Thermal Effect, Diffusion, and Leaching of Health-Promoting Phytochemicals in Commercial Canning Process of Mango (Mangifera indica L.) and Pineapple (Ananas comosus L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Chemicals
2.2. Preparation of Fruits for Analysis
2.3. Analysis of Constituents
2.3.1. Determination of Total Soluble Solids (TSS)
2.3.2. Determination of Vitamin C Content
2.3.3. Determination of Total Polyphenolic Content
2.3.4. Antioxidant Activity
2.3.5. Determination of β-Carotene Content
2.3.6. Determination of Flavonoid Content
2.3.7. Mathematical Modelling
- Cc,i:
- fraction of intact cells (-),
- Cc,l:
- fraction of lysed cells (-),
- kl:
- lysis rate constant (min−1),
- t:
- time (min).
- Mw: mass of free water (g),
- Mf,0: initial mass of fruit (g).
- L: refers to the fact that this component represents the change due to leaching only,
- Cw: component concentration in the free water (µmol/g),
- Cf: component concentration in the intact part of the fruit (µmol/g).
- B: refers to the fact that this component is the change due to breakdown only,
- kd,f: breakdown rate constant in fruit (min−1),
- kd,w: breakdown rate constant in water (min−1).
3. Results and Discussion
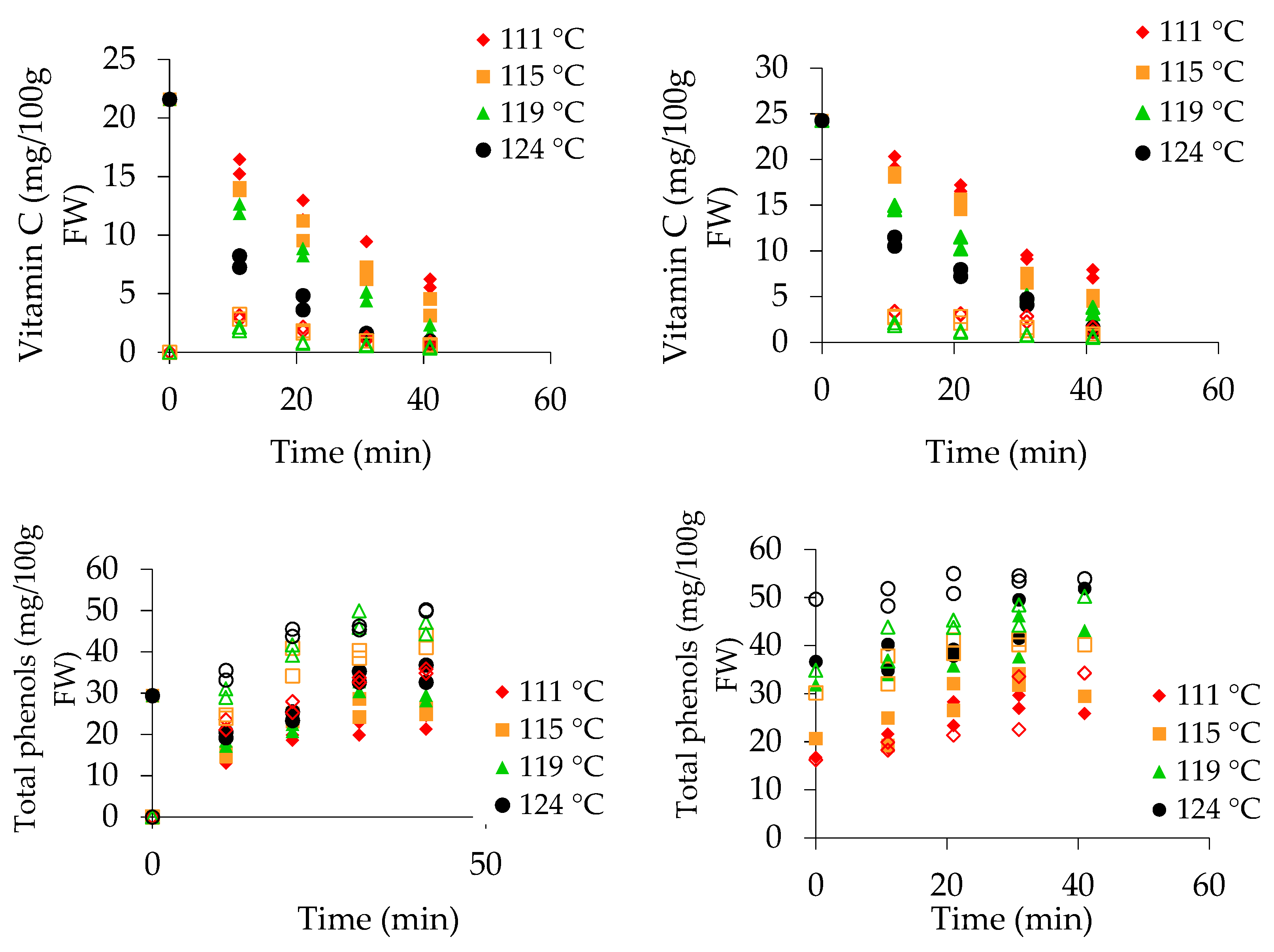
3.1. Vitamin C Content
3.2. Polyphenol Content
3.3. β-Carotene
3.4. Antioxidant Activity (TEAC)
3.5. Flavonoids
3.5.1. Mango Fruit Pieces
3.5.2. Pineapple Pieces
3.5.3. Sugar Syrup in Cans
3.6. Kinetics of Compounds
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rattanathanalerk, M.; Chiewchan, N.; Srichumpoung, W. Effect of thermal processing on the quality loss of pineapple juice. J. Food Eng. 2005, 66, 259–265. [Google Scholar] [CrossRef]
- Nicoli, M.; Anese, M.; Parpinel, M. Influence of processing on the antioxidant properties of fruit and vegetables. Trends Food Sci. Technol. 1999, 10, 94–100. [Google Scholar] [CrossRef]
- Dewanto, V.; Xianzhong, W.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef] [PubMed]
- Sablani, S.S.; Andrews, P.K.; Davies, N.M.; Walters, T.; Saez, H.; Syamaladevi, R.M.; Mohekar, P.R. Effect of thermal treatments on phytochemicals in conventionally and organically grown berries. J. Sci. Food Agric. 2010, 90, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Padmavati, R.; Anandharamakrishnan, C. Computational Fluid Dynamics Modeling of the Thermal Processing of Canned Pineapple Slices and Titbits. Food Bioprocess Technol. 2013. [Google Scholar] [CrossRef]
- Ismail, I.M. Optimizing the sterilization process of canned food using temperature distribution studies. IOSR J. Agric. Vet. Sci. 2013. [Google Scholar] [CrossRef]
- Vatankhah, H.; Zamindar, N.; Shahedi Baghekhandan, M. Heat transfer simulation and retort program adjustment for thermal processing of wheat based Haleem in semi-rigid aluminum containers. J. Food Sci. Technol. 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adnan, S.; Bhattacharjee, S.; Akter, S.; Chakraborty, D.; Ahmad, M. Development and Quality Evaluation of anned Pineapple. J. Environ. Sci. Nat. Resour. 2018. [Google Scholar] [CrossRef]
- Chutintrasri, B.; Noomhorm, A. Thermal inactivation of polyphenoloxidase in pineapple puree. LWT—Food Sci. Technol. 2006. [Google Scholar] [CrossRef]
- Nayak, B.; Liu, R.H.; Tang, J. Effect of Processing on Phenolic Antioxidants of Fruits, Vegetables, and Grains—A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 887–919. [Google Scholar] [CrossRef]
- Maldonado-Celis, M.E.; Yahia, E.M.; Bedoya, R.; Landázuri, P.; Loango, N.; Aguillón, J.; Restrepo, B.; Ospina, J.C.G. Chemical Composition of Mango (Mangifera indica L.) Fruit: Nutritional and Phytochemical Compounds. Front. Plant Sci. 2019. [Google Scholar] [CrossRef] [PubMed]
- van Breda, S.G.J.; de Kok, T.M.C.M.; van Delft, J.H.M. Mechanisms of colorectal and lung cancer prevention by vegetables: A genomic approach. J. Nutr. Biochem. 2008, 19, 139–157. [Google Scholar] [CrossRef] [PubMed]
- Bueno, J.M.; Ramos-Escudero, F.; Sáez-Plaza, P.; Muñoz, A.M.; Navas, M.J.; Asuero, A.G. Analysis and Antioxidant Capacity of Anthocyanin Pigments. Part I: General Considerations Concerning Polyphenols and Flavonoids. Crit. Rev. Anal. Chem. 2012, 42, 102–125. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Brecht, J.K.; Talcott, S.T. Antioxidant phytochemical and fruit quality changes in mango (Mangifera indica L.) following hot water immersion and controlled atmosphere storage. Food Chem. 2007, 105, 1327–1334. [Google Scholar] [CrossRef]
- Dar, M.S.; Oak, P.; Chidley, H.; Deshpande, A.; Giri, A.; Gupta, V. Nutrient and Flavor Content of Mango (Mangifera indica L.) Cultivars: An Appurtenance to the List of Staple Foods. In Nutritional Composition of Fruit Cultivars; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Cervo, M.M.C.; Llido, L.O.; Barrios, E.B.; Panlasigui, L.N. Effects of canned pineapple consumption on nutritional status, immunomodulation, and physical health of selected school children. J. Nutr. Metab. 2014. [Google Scholar] [CrossRef]
- Van Boekel, M.A.J.S. Kinetic Modeling of Food Quality: A Critical Review. Compr. Rev. Food Sci. Food Saf. 2008, 7, 144–158. [Google Scholar] [CrossRef]
- Manso, M.C.; Oliveira, F.A.R.; Oliveira, J.C.; Frías, J.M. Modelling ascorbic acid thermal degradation and browning in orange juice under aerobic conditions. Int. J. Food Sci. Technol. 2001. [Google Scholar] [CrossRef]
- Summen, M.A.; Erge, H.S. Thermal Degradation Kinetics of Bioactive Compounds and Visual Color in Raspberry Pulp. J. Food Process. Preserv. 2004, 38, 551–557. [Google Scholar] [CrossRef]
- Durance, T.D. Improving canned food quality with variable retort temperature processes. Trends Food Sci. Technol. 1997, 8, 113–118. [Google Scholar] [CrossRef]
- Miri, T.; Tsoukalas, A.; Bakalis, S.; Pistikopoulos, E.; Rustem, B.; Fryer, P. Global optimization of process conditions in batch thermal sterilization of food. J. Food Eng. 2008. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1998, 299, 152–178. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A procedure to measure the antiradical efficiency of polyphenols. J. Sci. Food Agric. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Jiménez-Escrig, A.; Jiménez-Jiménez, I.; Sánchez-Moreno, C.; Saura-Calixto, F. Evaluation of free radical scavenging of dietary carotenoids by the stable radical 2,2-diphenyl-1-picrylhydrazyl. J. Sci. Food Agric. 2000, 80, 1686–1690. [Google Scholar] [CrossRef]
- Bushway, R.J. Separation of carotenoids in fruits and vegetables by high performance liquid chromatography. J. Liq. Chromatogr. 1985, 8, 1527–1547. [Google Scholar] [CrossRef]
- Bushway, R.J.; Wilson, A.M. Determination of α– and β–Carotene in Fruit and Vegetables by High Performance Liquid Chromatography. Can. Inst. Food Sci. Technol. J. 1982, 15, 165–169. [Google Scholar] [CrossRef]
- Arampath, P.C.; Dekker, M. Bulk storage of mango (Mangifera indica L.) and pineapple (Ananas comosus L.) pulp: Effect of pulping and storage temperature on phytochemicals and antioxidant activity. J. Sci. Food Agric. 2019. [Google Scholar] [CrossRef]
- Sarvan, I.; Verkerk, R.; Dekker, M. Modelling the fate of glucosinolates during thermal processing of Brassica vegetables. LWT—Food Sci. Technol. 2012, 49, 178–183. [Google Scholar] [CrossRef]
- Oerlemans, K.; Barrett, D.M.; Suades, C.B.; Verkerk, R.; Dekker, M. Thermal degradation of glucosinolates in red cabbage. Food Chem. 2006, 95, 19–29. [Google Scholar] [CrossRef]
- Stewart, W.E.; Caracotsios, M.; Sørensen, J.P. Parameter estimation from multiresponse data. AIChE J. 1992, 38, 641–650. [Google Scholar] [CrossRef]
- Hernandez, Y.; Lobo, M.G.; Gonzalez, M. Determination of vitamin C in tropical fruits: A comparative evaluation of methods. Food Chem. 2006, 96, 654–664. [Google Scholar] [CrossRef]
- Leong, L.P.; Shui, G. An investigation of antioxidant capacity of fruits in Singapore markets. Food Chem. 2002, 76, 69–75. [Google Scholar] [CrossRef]
- Hassimotto, N.M.A.; Genovese, M.I.; Lajolo, F.M. Antioxidant activity of dietary fruits, vegetables, and commercial frozen fruit pulps. J. Agric. Food Chem. 2005, 53, 2928–2935. [Google Scholar] [CrossRef]
- Luximon-Ramma, A.; Bahorun, T.; Crozier, A. Antioxidant actions and phenolic and vitamin C contents of common Mauritian exotic fruits. J. Sci. Food Agric. 2003, 83, 496–502. [Google Scholar] [CrossRef]
- Lima, J.R.; Elizondo, N.J.; Bohuon, P. Kinetics of ascorbic acid degradation and colour change in ground cashew apples treated at high temperatures (100–180 °C). Int. J. Food Sci. Technol. 2010, 45, 1724–1731. [Google Scholar] [CrossRef]
- Dhuique-Mayer, C.; Tbatou, M.; Carail, M.; Caris-Veyrat, C.; Dornier, M.; Amiot, M.J. Thermal degradation of antioxidant micronutrients in Citrus juice: Kinetics and newly formed compounds. J. Agric. Food Chem. 2007, 55, 4209–4216. [Google Scholar] [CrossRef]
- Singh, U.P.; Singh, D.P.; Singh, M.; Maurya, S.; Srivastava, J.S.; Singh, R.B.; Singh, S.P. Characterization of phenolic compounds in some Indian mango cultivars. Int. J. Food Sci. Nutr. 2004, 55, 163–169. [Google Scholar] [CrossRef]
- Sun, J.; Chu, Y.F.; Wu, X.; Liu, R.H. Antioxidant and antiproliferative activities of common fruits. J. Agric. Food Chem. 2002, 50, 7449–7454. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B.; Kimura, M.; Godoy, H.T.; Amaya-Farfan, J. Updated Brazilian database on food carotenoids: Factors affecting carotenoid composition. J. Food Compos. Anal. 2008, 21, 445–463. [Google Scholar] [CrossRef]
- Brat, P.; Hoang, L.N.T.; Soler, A.; Reynes, M.; Brillouet, J.-M. Physicochemical characterization of a new pineapple hybrid (FLHORAN41 Cv.). J. Agric. Food Chem. 2004, 52, 6170–6177. [Google Scholar] [CrossRef]
- Chavasit, V.; Pisaphab, R.; Sungpuag, P.; Jittinandana, S.; Wasantwisut, E. Changes in β-carotene and vitamin A contents of vitamin A-rich foods in Thailand during preservation and storage. J. Food Sci. 2003, 67, 375–379. [Google Scholar] [CrossRef]
- Masibo, M.; He, Q. In vitro antimicrobial activity and the major polyphenol in leaf extract of Mangifera indica L. Malays J. Microbiol. 2009, 5, 73–80. [Google Scholar]
- Schieber, A.; Ullrich, W.; Carle, R. Characterization of polyphenols in mango puree concentrate by HPLC with diode array and mass spectrometric detection. Innov. Food Sci. Emerg. Technol. 2002, 1, 161–166. [Google Scholar] [CrossRef]
- Burdurlu, H.S.; Koca, N.; Karadeniz, F. Degradation of vitamin C in citrus juice concentrates during storage. J. Food Eng. 2006, 74, 211–216. [Google Scholar] [CrossRef]
- Van Den Broeck, I.; Ludikhuyze, L.; Weemaes, C.; Van Loey, A.; Hendrickx, M. Kinetics for Isobaric-Isothermal Degradation of L-Ascorbic Acid. J. Agric. Food Chem. 1998, 46, 2001–2006. [Google Scholar] [CrossRef]
- Ahmed, J.; Shivhare, U.S.; Kaur, M. Thermal colour degradation kinetics of mango puree thermal colour degradation kinetics of mango puree. Int. J. Food Prop. 2002. [Google Scholar] [CrossRef]
- Chutintrasri, B.; Noomhorm, A. Color degradation kinetics of pineapple puree during thermal processing. LWT—Food Sci. Technol. 2007, 40, 300–306. [Google Scholar] [CrossRef]
- Karhan, M.; Aksu, M.; Tetik, N.; Turhan, I. Kinetic modeling of anaerobic thermal degradation of ascorbic acid in rose HIP (Rosa canina L) pulp. J. Food Qual. 2004, 27, 311–319. [Google Scholar] [CrossRef]
- Eberhardt, M.V.; Lee, C.Y.; Liu, R.H. Antioxidant activity of fresh apples. Nature 2000, 405, 903–904. [Google Scholar] [CrossRef]
- Chuah, A.M.; Lee, Y.C.; Yamaguchi, T.; Takamura, H.; Yin, L.J.; Matoba, T. Effect of cooking on the antioxidant properties of coloured peppers. Food Chem. 2008, 111, 20–28. [Google Scholar] [CrossRef]





| Compound | Internal Can Temperature (°C) | |||
|---|---|---|---|---|
| 111 | 115 | 119 | 124 | |
| Vitamin C | 7.87(0.55) | 12.22(0.20) | 4.01(0.91) | 5.43(0.79) |
| TEAC | 6.03(0.74) | 8.60(07) | 5.77(0.76) | 19.20(0.02) |
| Constituent | Pineapple | Mango | ||
|---|---|---|---|---|
| kd,111 (10−2) min−1 | Ea kJ mol−1 | kd,111 (10−2) min−1 | Ea kJ mol−1 | |
| Vitamin C | 2.4 ± 0.1 | 81 ± 6 | 2.4 ± 0.1 | 109 ± 6 |
| β-carotene | 1.3 ± 0.1 | 92 ± 5 | 0.9 ± 0.0 | 148 ± 4 |
| Catechin | 3.5 ± 0.1 | 35 ± 3 | 1.4 ± 0.1 | 62 ± 4 |
| Tannic acid | 3.5 ± 0.1 | 44 ± 2 | 1.0 ± 0.0 | 83 ± 4 |
| Chlorogenic acid | 2.7 ± 0.1 | 31 ± 3 | 1.2 ± 0.0 | 96 ± 4 |
| Epicatechin | 2.1 ± 0.0 | 25 ± 2 | 0.9 ± 0.0 | 82 ± 3 |
| Gallic acid | 2.5 ± 0.1 | 73 ± 3 | 1.4 ± 0.0 | 51 ± 4 |
| TEAC | 2.2 ± 0.1 | 106 ± 8 | 1.5 ± 0.1 | 61 ± 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arampath, P.C.; Dekker, M. Thermal Effect, Diffusion, and Leaching of Health-Promoting Phytochemicals in Commercial Canning Process of Mango (Mangifera indica L.) and Pineapple (Ananas comosus L.). Foods 2021, 10, 46. https://doi.org/10.3390/foods10010046
Arampath PC, Dekker M. Thermal Effect, Diffusion, and Leaching of Health-Promoting Phytochemicals in Commercial Canning Process of Mango (Mangifera indica L.) and Pineapple (Ananas comosus L.). Foods. 2021; 10(1):46. https://doi.org/10.3390/foods10010046
Chicago/Turabian StyleArampath, Palitha C., and Matthijs Dekker. 2021. "Thermal Effect, Diffusion, and Leaching of Health-Promoting Phytochemicals in Commercial Canning Process of Mango (Mangifera indica L.) and Pineapple (Ananas comosus L.)" Foods 10, no. 1: 46. https://doi.org/10.3390/foods10010046
APA StyleArampath, P. C., & Dekker, M. (2021). Thermal Effect, Diffusion, and Leaching of Health-Promoting Phytochemicals in Commercial Canning Process of Mango (Mangifera indica L.) and Pineapple (Ananas comosus L.). Foods, 10(1), 46. https://doi.org/10.3390/foods10010046







