.M.; Wessjohann, L.; Gazzino, R.; Costantino, P.; et al. Anti-Inflammatory Activity of A Polyphenolic Extract from Arabidopsis thaliana in In Vitro and In Vivo Models of Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 708. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.D.; Dong, C.M.; Ho, L.C.; Lam, C.T.W.; Zhou, X.D.; Wu, E.X.; Zhou, Z.J.; Wang, X.M.; Zhang, Z.J. Resveratrol, a natural polyphenol, prevents chemotherapy-induced cognitive impairment: Involvement of cytokine modulation and neuroprotection. Neurobiol. Dis. 2018, 114, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, G.; Szeto, S.S.W.; Chong, C.M.; Quan, Q.; Huang, C.; Cui, W.; Guo, B.; Wang, Y.; Han, Y.; et al. Examining the neuroprotective effects of protocatechuic acid and chrysin on in vitro and in vivo models of Parkinson disease. Free Radic. Biol. Med. 2015, 84, 331–343. [Google Scholar] [CrossRef]
- Balez, R.; Steiner, N.; Engel, M.; Muñoz, S.S.; Lum, J.S.; Wu, Y.; Wang, D.; Vallotton, P.; Sachdev, P.; O’Connor, M.; et al. Neuroprotective effects of apigenin against inflammation, neuronal excitability and apoptosis in an induced pluripotent stem cell model of Alzheimer’s disease. Sci. Rep. 2016, 6, 31450. [Google Scholar] [CrossRef]
- Maciel, R.M.; Carvalho, F.B.; Olabiyi, A.A.; Schmatz, R.; Gutierres, J.M.; Stefanello, N.; Zanini, D.; Rosa, M.M.; Andrade, C.M.; Rubin, M.A.; et al. Neuroprotective effects of quercetin on memory and anxiogenic-like behavior in diabetic rats: Role of ectonucleotidases and acetylcholinesterase activities. Biomed. Pharmacother. 2016, 84, 559–568. [Google Scholar] [CrossRef]
- Sugumar, M.; Sevanan, M.; Sekar, S. Neuroprotective effect of naringenin against MPTP-induced oxidative stress. Int. J. Neurosci. 2019, 129, 534–539. [Google Scholar] [CrossRef]
- Strathearn, K.E.; Yousef, G.G.; Grace, M.H.; Roy, S.L.; Tambe, M.A.; Ferruzzi, M.G.; Wu, Q.-L.; Simon, J.E.; Lila, M.A.; Rochet, J.-C. Neuroprotective effects of anthocyanin- and proanthocyanidin-rich extracts in cellular models of Parkinson׳s disease. Brain Res. 2014, 1555, 60–77. [Google Scholar] [CrossRef]
- Dias, R.; Oliveira, H.; Fernandes, I.; Simal-Gandara, J.; Perez-Gregorio, R. Recent advances in extracting phenolic compounds from food and their use in disease prevention and as cosmetics. Crit. Rev. Food Sci. Nutr. 2020, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Grgic, J.; Selo, G.; Planinic, M.; Tisma, M.; Bucic-Kojic, A. Role of the Encapsulation in Bioavailability of Phenolic Compounds. Antioxidants (Basel) 2020, 9, 923. [Google Scholar] [CrossRef] [PubMed]
- Thakur, N.; Raigond, P.; Singh, Y.; Mishra, T.; Singh, B.; Lal, M.K.; Dutt, S. Recent updates on bioaccessibility of phytonutrients. Trends Food Sci. Technol. 2020, 97, 366–380. [Google Scholar] [CrossRef]
- McClements, D.J. Advances in nanoparticle and microparticle delivery systems for increasing the dispersibility, stability, and bioactivity of phytochemicals. Biotechnol. Adv. 2020, 38, 107287. [Google Scholar] [CrossRef] [PubMed]
- Kamiloglu, S. Effect of different freezing methods on the bioaccessibility of strawberry polyphenols. Int. J. Food Sci. Tech. 2019, 54, 2652–2660. [Google Scholar] [CrossRef]
- Del Bo, C.; Riso, P.; Brambilla, A.; Gardana, C.; Rizzolo, A.; Simonetti, P.; Bertolo, G.; Klimis-Zacas, D.; Porrini, M. Blanching improves anthocyanin absorption from highbush blueberry (Vaccinium corymbosum L.) puree in healthy human volunteers: A pilot study. J. Agric. Food. Chem. 2012, 60, 9298–9304. [Google Scholar] [CrossRef]
- Lamothe, S.; Langlois, A.; Bazinet, L.; Couillard, C.; Britten, M. Antioxidant activity and nutrient release from polyphenol-enriched cheese in a simulated gastrointestinal environment. Food Funct. 2016, 7, 1634–1644. [Google Scholar] [CrossRef]
- Pineda-Vadillo, C.; Nau, F.; Guerin-Dubiard, C.; Jardin, J.; Lechevalier, V.; Sanz-Buenhombre, M.; Guadarrama, A.; Toth, T.; Csavajda, E.; Hingyi, H.; et al. The food matrix affects the anthocyanin profile of fortified egg and dairy matrices during processing and in vitro digestion. Food Chem. 2017, 214, 486–496. [Google Scholar] [CrossRef]
- Lagoa, R.; Silva, J.; Rodrigues, J.R.; Bishayee, A. Advances in phytochemical delivery systems for improved anticancer activity. Biotechnol. Adv. 2020, 38, 107382. [Google Scholar] [CrossRef]
- Bacelo, H.A.M.; Santos, S.C.R.; Botelho, C.M.S. Tannin-based biosorbents for environmental applications—A review. Chem. Eng. J. 2016, 303, 575–587. [Google Scholar] [CrossRef]
- Jia, Y.; Liu, B.; Cheng, D.; Li, J.; Huang, F.; Lu, Y. Dyeing characteristics and functionability of tussah silk fabric with oak bark extract. Text. Res. J. 2016, 87, 1806–1817. [Google Scholar] [CrossRef]
- Bonet-Aracil, M.Á.; Díaz-García, P.; Bou-Belda, E.; Sebastiá, N.; Montoro, A.; Rodrigo, R. UV protection from cotton fabrics dyed with different tea extracts. Dyes Pigments 2016, 134, 448–452. [Google Scholar] [CrossRef]
- Das, A.K.; Rajkumar, V.; Nanda, P.K.; Chauhan, P.; Pradhan, S.R.; Biswas, S. Antioxidant Efficacy of Litchi (Litchi chinensis Sonn.) Pericarp Extract in Sheep Meat Nuggets. Antioxidants (Basel) 2016, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Martillanes, S.; Rocha-Pimienta, J.; Gil, M.V.; Ayuso-Yuste, M.C.; Delgado-Adamez, J. Antioxidant and antimicrobial evaluation of rice bran (Oryza sativa L.) extracts in a mayonnaise-type emulsion. Food Chem. 2020, 308, 125633. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, B.R.; Oliveira, M.B.P.P.; Barros, L.; Ferreira, I.C.F.R. Could fruits be a reliable source of food colorants? Pros and cons of these natural additives. Crit. Rev. Food Sci. Nutr. 2020, 1–31. [Google Scholar] [CrossRef]
- Baldin, J.C.; Michelin, E.C.; Polizer, Y.J.; Rodrigues, I.; de Godoy, S.H.; Fregonesi, R.P.; Pires, M.A.; Carvalho, L.T.; Fávaro-Trindade, C.S.; de Lima, C.G.; et al. Microencapsulated jabuticaba (Myrciaria cauliflora) extract added to fresh sausage as natural dye with antioxidant and antimicrobial activity. Meat Sci. 2016, 118, 15–21. [Google Scholar] [CrossRef]
- Wang, S.; Xia, P.; Wang, S.; Liang, J.; Sun, Y.; Yue, P.; Gao, X. Packaging films formulated with gelatin and anthocyanins nanocomplexes: Physical properties, antioxidant activity and its application for olive oil protection. Food Hydrocoll. 2019, 96, 617–624. [Google Scholar] [CrossRef]
- Zeng, P.; Chen, X.; Qin, Y.R.; Zhang, Y.H.; Wang, X.P.; Wang, J.Y.; Ning, Z.X.; Ruan, Q.J.; Zhang, Y.S. Preparation and characterization of a novel colorimetric indicator film based on gelatin/polyvinyl alcohol incorporating mulberry anthocyanin extracts for monitoring fish freshness. Food Res. Int. 2019, 126, 108604. [Google Scholar] [CrossRef]
- Cano, A.; Andres, M.; Chiralt, A.; González-Martinez, C. Use of tannins to enhance the functional properties of protein based films. Food Hydrocoll. 2020, 100, 105443. [Google Scholar] [CrossRef]
- Kamma, M.; Lin, W.C.; Lau, S.-C.; Chansakaow, S.; Leelapornpisid, P. Anti-aging Cosmeceutical Product Containing of Nymphaea rubra Roxb. ex Andrews Extract. Chiang Mai J. Sci. 2019, 46, 1143–1160. [Google Scholar]
- Taofiq, O.; Heleno, S.A.; Calhelha, R.C.; Fernandes, I.P.; Alves, M.J.; Barros, L.; González-Paramás, A.M.; Ferreira, I.C.F.R.; Barreiro, M.F. Phenolic acids, cinnamic acid, and ergosterol as cosmeceutical ingredients: Stabilization by microencapsulation to ensure sustained bioactivity. Microchem. J. 2019, 147, 469–477. [Google Scholar] [CrossRef]
- Yamate, Y.; Hiramoto, K.; Sato, E.F. The Preventive Effect of Coffee Compounds on Dermatitis and Epidermal Pigmentation after Ultraviolet Irradiation in Mice. Skin Pharmacol. Physiol. 2017, 30, 24–35. [Google Scholar] [CrossRef]
- Mota, M.D.; da Boa Morte, A.N.; e Silva, L.C.R.C.; Chinalia, F.A. Sunscreen protection factor enhancement through supplementation with Rambutan (Nephelium lappaceum L) ethanolic extract. J. Photochem. Photobiol. B Biol. 2020, 205, 111837. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.S.; Han, J.H.; Yoo, H.G.; Chung, J.H.; Cho, K.H.; Eun, H.C.; Kim, K.H. Human hair growth enhancement in vitro by green tea epigallocatechin-3-gallate (EGCG). Phytomedicine 2007, 14, 551–555. [Google Scholar] [CrossRef]
- Riccio, G.; Sommella, E.; Badolati, N.; Salviati, E.; Bottone, S.; Campiglia, P.; Dentice, M.; Tenore, G.C.; Stornaiuolo, M.; Novellino, E. Annurca Apple Polyphenols Protect Murine Hair Follicles from Taxane Induced Dystrophy and Hijacks Polyunsaturated Fatty Acid Metabolism toward beta-Oxidation. Nutrients 2018, 10, 1808. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Williams, M.; Cauchi, M.; Berkovitz, S.; Smith, S.A. A double-blind, randomised trial of a polyphenolic-rich nail bed balm for chemotherapy-induced onycholysis: The UK polybalm study. Breast Cancer Res. Treat. 2018, 171, 103–110. [Google Scholar] [CrossRef]
- Casado, N.; Perestrelo, R.; Silva, C.L.; Sierra, I.; Câmara, J.S. Comparison of high-throughput microextraction techniques, MEPS and μ-SPEed, for the determination of polyphenols in baby food by ultrahigh pressure liquid chromatography. Food Chem. 2019, 292, 14–23. [Google Scholar] [CrossRef]
- Pereira, J.A.M.; Goncalves, J.; Porto-Figueira, P.; Figueira, J.A.; Alves, V.; Perestrelo, R.; Medina, S.; Camara, J.S. Current trends on microextraction by packed sorbent—Fundamentals, application fields, innovative improvements and future applications. Analyst 2019, 144, 5048–5074. [Google Scholar] [CrossRef]
- Perestrelo, R.; Silva, P.; Porto-Figueira, P.; Pereira, J.A.M.; Silva, C.; Medina, S.; Camara, J.S. QuEChERS—Fundamentals, relevant improvements, applications and future trends. Anal. Chim. Acta 2019, 1070, 1–28. [Google Scholar] [CrossRef]
- Casado, N.; Perestrelo, R.; Silva, C.L.; Sierra, I.; Câmara, J.S. An improved and miniaturized analytical strategy based on μ-QuEChERS for isolation of polyphenols. A powerful approach for quality control of baby foods. Microchem. J. 2018, 139, 110–118. [Google Scholar] [CrossRef]
- Rahimi, A.; Hashemi, P.; Badiei, A.; Safdarian, M.; Rashidipour, M. Microextraction of Rosmarinic Acid Using CMK-3 Nanoporous Carbon in a Packed Syringe. Chromatographia 2013, 76, 857–860. [Google Scholar] [CrossRef]
- Gonçalves, J.; Silva, C.L.; Castilho, P.C.; Câmara, J.S. An attractive, sensitive and high-throughput strategy based on microextraction by packed sorbent followed by UHPLC-PDA analysis for quantification of hydroxybenzoic and hydroxycinnamic acids in wines. Microchem. J. 2013. [Google Scholar] [CrossRef]
- Gonçalves, J.L.; Alves, V.L.; Rodrigues, F.P.; Figueira, J.A.; Câmara, J.S. A semi-automatic microextraction in packed sorbent, using a digitally controlled syringe, combined with ultra-high pressure liquid chromatography as a new and ultra-fast approach for the determination of prenylflavonoids in beers. J. Chromatogr. A 2013, 1304, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Casado, N.; Pérez-Quintanilla, D.; Morante-Zarcero, S.; Sierra, I. Current development and applications of ordered mesoporous silicas and other sol–gel silica-based materials in food sample preparation for xenobiotics analysis. TrAC Trends Anal. Chem. 2017, 88, 167–184. [Google Scholar] [CrossRef]
- Porto-Figueira, P.; Figueira, J.A.; Pereira, J.A.; Câmara, J.S. A fast and innovative microextraction technique, μSPEed, followed by ultrahigh performance liquid chromatography for the analysis of phenolic compounds in teas. J. Chromatogr. A 2015, 1424, 1–9. [Google Scholar] [CrossRef]
- Aresta, A.; Cotugno, P.; Massari, F.; Zambonin, C. Determination of Trans-resveratrol in Wines, Spirits, and Grape Juices Using Solid-Phase Micro Extraction Coupled to Liquid Chromatography with UV Diode-Array Detection. Food Anal. Methods 2018, 11, 426–431. [Google Scholar] [CrossRef]
- Pei, M.; Huang, X. Determination of trace phenolic acids in fruit juice samples using multiple monolithic fiber solid-phase microextraction coupled with high-performance liquid chromatography. Anal. Methods 2016, 8, 3831–3838. [Google Scholar] [CrossRef]
- Mirnaghi, F.S.; Mousavi, F.; Rocha, S.M.; Pawliszyn, J. Automated determination of phenolic compounds in wine, berry, and grape samples using 96-blade solid phase microextraction system coupled with liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2013, 1276, 12–19. [Google Scholar] [CrossRef]
- Casado, N.; Morante-Zarcero, S.; Pérez-Quintanilla, D.; Câmara, J.S.; Sierra, I. Dispersive Solid-Phase Extraction of Polyphenols from Juice and Smoothie Samples Using Hybrid Mesostructured Silica Followed by Ultra-high-Performance Liquid Chromatography-Ion-Trap Tandem Mass Spectrometry. J. Agric. Food. Chem. 2019, 67, 955–967. [Google Scholar] [CrossRef]
- Cacho, J.I.; Campillo, N.; Viñas, P.; Hernández-Córdoba, M. Stir bar sorptive extraction with gas chromatography–mass spectrometry for the determination of resveratrol, piceatannol and oxyresveratrol isomers in wines. J. Chromatogr. A 2013, 1315, 21–27. [Google Scholar] [CrossRef]
- Xu, J.-J.; Yang, R.; Ye, L.-H.; Cao, J.; Cao, W.; Hu, S.-S.; Peng, L.-Q. Application of ionic liquids for elution of bioactive flavonoid glycosides from lime fruit by miniaturized matrix solid-phase dispersion. Food Chem. 2016, 204, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.Q.; Li, Q.; Chang, Y.X.; An, M.; Yang, R.; Tan, Z.; Hao, J.; Cao, J.; Xu, J.J.; Hu, S.S. Determination of natural phenols in olive fruits by chitosan assisted matrix solid-phase dispersion microextraction and ultrahigh performance liquid chromatography with quadrupole time-of-flight tandem mass spectrometry. J. Chromatogr. A 2016, 1456, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Barfi, B.; Asghari, A.; Rajabi, M.; Barfi, A.; Saeidi, I. Simplified miniaturized ultrasound-assisted matrix solid phase dispersion extraction and high performance liquid chromatographic determination of seven flavonoids in citrus fruit juice and human fluid samples: Hesperetin and naringenin as biomarkers. J. Chromatogr. A 2013, 1311, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Tadeo, J.L.; Sanchez-Brunete, C.; Albero, B.; Garcia-Valcarcel, A.I. Application of ultrasound-assisted extraction to the determination of contaminants in food and soil samples. J. Chromatogr. A 2010, 1217, 2415–2440. [Google Scholar] [CrossRef] [PubMed]
- Corbin, C.; Fidel, T.; Leclerc, E.A.; Barakzoy, E.; Sagot, N.; Falguieres, A.; Renouard, S.; Blondeau, J.P.; Ferroud, C.; Doussot, J.; et al. Development and validation of an efficient ultrasound assisted extraction of phenolic compounds from flax (Linum usitatissimum L.) seeds. Ultrason. Sonochem. 2015, 26, 176–185. [Google Scholar] [CrossRef]
- Zhou, T.; Xu, D.P.; Lin, S.J.; Li, Y.; Zheng, J.; Zhou, Y.; Zhang, J.J.; Li, H.B. Ultrasound-Assisted Extraction and Identification of Natural Antioxidants from the Fruit of Melastoma sanguineum Sims. Molecules 2017, 22, 306. [Google Scholar] [CrossRef]
- Routray, W.; Orsat, V. MAE of phenolic compounds from blueberry leaves and comparison with other extraction methods. Ind. Crop. Prod. 2014, 58, 36–45. [Google Scholar] [CrossRef]
- Gadkari, P.; Manohar, B. Polyphenols from fresh frozen tea leaves (Camellia assamica L.,) by supercritical carbon dioxide extraction with ethanol entrainer-application of response surface methodology. J. Food Sci. Technol.-Mysore 2013, 1–11. [Google Scholar] [CrossRef][Green Version]
- King, J.W. Isolation of Polyphenolic Compounds from Fruits or Vegetables Utilizing Sub-critical Water Extraction. United States Department of Agriculture Patents. U.S. Patent No 2208,2181 B2001, 24 April 2007. [Google Scholar]
- Hasbay, İ.; Çetin, H.; Yener, E.; Bayindirli, A. Subcritical (carbon dioxide + ethanol) extraction of polyphenols from apple and peach pomaces, and determination of the antioxidant activities of the extracts. J. Supercrit. Fluids 2007, 43, 55–63. [Google Scholar] [CrossRef]
- Sampedro, F.; Rodrigo, D. Pulsed Electric Fields (PEF) Processing of Milk and Dairy Products. In Emerging Dairy Processing Technologies: Opportunities for the Dairy Industry; Datta, N., Tomasula, P.M., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2015; pp. 115–148. [Google Scholar]
- Barba, F.J.; Ana, F.; Charis, M.G.; Eugène, V.; Maria, J.E. Potential use of pulsed electric technologies and ultrasounds to improve the recovery of high-added value compounds from blackberries. J. Food Eng. 2015, 167, 38–44. [Google Scholar] [CrossRef]
- Clark, J.P. Processing-pulsed electric field processing. Food Technol. Mag. 2006, 60, 66–67. [Google Scholar]
- Cabrera, L.D.C.; Caldas, S.S.; Prestes, O.D.; Primel, E.G.; Zanella, R. Evaluation of alternative sorbents for dispersive solid-phase extraction clean-up in the QuEChERS method for the determination of pesticide residues in rice by liquid chromatography with tandem mass spectrometry. J. Sep. Sci. 2016, 39, 1945–1954. [Google Scholar] [CrossRef] [PubMed]
- Hamed, A.M.; Moreno-González, D.; Gámiz-Gracia, L.; García-Campaña, A.M. Evaluation of a new modified QuEChERS method for the monitoring of carbamate residues in high-fat cheeses by using UHPLC-MS/MS. J. Sep. Sci. 2017, 40, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Arias, J.L.O.; Schneider, A.; Batista-Andrade, J.A.; Vieira, A.A.; Caldas, S.S.; Primel, E.G. Chitosan from shrimp shells: A renewable sorbent applied to the clean-up step of the QuEChERS method in order to determine multi-residues of veterinary drugs in different types of milk. Food Chem. 2018, 240, 1243–1253. [Google Scholar] [CrossRef]
- Soares, K.L.; Cerqueira, M.B.R.; Caldas, S.S.; Primel, E.G. Evaluation of alternative environmentally friendly matrix solid phase dispersion solid supports for the simultaneous extraction of 15 pesticides of different chemical classes from drinking water treatment sludge. Chemosphere 2017, 182, 547–554. [Google Scholar] [CrossRef]
- Wang, X.C.; Shu, B.; Li, S.; Yang, Z.G.; Qiu, B. QuEChERS followed by dispersive liquid-liquid microextraction based on solidification of floating organic droplet method for organochlorine pesticides analysis in fish. Talanta 2017, 162, 90–97. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, C.; Mendiola, J.A.; Quirantes-Piné, R.; Ibáñez, E.; Segura-Carretero, A. Green downstream processing using supercritical carbon dioxide, CO2-expanded ethanol and pressurized hot water extractions for recovering bioactive compounds from Moringa oleifera leaves. J. Supercrit. Fluids 2016, 116, 90–100. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, C.; Quirantes-Piné, R.; Contreras Mdel, M.; Uberos, J.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Assessment of the stability of proanthocyanidins and other phenolic compounds in cranberry syrup after gamma-irradiation treatment and during storage. Food Chem. 2015, 174, 392–399. [Google Scholar] [CrossRef]
- López-Cobo, A.; Alberto, F.-G.; Ana María, G.-C.; Antonio, S.-C.; Federica, P.; María Fiorenza, C. HPLC-DAD-ESI-QTOF-MS and HPLC-FLD-MS as valuable tools for the determination of phenolic and other polar compounds in the edible part and by-products of avocado. LWT 2016, 73, 505–513. [Google Scholar] [CrossRef]
- Brown, H.M.; McDaniel, T.J.; Fedick, P.W.; Mulligan, C.C. The current role of mass spectrometry in forensics and future prospects. Anal. Methods 2020, 12, 3974–3997. [Google Scholar] [CrossRef]
- Nizzia, J.L.; O’Leary, A.E.; Ton, A.T.; Mulligan, C.C. Screening of cosmetic ingredients from authentic formulations and environmental samples with desorption electrospray ionization mass spectrometry. Anal. Methods 2013, 5, 394–401. [Google Scholar] [CrossRef]
- Farré, M.; Picó, Y.; Barceló, D. Direct analysis in real-time high-resolution mass spectrometry as a valuable tool for polyphenols profiling in olive oil. Anal. Methods 2019, 11, 472–482. [Google Scholar] [CrossRef]
- Lara-Ortega, F.J.; Beneito-Cambra, M.; Robles-Molina, J.; García-Reyes, J.F.; Gilbert-López, B.; Molina-Díaz, A. Direct olive oil analysis by mass spectrometry: A comparison of different ambient ionization methods. Talanta 2018, 180, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.; Swiner, D.J.; Capone, P.C.; Badu-Tawiah, A.K. Thread spray mass spectrometry for direct analysis of capsaicinoids in pepper products. Anal. Chim. Acta 2018, 1023, 81–88. [Google Scholar] [CrossRef]
- Fatigante, W.L.; Mukta, S.; Lawton, Z.E.; Bruno, A.M.; Traub, A.; Gasa, A.J.; Stelmack, A.R.; Wilson-Frank, C.R.; Mulligan, C.C. Filter Cone Spray Ionization Coupled to a Portable MS System: Application to On-Site Forensic Evidence and Environmental Sample Analysis. J. Am. Soc. Mass Spectrom. 2020, 31, 336–346. [Google Scholar] [CrossRef]









| (A) | |||||||
| Polyphenols | Cancer Type | Type of Assay | Reference | ||||
| In Vitro Assay | In Vivo Assay | ||||||
| Classes | Compounds | Cell-Lines | Concentration * | Model | Effects | ||
| Anthocyanins | Colorectal cancer | HT-29 | 400 μg/mL 2 | Mouse model | ↓ tumour size; ↓ inflammation | [72] | |
| Melanoma | Mouse model | Inhibited tumour growth and lung metastasis. | [64] | ||||
| Flavanones | Naringin | Oesophageal cancer | YMI | 354 μM 1 | Mouse model | ↓tumour size. | [68] |
| Flavones | Hispidulin | Acute myeloid leukaemia | MOLM-13 | 6.4 μM 1 | - | - | [62] |
| MV4-11 | 8.2 μM 1 | - | - | [62] | |||
| Luteolin | Lung carcinoma | A549 | 25–100 μM 2 | [73] | |||
| Acute myeloid leukaemia | MOLM-13 | 4.5 μM 1 | [62] | ||||
| MV4-11 | 3.1 μM 1 | [62] | |||||
| Tangeretin | Colorectal carcinoma | HCT116 | 12.5 μM 2 | Mouse model | ↓ tumour incidence; ↓ pathological symptoms | [74] | |
| Flavonols | Kaempferol | Breast carcinoma | Mouse model | ↓ Tumour size | [75] | ||
| Isoflavones | Alpinumisoflavone | Leukaemia | CEM/ADR5000 | 5.91 μM 1 | [65] | ||
| Breast carcinoma | MDA-MB-231-BCRP | 65.65 μM 1 | [65] | ||||
| Lignans | Pycnanthulignene | Leukaemia | CEM/ADR5000 | 5.84 μM 1 | [65] | ||
| Colon carcinoma | HCT116 | 65.32 μM 1 | [65] | ||||
| Phenolic acid | |||||||
| Stilbenes | Pterostilbene | Cervical cancer | TC1 | 15.61 μM 1 | Mouse model | ↓ Tumour size; | [76] |
| Pancreatic cancer | - | - | Mouse model | Inhibited tumour growth | [77] | ||
| Resveratrol | Cervical cancer | TC1 | 34.46 μM 1 | Mouse model | ↓ Tumour size; | [76] | |
| Melanoma | B16F10, A375 and B6 | 40 μM 2 | - | - | [63] | ||
| Lung cancer metastasis | - | Mouse model | ↓ Angiogenesis; ↑ apoptosis metastatic colonies in the lungs | [63] | |||
| Phenolic extracts from: | |||||||
| Cynara cardunculus L. | Oral carcinoma | SCC-25 | 184.81 μg/mL 1 | - | - | [78] | |
| Orange peel | Oesophageal cancer | YMI | Mouse model | ↓ Tumour size. | |||
| (B) | |||||||
| Compounds | Type of Cancer | Cancer Drug | Effect | Reference | |||
| Quercetin | Lung cancer | Paclitaxel-resistant cells | Reversal of PTX resistance mitochondrial membrane potential MMP depolarization | [79] | |||
| Luteolin | Lung cancer | TRIAL | Positive effect synergetic | [67] | |||
| Orange peel extract | Oesophageal cancer | Doxorubicin | Decreased the side effects of chemotherapeutic treatment | [68] | |||
| Polyphenols | Main Cardioprotective Effects | Type of Assay | Reference | |
|---|---|---|---|---|
| Classes | Compounds | |||
| Phenolic acids | Gallic acid | ↑ Glucose tolerance | Animal model | [82] |
| p-Coumaric acid | ↑ Glucose tolerance | Animal model | [82] | |
| Anthocyanins | ↓ Platelet chemokines; ↓ LDL-C | Clinical trial | [70] | |
| Flavan-3-ols | Catechins | ↓ TC; ↓ LDL-C | Clinical trial | [71] |
| ↓ CK; ↓ CK-MB; ↓ LDH; ↓ cTnT; ↑ LVEF; ↓ LVIDs | Animal model | [83] | ||
| Flavanones | Hesperidin | ↓ CK; ↓ cTnT; ↓ oxidative stress; ↓ cardiac tissue lesions | Animal model | [84] |
| Flavones | Apigenin | Anti-cardiac fibrosis | In vitro | [79] |
| Flavonols | Quercetin | Anti-platelet properties | In vitro | [85] |
| ↑ Vasodilatation | Clinical trial | [86] | ||
| Kaempferol | Anti-platelet properties | In vitro | [85] | |
| ↓ LDL-C | Clinical trial | [87] | ||
| Isoflavones | ↓ Blood pressure | Clinical trial | [88] | |
| Stilbenes | Resveratrol | ↑ Antioxidant activity in the blood; ↓ Diabetic body weight | Clinical trial | [80] |
| ↓ TC; ↓ blood pressure; ↓ glucose; | Clinical trial | [89] | ||
| ↓ Blood glucose; ↓body weight; ↑ plasma insulin; ↓inflammation factors; ↓ oxidative stress; ↑ eNOS | Animal model | [81] | ||
| Curcuminoids | Curcumin | ↓ Inflammation factors; ↓LDL-C; ↑ Nrf2, ↓ At1R; ↓ NF-kB | Clinical trial | [69] |
| Polyphenols | Target Diseases | Neuroprotective Effects | Type of Assay | Reference | |
|---|---|---|---|---|---|
| Classes | Compounds | ||||
| Phenolic acids | protocatechuic acid + chrysin (flavone) | PD | ↓Neuroinflammation; ↓Dopamine neurons death; ↑NRF2 and NF-κB; ↓oxidative stress | In vitro/Animal model | [95] |
| Flavonols | Apigenin | AD | ↓Neuroinflammation; ↓Apoptosis | In vitro | [96] |
| Quercetin | Cognitive deficit associated with diabetes | ↑Memory; ↓oxidative stress; | Animal model | [97] | |
| Flavanone | Naringenin | PD | ↓Oxidative stress; ↓Neuroinflammation ↑Motor performance | Animal model | [98] |
| Stilbene | Resveratrol | Chemobrain | Prevents cognitive decline | Animal model | [94] |
| Polyphenol extract | Main compounds | ||||
| White grape juice | Quercetin derivatives; proanthocyanidins; | Autoimmune encephalomyelitis; Multiple sclerosis | ↓Neuroinflammation; | Animal model | [91] |
| Blueberry | Anthocyanins; phenolic acids; proanthocyanidins | PD | ↓Dopamine neurons death | In vitro | [99] |
| Grape seeds | Proanthocyanidins | PD | ↓Dopamine neurons death | In vitro | [99] |
| Arabidopsis thaliana | Phenolic acids, quercetin derivatives; kaempferol derivatives | AD | ↓Neuroinflammation; ↓ oxidative stress; restored the locomotor activity | In vitro/animal model | [93] |
| Cherry juice | Anthocyanins | Mild-to-moderate dementia | ↑Verbal fluency; ↑Shot- and long-term memory | Clinical trial | [92] |
| Extraction Technique | Sorbent (Amount) | Food Matrix | Target Analytes | Methodology | Recoveries (%) | Ref. |
|---|---|---|---|---|---|---|
| µ-QuEChERS | ||||||
| MgSO4 (75 mg), PSA (12.5 mg) | Baby foods | 12 polyphenols | UHPLC-PDA | 71–100 | [129] | |
| QuEChERS-USAE | ||||||
| PSA (25 mg), C18 sorbent (25 mg), and MgSO4 (150 mg) | Fruits and Vegetables | 12 polyphenols | UHPLC-PDA | [30] | ||
| MEPS | ||||||
| CMK-3 nanoporous carbon (2 mg) | Rosemary | Rosmarinic acid | HPLC-UV/VIS | 94–106 | [130] | |
| C18 (4 mg) | Beer | 2 prenylflavonoids | UHPLC-PDA | 67–100 | [131] | |
| C8 (4 mg) | Wines | 10 phenolic acids | UHPLC-PDA | 77–100 | [132] | |
| μSPEed | ||||||
| PS/DVB-RP | Baby Foods | 12 polyphenols | UHPLC-PDA | 67–97 | [133] | |
| PS/DVB-RP | Tea | 8 polyphenols | UHPLC-PDA | 89–103 | [134] | |
| SPME | ||||||
| PA fiber | Wines, Spirits, and Grape Juices | Resveratrol | HPLC-DAD | 92–99 | [135] | |
| VIED/MMF-SPME | Fruit juice | 4 phenolic acids | HPLC–DAD | 70–118 | [136] | |
| PS-DVB-PAN | Grapes, Berries, and Wine | 8 polyphenols | HPLC-TQ-MS/MS | 69–82 | [137] | |
| dSPE | ||||||
| HMS-C18 (50 mg) | Fruit and vegetables juices and smoothies | 20 Polyphenols | UHPLC-IT-MS/MS | 57–99 | [138] | |
| SBSE | ||||||
| PDMS | Wine | 6 stilbenes | GC-Q-MS | 79–109 | [139] | |
| μ-MSPD | ||||||
| Florisil (150 mg) | Lime fruit | 2 flavonoids | UHPLC-UV | 90–96 | [140] | |
| Middle-molecular-weight chitosan (25 mg) | Olive fruits | 7 polyphenols | UHPLC-Q-TOF-MS/MS | 80–113 | [141] | |
| Silica-based C18 (200 mg) | Citrus fruit juice | 7 flavonoids | HPLC-UV | 86–94 | [142] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Câmara, J.S.; Albuquerque, B.R.; Aguiar, J.; Corrêa, R.C.G.; Gonçalves, J.L.; Granato, D.; Pereira, J.A.M.; Barros, L.; Ferreira, I.C.F.R. Food Bioactive Compounds and Emerging Techniques for Their Extraction: Polyphenols as a Case Study. Foods 2021, 10, 37. https://doi.org/10.3390/foods10010037
Câmara JS, Albuquerque BR, Aguiar J, Corrêa RCG, Gonçalves JL, Granato D, Pereira JAM, Barros L, Ferreira ICFR. Food Bioactive Compounds and Emerging Techniques for Their Extraction: Polyphenols as a Case Study. Foods. 2021; 10(1):37. https://doi.org/10.3390/foods10010037
Chicago/Turabian StyleCâmara, José S., Bianca R. Albuquerque, Joselin Aguiar, Rúbia C. G. Corrêa, João L. Gonçalves, Daniel Granato, Jorge A. M. Pereira, Lillian Barros, and Isabel C. F. R. Ferreira. 2021. "Food Bioactive Compounds and Emerging Techniques for Their Extraction: Polyphenols as a Case Study" Foods 10, no. 1: 37. https://doi.org/10.3390/foods10010037
APA StyleCâmara, J. S., Albuquerque, B. R., Aguiar, J., Corrêa, R. C. G., Gonçalves, J. L., Granato, D., Pereira, J. A. M., Barros, L., & Ferreira, I. C. F. R. (2021). Food Bioactive Compounds and Emerging Techniques for Their Extraction: Polyphenols as a Case Study. Foods, 10(1), 37. https://doi.org/10.3390/foods10010037