Bioactive Ingredients and Medicinal Values of Grifola frondosa (Maitake)
Abstract
:1. Introduction
2. Chemical and Nutritional Compositions
2.1. Proximate Composition
2.2. Soluble Sugar Content
2.3. Free Amino Acid Content
3. Bioactive Ingredients
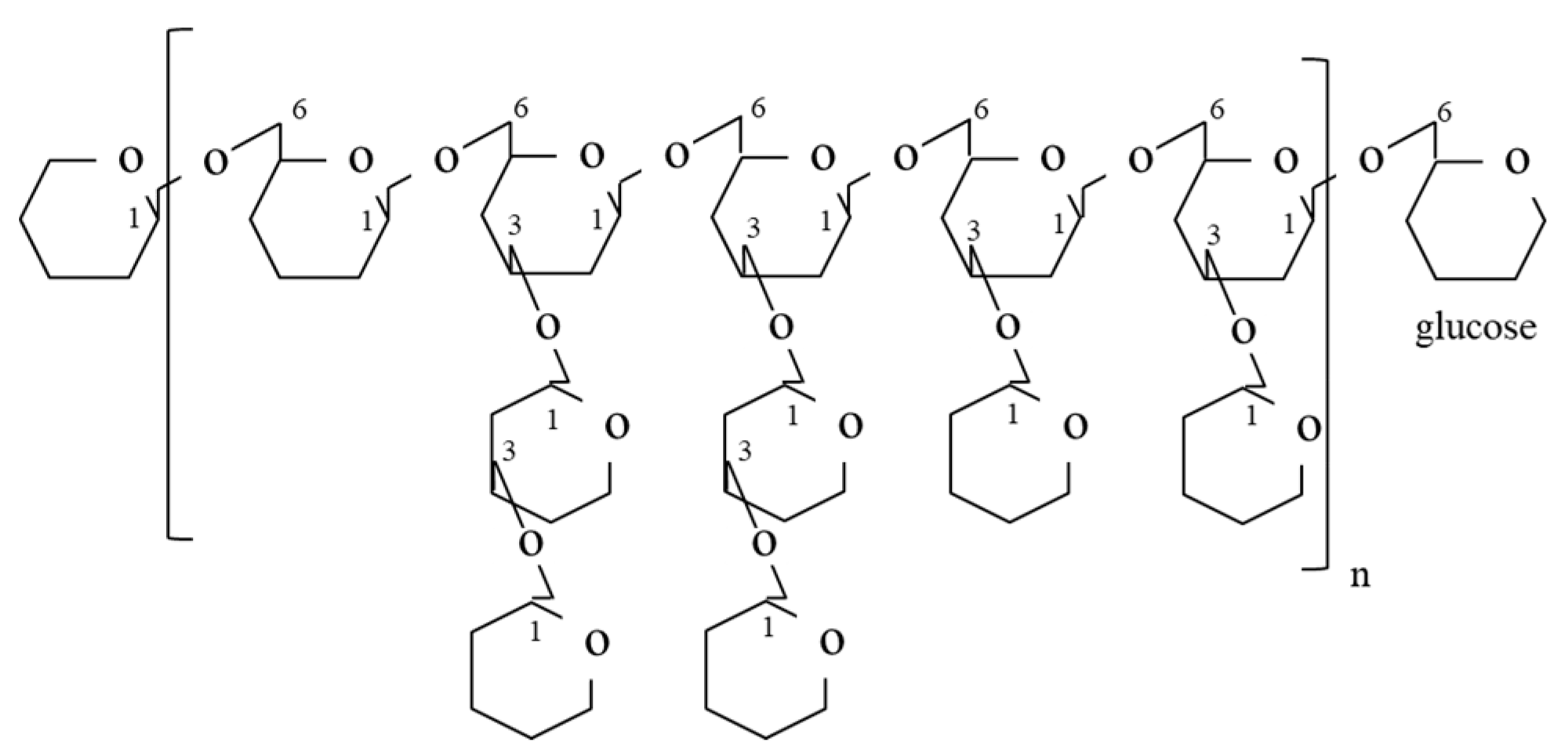
3.1. Polysaccharides
3.2. Proteins and Peptides
3.3. Other Bioactive Molecules
4. Biological Activities and Medicinal Properties
4.1. Antitumor Effects
4.2. Immunomodulation
4.3. Antiviral and Antibacterial Effects
4.4. Antidiabetic Activity
4.5. Lipid Metabolism Regulation and Anti-Hypertension Effects
4.6. Antioxidant Activities
4.7. Gut Microbiota Regulation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| B. acidifaciens: | Bacteroides acidifaciens |
| EV71: | enterovirus 71 |
| FBG: | fasting blood glucose |
| FSG: | fasting serum glucose |
| G. frondosa: | Grifola frondosa |
| GFP: | Grifola frondosa polysaccharide |
| GRN: | Grifolan |
| HBV: | hepatitis B virus |
| HFD: | high-fat diet |
| HIV: | human immunodeficiency virus |
| IFN: | interferons |
| IL: | interleukins |
| IR: | insulin receptor |
| IRS-1: | insulin receptor substrate 1 |
| HSV: | herpes simplex virus |
| L. acidophilus: | Lactobacillus acidophilus |
| L. edodes: | Lentinus edodes |
| NAFLD: | non-alcoholic fatty liver disease |
| NK cell: | natural killer cell |
| SBP: | systolic blood pressure |
| SSF: | solid-state fermentation |
| T2DM: | type 2 diabetes mellitus |
| TNF: | tumor necrosis factors |
References
- Mayell, M. Maitake extracts and their therapeutic potential—A review. Altern. Med. Rev. 2001, 6, 48–60. [Google Scholar] [PubMed]
- Mayuzumi, Y.; Mizuno, T., III. Cultivation methods of maitake (Grifola frondosa). Food Rev. Int. 1997, 13, 357–364. [Google Scholar] [CrossRef]
- Montoya Barreto, S.; Orrego Alzate, C.E.; Levin, L. Modeling Grifola frondosa fungal growth during solid-state fermentation. Eng. Life Sci. 2011, 11, 316–321. [Google Scholar] [CrossRef]
- Lee, B.C.; Bae, J.T.; Pyo, H.B.; Choe, T.B.; Kim, S.W.; Hwang, H.J.; Yun, J.W. Submerged culture conditions for the production of mycelial biomass and exopolysaccharides by the edible Basidiomycete Grifola frondosa. Enzym. Microb. Technol. 2004, 35, 369–376. [Google Scholar] [CrossRef]
- Takama, F.; Minomiya, S.; Yoda, R.; Ishii, H.; Muraki, S. Parenchyma cells, chemical components of maitake mushroom (Grifola frondosa SF Gray) cultured artificially, and their changes by storage and boiling. In Proceedings of the Eleventh International Scientific Congress on the Cultivation of Edible Fungi, Sydney, Australia, 14–19 August 1981. [Google Scholar]
- Shih, L.; Chou, B.-W.; Chen, C.-C.; Wu, J.-Y.; Hsieh, C. Study of mycelial growth and bioactive polysaccharide production in batch and fed-batch culture of Grifola frondosa. Bioresour. Technol. 2008, 99, 785–793. [Google Scholar] [CrossRef]
- Cohen, N.; Cohen, J.; Asatiani, M.D.; Varshney, V.K.; Yu, H.-T.; Yang, Y.-C.; Li, Y.-H.; Mau, J.-L.; Wasser, S.P. Chemical composition and nutritional and medicinal value of fruit bodies and submerged cultured mycelia of culinary-medicinal higher Basidiomycetes mushrooms. Int. J. Med. Mushrooms 2014, 16, 273–291. [Google Scholar] [CrossRef]
- Kurasawa, S.-I.; Sugahara, T.; Hayashi, J. Proximate and dietary fibre analysis of mushrooms. Nippon Shokuhin Kogyo Gakkaishi 1982, 29, 400–406. [Google Scholar] [CrossRef]
- Muratsubaki, T.; Sayama, K.; Sato, K. Change of constituents in fruit body formation of Grifola frondosa. Nippon Shokuhin Kogyo Gakkaishi 1986, 33, 181–185. [Google Scholar] [CrossRef] [Green Version]
- Mau, J.-L.; Lin, H.-C.; Ma, J.-T.; Song, S.-F. Non-volatile taste components of several speciality mushrooms. Food Chem. 2001, 73, 461–466. [Google Scholar] [CrossRef]
- Huang, S.-J.; Tsai, S.-Y.; Lin, S.-Y.; Liang, C.-H.; Mau, J.-L. Nonvolatile taste components of culinary-medicinal Maitake mushroom, Grifola frondosa (Dicks.:Fr.) S.F. Gray. Int. J. Med. Mushrooms 2011, 13, 265–272. [Google Scholar] [CrossRef]
- Kawai, H.; Matsuzawa, M.; Tsutagawa, Y.; Sasaki, H.; Kasuga, A.; Aoyagi, Y. Relationship between fruiting bodies compositions and substrate in Hiratake and Maitake mushrooms cultivated on sawdust substrate beds. Nippon Shokuhin Kogyo Gakkaishi 1994, 41, 419–424. [Google Scholar] [CrossRef]
- Tabata, T.; Yamasaki, Y.; Ogura, T. Comparison of chemical compositions of Maitake (Grifola frondosa (Fr.) SF Gray) cultivated on logs and sawdust substrate. Food Sci. Technol. Res. 2004, 10, 21–24. [Google Scholar] [CrossRef] [Green Version]
- Phillips, K.M.; Ruggio, D.M.; Horst, R.L.; Minor, B.; Simon, R.R.; Feeney, M.J.; Byrdwell, W.C.; Haytowitz, D.B. Vitamin D and sterol composition of 10 types of mushrooms from retail suppliers in the United States. J. Agric. Food Chem. 2011, 59, 7841–7853. [Google Scholar] [CrossRef] [PubMed]
- USDA. FoodData Central Search Results. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/169403/nutrients (accessed on 4 January 2019).
- Miyazaki, T.; Yadomae, T.; Suzuki, I.; Nishijima, M.; Yui, S.; Oikawa, S.; Sato, K. Antitumor activity of fruiting bodies of cultured Grifola frondosa. Jpn. J. Med Mycol. 1982, 23, 261–263. [Google Scholar] [CrossRef]
- Ohno, N.; Suzuki, I.; Oikawa, S.; Sato, K.; Miyazaki, T.; Yadomae, T. Antitumor activity and structural characterization of glucans extracted from cultured fruit bodies of Grifola frondosa. Chem. Pharm. Bull. 1984, 32, 1142–1151. [Google Scholar] [CrossRef]
- Iino, K.; Ohno, N.; Suzuki, I.; Miyazaki, T.; Yadomae, T.; Oikawa, S.; Sato, K. Structural characterisation of a neutral antitumour β-d-glucan extracted with hot sodium hydroxide from cultured fruit bodies of Grifola frondosa. Carbohydr. Res. 1985, 141, 111–119. [Google Scholar] [CrossRef]
- Ohno, N.; Adachi, Y.; Suzuki, I.; Sato, K.; Oikawa, S.; Yadomae, T. Characterization of the antitumor glucan obtained from liquid-cultured Grifola frondosa. Chem. Pharm. Bull. 1986, 34, 1709–1715. [Google Scholar] [CrossRef] [Green Version]
- Nanba, H.; Hamaguchi, A.; Kuroda, H. The chemical structure of an antitumor polysaccharide in fruit bodies of Grifola frondosa (Maitake). Chem. Pharm. Bull. 1987, 35, 1162–1168. [Google Scholar] [CrossRef] [Green Version]
- Hishida, I.; Nanba, H.; Kuroda, H. Antitumor activity exhibited by orally administered extract from fruit body of Grifola frondosa (Maitake). Chem. Pharm. Bull. 1988, 36, 1819–1827. [Google Scholar] [CrossRef] [Green Version]
- Nanba, H.; Kubo, K. Antitumor Substance Extracted from Grifola. U.S. Patent 5,854,404, 29 December 1998. [Google Scholar]
- Kubo, K.; Aoki, H.; Nanba, H. Anti-diabetic activity present in the fruit body of Grifola frondosa (Maitake). I. Biol. Pharm. Bull. 1994, 17, 1106–1110. [Google Scholar] [CrossRef] [Green Version]
- Adachi, Y.; Okazaki, M.; Ohno, N.; Yadomae, T. Enhancement of cytokine production by macrophages stimulated with (1→3)-β-D-glucan, grifolan (GRN), isolated from Grifola frondosa. Biol. Pharm. Bull. 1994, 17, 1554–1560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masuda, Y.; Kodama, N.; Nanba, H. Macrophage J774. 1 cell is activated by MZ-Fraction (Klasma-MZ) polysaccharide in Grifola frondosa. Mycoscience 2006, 47, 360–366. [Google Scholar] [CrossRef]
- Lei, H.; Ma, X.; Wu, W. Anti-diabetic effect of an α-glucan from fruit body of maitake (Grifola frondosa) on KK-Ay mice. J. Pharm. Pharmacol. 2007, 59, 575–582. [Google Scholar] [CrossRef]
- Zhao, C.; Gao, L.; Wang, C.; Liu, B.; Jin, Y.; Xing, Z. Structural characterization and antiviral activity of a novel heteropolysaccharide isolated from Grifola frondosa against enterovirus 71. Carbohydr. Polym. 2016, 144, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Su, C.-H.; Lu, M.-K.; Lu, T.-J.; Lai, M.-N.; Ng, L.-T. A (1→6)-Branched (1→4)-β-d-Glucan from Grifola frondosa Inhibits Lipopolysaccharide-Induced Cytokine Production in RAW264. 7 Macrophages by Binding to TLR2 Rather than Dectin-1 or CR3 Receptors. J. Nat. Prod. 2020, 83, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, M.; Yu, M.; Shen, M.; Wang, Q.; Yu, Y.; Xie, J. Natural polysaccharides exhibit anti-tumor activity by targeting gut microbiota. Int. J. Biol. Macromol. 2019, 121, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zeng, F.; Huang, Y.; Liu, B. The positive effects of Grifola frondosa heteropolysaccharide on NAFLD and regulation of the gut microbiota. Int. J. Mol. Sci. 2019, 20, 5302. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Liu, D.; Wang, D.; Lai, S.; Zhong, R.; Liu, Y.; Yang, C.; Liu, B.; Sarker, M.R.; Zhao, C. Hypoglycemic activity and gut microbiota regulation of a novel polysaccharide from Grifola frondosa in type 2 diabetic mice. Food Chem. Toxicol. 2019, 126, 295–302. [Google Scholar] [CrossRef]
- Cui, F.; Zan, X.; Li, Y.; Yang, Y.; Sun, W.; Zhou, Q.; Yu, S.; Dong, Y. Purification and partial characterization of a novel anti-tumor glycoprotein from cultured mycelia of Grifola frondosa. Int. J. Biol. Macromol. 2013, 62, 684–690. [Google Scholar] [CrossRef]
- Tsao, Y.-W.; Kuan, Y.-C.; Wang, J.-L.; Sheu, F. Characterization of a novel maitake (Grifola frondosa) protein that activates natural killer and dendritic cells and enhances antitumor immunity in mice. J. Agric. Food Chem. 2013, 61, 9828–9838. [Google Scholar] [CrossRef]
- Zhuang, C.; Kawagishi, H.; Preuss, H.G. Glycoprotein with Antidiabetic, Antihypertensive, Antiobesity and Antihyperlipidemic Effects from Grifola frondosa, and a Method for Preparing Same. U.S. Patent 7,214,778, 8 May 2007. [Google Scholar]
- Gu, C.-Q.; Li, J.-W.; Chao, F.; Jin, M.; Wang, X.-W.; Shen, Z.-Q. Isolation, identification and function of a novel anti-HSV-1 protein from Grifola frondosa. Antivir. Res. 2007, 75, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Cui, B. Pharmacological and pharmacokinetic studies with agaricoglycerides, extracted from Grifola frondosa, in animal models of pain and inflammation. Inflammation 2012, 35, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yong, T.; Xiao, C.; Su, J.; Zhang, Y.; Jiao, C.; Xie, Y. Pyrrole alkaloids and ergosterols from Grifola frondosa exert anti-α-glucosidase and anti-proliferative activities. J. Funct. Foods 2018, 43, 196–205. [Google Scholar] [CrossRef]
- Lin, J.-T.; Liu, W.-H. ο-Orsellinaldehyde from the submerged culture of the edible mushroom Grifola frondosa exhibits selective cytotoxic effect against Hep 3B cells through apoptosis. J. Agric. Food Chem. 2006, 54, 7564–7569. [Google Scholar] [CrossRef]
- Yeh, J.-Y.; Hsieh, L.-H.; Wu, K.-T.; Tsai, C.-F. Antioxidant properties and antioxidant compounds of various extracts from the edible basidiomycete Grifola frondosa (Maitake). Molecules 2011, 16, 3197–3211. [Google Scholar] [CrossRef] [Green Version]
- Sim, K.Y.; Liew, J.Y.; Ding, X.Y.; Choong, W.S.; Intan, S. Effect of vacuum and oven drying on the radical scavenging activity and nutritional contents of submerged fermented Maitake (Grifola frondosa) mycelia. Food Sci. Technol. 2017, 37, 131–135. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.-T.; Hayes, W.A. The Biology and Cultivation of Edible Mushrooms; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Ajlouni, S.O.; Beelman, R.B.; Thompson, D.B.; Mau, J.-L. Changes in soluble sugars in various tissues of cultivated mushrooms, Agaricus bisporus, during postharvest storage. In Developments in Food Science; Charalambous, G., Ed.; Elsevier: Amsterdam, The Netherlands, 1995; Volume 37, pp. 1865–1880. [Google Scholar]
- Yoshida, H.; Sasaki, H.; Fujimoto, S.; Sugahara, T. The chemical components of the vegetative mycelia of Basidiomycetes. Nippon Shokuhin Kagaku Kogaku Kaishi 1996, 43, 748–755. [Google Scholar] [CrossRef]
- Tsai, S.-Y.; Weng, C.-C.; Huang, S.-J.; Chen, C.-C.; Mau, J.-L. Nonvolatile taste components of Grifola frondosa, Morchella esculenta and Termitomyces albuminosus mycelia. LWT Food Sci. Technol. 2006, 39, 1066–1071. [Google Scholar] [CrossRef]
- Sanmee, R.; Dell, B.; Lumyong, P.; Izumori, K.; Lumyong, S. Nutritive value of popular wild edible mushrooms from northern Thailand. Food Chem. 2003, 82, 527–532. [Google Scholar] [CrossRef]
- Su, C.-H.; Lai, M.-N.; Lin, C.-C.; Ng, L.-T. Comparative characterization of physicochemical properties and bioactivities of polysaccharides from selected medicinal mushrooms. Appl. Microbiol. Biotechnol. 2016, 100, 4385–4393. [Google Scholar] [CrossRef]
- Mizuno, T.; Zhuang, C. Maitake, Grifola frondosa: Pharmacological effects. Food Rev. Int. 1995, 11, 135–149. [Google Scholar] [CrossRef]
- Alonso, E.N.; Ferronato, M.J.; Gandini, N.A.; Fermento, M.E.; Obiol, D.J.; López Romero, A.; Arévalo, J.; Villegas, M.E.; Facchinetti, M.M.; Curino, A.C. Antitumoral effects of D-fraction from Grifola frondosa (maitake) mushroom in breast cancer. Nutr. Cancer 2017, 69, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Kodama, N.; Komuta, K.; Sakai, N.; Nanba, H. Effects of D-Fraction, a polysaccharide from Grifola frondosa on tumor growth involve activation of NK cells. Biol. Pharm. Bull. 2002, 25, 1647–1650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nanba, H.; Kodama, N.; Schar, D.; Turner, D. Effects of Maitake (Grifola frondosa) glucan in HIV-infected patients. Mycoscience 2000, 41, 293–295. [Google Scholar] [CrossRef]
- Ji, H.-Y.; Yu, J.; Liu, A.-J. Structural characterization of a low molecular weight polysaccharide from Grifola frondosa and its antitumor activity in H22 tumor-bearing mice. J. Funct. Foods 2019, 61, 103472. [Google Scholar] [CrossRef]
- Lei, H.; Wang, W.; Wang, Q.; Guo, S.; Wu, L. Antioxidant and immunomodulatory effects of a α-glucan from fruit body of maitake (Grifola frondosa). Food Agric. Immunol. 2013, 24, 409–418. [Google Scholar]
- Lei, H.; Guo, S.; Han, J.; Wang, Q.; Zhang, X.; Wu, W. Hypoglycemic and hypolipidemic activities of MT-α-glucan and its effect on immune function of diabetic mice. Carbohydr. Polym. 2012, 89, 245–250. [Google Scholar] [CrossRef]
- Ma, X.; Meng, M.; Han, L.; Cheng, D.; Cao, X.; Wang, C. Structural characterization and immunomodulatory activity of Grifola frondosa polysaccharide via toll-like receptor 4–mitogen-activated protein kinases–nuclear factor κB pathways. Food Funct. 2016, 7, 2763–2772. [Google Scholar] [CrossRef]
- Chen, X.; Ji, H.; Zhang, C.; Yu, J.; Liu, A. Structural characterization and antitumor activity of a novel polysaccharide from Grifola frondosa. J. Food Meas. Charact. 2020, 14, 272–282. [Google Scholar] [CrossRef]
- Yu, J.; Ji, H.-Y.; Liu, C.; Liu, A.-J. The structural characteristics of an acid-soluble polysaccharide from Grifola frondosa and its antitumor effects on H22-bearing mice. Int. J. Biol. Macromol. 2020, 158, 1288–1298. [Google Scholar] [CrossRef]
- Cui, F.; Tao, W.; Xu, Z.; Guo, W.; Xu, H.; Ao, Z.; Jin, J.; Wei, Y. Structural analysis of anti-tumor heteropolysaccharide GFPS1b from the cultured mycelia of Grifola frondosa GF9801. Bioresour. Technol. 2007, 98, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.-K.; Gu, Y.-A.; Jeong, Y.-T.; Jeong, H.; Song, C.-H. Chemical characteristics and immuno-modulating activities of exo-biopolymers produced by Grifola frondosa during submerged fermentation process. Int. J. Biol. Macromol. 2007, 41, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Masuda, Y.; Matsumoto, A.; Toida, T.; Oikawa, T.; Ito, K.; Nanba, H. Characterization and antitumor effect of a novel polysaccharide from Grifola frondosa. J. Agric. Food Chem. 2009, 57, 10143–10149. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Wang, Y.; Lv, X.; Shen, X.; Ni, X.; Ding, K. Structure of a β-glucan from Grifola frondosa and its antitumor effect by activating Dectin-1/Syk/NF-κB signaling. Glycoconj. J. 2012, 29, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fang, J.; Ni, X.; Li, J.; Liu, Q.; Dong, Q.; Duan, J.; Ding, K. Inducement of cytokine release by GFPBW2, a novel polysaccharide from fruit bodies of Grifola frondosa, through dectin-1 in macrophages. J. Agric. Food Chem. 2013, 61, 11400–11409. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, X.; Liao, W.; Fang, J.; Chen, X.; Dong, Q.; Ding, K. A heteropolysaccharide, l-fuco-d-manno-1, 6-α-d-galactan extracted from Grifola frondosa and antiangiogenic activity of its sulfated derivative. Carbohydr. Polym. 2014, 101, 631–641. [Google Scholar] [CrossRef]
- Xiao, C.; Wu, Q.; Xie, Y.; Zhang, J.; Tan, J. Hypoglycemic effects of Grifola frondosa (Maitake) polysaccharides F2 and F3 through improvement of insulin resistance in diabetic rats. Food Funct. 2015, 6, 3567–3575. [Google Scholar] [CrossRef]
- Mao, G.-H.; Ren, Y.; Feng, W.-W.; Li, Q.; Wu, H.-Y.; Jin, D.; Zhao, T.; Xu, C.-Q.; Yang, L.-Q.; Wu, X.-Y. Antitumor and immunomodulatory activity of a water-soluble polysaccharide from Grifola frondosa. Carbohydr. Polym. 2015, 134, 406–412. [Google Scholar] [CrossRef]
- Bie, N.; Han, L.; Wang, Y.; Wang, X.; Wang, C. A polysaccharide from Grifola frondosa fruit body induces HT-29 cells apoptosis by PI3K/AKT-MAPKs and NF-κB-pathway. Int. J. Biol. Macromol. 2020, 147, 79–88. [Google Scholar] [CrossRef]
- Mao, G.-H.; Ren, Y.; Li, Q.; Wu, H.-Y.; Jin, D.; Zhao, T.; Xu, C.-Q.; Zhang, D.-H.; Jia, Q.-D.; Bai, Y.-P. Anti-tumor and immunomodulatory activity of selenium (Se)-polysaccharide from Se-enriched Grifola frondosa. Int. J. Biol. Macromol. 2016, 82, 607–613. [Google Scholar] [CrossRef]
- Li, Q.; Wang, W.; Zhu, Y.; Chen, Y.; Zhang, W.; Yu, P.; Mao, G.; Zhao, T.; Feng, W.; Yang, L. Structural elucidation and antioxidant activity a novel Se-polysaccharide from Se-enriched Grifola frondosa. Carbohydr. Polym. 2017, 161, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Meng, M.; Cheng, D.; Han, L.; Chen, Y.; Wang, C. Isolation, purification, structural analysis and immunostimulatory activity of water-soluble polysaccharides from Grifola Frondosa fruiting body. Carbohydr. Polym. 2017, 157, 1134–1143. [Google Scholar] [CrossRef]
- Zhang, A.; Deng, J.; Yu, S.; Zhang, F.; Linhardt, R.J.; Sun, P. Purification and structural elucidation of a water-soluble polysaccharide from the fruiting bodies of the Grifola frondosa. Int. J. Biol. Macromol. 2018, 115, 221–226. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, F.; Chen, G.; Chen, Y.; Zhang, W.; Mao, G.; Zhao, T.; Zhang, M.; Yang, L.; Wu, X. Purification, characterization and immunomodulatory activity of a novel polysaccharide from Grifola frondosa. Int. J. Biol. Macromol. 2018, 111, 1293–1303. [Google Scholar] [CrossRef] [PubMed]
- Kawagishi, H.; Nomura, A.; Mizuno, T.; Kimura, A.; Chiba, S. Isolation and characterization of a lectin from Grifola frondosa fruiting bodies. BBA Gen. Subj. 1990, 1034, 247–252. [Google Scholar] [CrossRef]
- Chan, J.Y.-Y.; Chan, E.; Chan, S.-W.; Sze, S.-Y.; Chan, M.-F.; Tsui, S.-H.; Leung, K.-Y.; Chan, R.Y.-K.; Chung, I.Y.-M. Enhancement of in vitro and in vivo anticancer activities of polysaccharide peptide from Grifola frondosa by chemical modifications. Pharm. Biol. 2011, 49, 1114–1120. [Google Scholar] [CrossRef]
- Zhang, Y.; Mills, G.L.; Nair, M.G. Cyclooxygenase inhibitory and antioxidant compounds from the mycelia of the edible mushroom Grifola frondosa. J. Agric. Food Chem. 2002, 50, 7581–7585. [Google Scholar] [CrossRef]
- He, X.; Du, X.; Zang, X.; Dong, L.; Gu, Z.; Cao, L.; Chen, D.; Keyhani, N.O.; Yao, L.; Qiu, J. Extraction, identification and antimicrobial activity of a new furanone, grifolaone A, from Grifola frondosa. Nat. Prod. Res. 2016, 30, 941–947. [Google Scholar] [CrossRef]
- Wu, S.-J.; Tung, Y.-J.; Ng, L.-T. Anti-diabetic effects of Grifola frondosa bioactive compound and its related molecular signaling pathways in palmitate-induced C2C12 cells. J. Ethnopharmacol. 2020, 260, 112962. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, Y.-F.; Song, L.; Jin, J.-X.; Zhang, Y.-Q.; Gan, H.-Y.; Yang, K.-H. Synergistic apoptotic effect of d-fraction from Grifola frondosa and vitamin C on hepatocellular carcinoma SMMC-7721 cells. Integr. Cancer Ther. 2017, 16, 205–214. [Google Scholar] [CrossRef] [Green Version]
- Masuda, Y.; Inoue, H.; Ohta, H.; Miyake, A.; Konishi, M.; Nanba, H. Oral administration of soluble β-glucans extracted from Grifola frondosa induces systemic antitumor immune response and decreases immunosuppression in tumor-bearing mice. Int. J. Cancer 2013, 133, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Q.; Liu, A.-J. Relationship between heat treatment on structural properties and antitumor activity of the cold-water soluble polysaccharides from Grifola frondosa. Glycoconj. J. 2020, 37, 107–117. [Google Scholar] [CrossRef]
- Alonso, E.N.; Orozco, M.; Nieto, A.E.; Balogh, G.A. Genes related to suppression of malignant phenotype induced by Maitake D-Fraction in breast cancer cells. J. Med. Food 2013, 16, 602–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kodama, N.; Murata, Y.; Asakawa, A.; Inui, A.; Hayashi, M.; Sakai, N.; Nanba, H. Maitake D-Fraction enhances antitumor effects and reduces immunosuppression by mitomycin-C in tumor-bearing mice. Nutrition 2005, 21, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Shomori, K.; Yamamoto, M.; Arifuku, I.; Teramachi, K.; Ito, H. Antitumor effects of a water-soluble extract from Maitake (Grifola frondosa) on human gastric cancer cell lines. Oncol. Rep. 2009, 22, 615–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kodama, N.; Kakuno, T.; Nanba, H. Stimulation of the natural immune system in normal mice by polysaccharide from maitake mushroom. Mycoscience 2003, 44, 257–261. [Google Scholar] [CrossRef]
- Wu, M.-J.; Cheng, T.-L.; Cheng, S.-Y.; Lian, T.-W.; Wang, L.; Chiou, S.-Y. Immunomodulatory properties of Grifola frondosa in submerged culture. J. Agric. Food Chem. 2006, 54, 2906–2914. [Google Scholar] [CrossRef]
- Kodama, N.; Yamada, M.; Nanba, H. Addition of Maitake D-fraction reduces the effective dosage of Vancomycin for the treatment of Listeria-infected mice. Jpn. J. Pharmacol. 2001, 87, 327–332. [Google Scholar] [CrossRef] [Green Version]
- Gu, C.-Q.; Li, J.-W.; Chao, F.-H. Inhibition of hepatitis B virus by D-fraction from Grifola frondosa: Synergistic effect of combination with interferon-α in HepG2 2.2.15. Antivir. Res. 2006, 72, 162–165. [Google Scholar] [CrossRef]
- Shen, K.-P.; Su, C.-H.; Lu, T.-M.; Lai, M.-N.; Ng, L.-T. Effects of Grifola frondosa non-polar bioactive components on high-fat diet fed and streptozotocin-induced hyperglycemic mice. Pharm. Biol. 2015, 53, 705–709. [Google Scholar] [CrossRef] [Green Version]
- Konno, S.; Alexander, B.; Zade, J.; Choudhury, M. Possible hypoglycemic action of SX-fraction targeting insulin signal transduction pathway. Int. J. Gen. Med. 2013, 6, 181–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubo, K.; Nanba, H. Anti-hyperliposis effect of Maitake fruit body (Grifola frondosa). I. Biol. Pharm. Bull. 1997, 20, 781–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukushima, M.; Ohashi, T.; Fujiwara, Y.; Sonoyama, K.; Nakano, M. Cholesterol-lowering effects of maitake (Grifola frondosa) fiber, shiitake (Lentinus edodes) fiber, and enokitake (Flammulina velutipes) fiber in rats. Exp. Biol. Med. 2001, 226, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Preuss, H.G.; Echard, B.; Bagchi, D.; Perricone, N.V. Maitake mushroom extracts ameliorate progressive hypertension and other chronic metabolic perturbations in aging female rats. Int. J. Med Sci. 2010, 7, 169. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Gao, Z.; Hu, C.; Zhang, J.; Sun, X.; Rong, C.; Jia, L. Antioxidant, antibacterial and anti-aging activities of intracellular zinc polysaccharides from Grifola frondosa SH-05. Int. J. Biol. Macromol. 2017, 95, 778–787. [Google Scholar] [CrossRef]
- Lee, B.C.; Bae, J.T.; Pyo, H.B.; Choe, T.B.; Kim, S.W.; Hwang, H.J.; Yun, J.W. Biological activities of the polysaccharides produced from submerged culture of the edible Basidiomycete Grifola frondosa. Enzym. Microb. Technol. 2003, 32, 574–581. [Google Scholar] [CrossRef]
- Chen, G.-t.; Ma, X.-m.; Liu, S.-t.; Liao, Y.-l.; Zhao, G.-q. Isolation, purification and antioxidant activities of polysaccharides from Grifola frondosa. Carbohydr. Polym. 2012, 89, 61–66. [Google Scholar] [CrossRef]
- DonG, Y.; Qi, G.; YanG, Z.; WanG, H.; WanG, S.; CHen, G. Preparation, separation and antioxidant properties of hydrolysates derived from Grifola frondosa protein. Czech J. Food Sci. 2015, 33, 500–506. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Guo, W.-L.; Zhang, W.; Xu, J.-X.; Qian, M.; Bai, W.-D.; Zhang, Y.-Y.; Rao, P.-F.; Ni, L.; Lv, X.-C. Grifola frondosa polysaccharides ameliorate lipid metabolic disorders and gut microbiota dysbiosis in high-fat diet fed rats. Food Funct. 2019, 10, 2560–2572. [Google Scholar] [CrossRef]
- Yadav, H.; Jain, S.; Sinha, P. Antidiabetic effect of probiotic dahi containing Lactobacillus acidophilus and Lactobacillus casei in high fructose fed rats. Nutrition 2007, 23, 62–68. [Google Scholar] [CrossRef]
- Pan, Y.; Wan, X.; Zeng, F.; Zhong, R.; Guo, W.; Lv, X.-C.; Zhao, C.; Liu, B. Regulatory effect of Grifola frondosa extract rich in polysaccharides and organic acids on glycolipid metabolism and gut microbiota in rats. Int. J. Biol. Macromol. 2019, 155, 1030–1039. [Google Scholar] [CrossRef]
- Pan, Y.-Y.; Zeng, F.; Guo, W.-L.; Li, T.-T.; Jia, R.-B.; Huang, Z.-R.; Lv, X.-C.; Zhang, J.; Liu, B. Effect of Grifola frondosa 95% ethanol extract on lipid metabolism and gut microbiota composition in high-fat diet-fed rats. Food Funct. 2018, 9, 6268–6278. [Google Scholar] [CrossRef] [PubMed]
- Kodama, N.; Murata, Y.; Nanba, H. Administration of a polysaccharide from Grifola frondosa stimulates immune function of normal mice. J. Med. Food 2004, 7, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K.-I.; Miura, N.N.; Adachi, Y.; Ohno, N.; Yadomae, T. Relationship between solubility of Grifolan, a fungal 1,3-beta;-D-Glucan, and production of tumor necrosis factor by macrophages in vitro. Biosci. Biotechnol. Biochem. 2001, 65, 1993–2000. [Google Scholar] [CrossRef] [PubMed]
- Su, C.H.; Lu, T.M.; Lai, M.N.; Ng, L.T. Inhibitory potential of Grifola frondosa bioactive fractions on α-amylase and α-glucosidase for management of hyperglycemia. Biotechnol. Appl. Biochem. 2013, 60, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Kabir, Y.; Yamaguchi, M.; Kimura, S. Effect of Shiitake (Lentinus edodes) and Maitake (Grjfola frondosa) mushrooms on blood pressure and plasma lipids of spontaneously hypertensive rats. J. Nutr. Sci. Vitaminol. 1987, 33, 341–346. [Google Scholar] [CrossRef] [Green Version]
- Kabir, Y.; Kimura, S. Dietary mushrooms reduce blood pressure in spontaneously hypertensive rats (SHR). J. Nutr. Sci. Vitaminol. 1989, 35, 91–94. [Google Scholar] [CrossRef]
- Jayachandran, M.; Xiao, J.; Xu, B. A critical review on health promoting benefits of edible mushrooms through gut microbiota. Int. J. Mol. Sci. 2017, 18, 1934. [Google Scholar] [CrossRef] [Green Version]
- Cotillard, A.; Kennedy, S.P.; Kong, L.C.; Prifti, E.; Pons, N.; Le Chatelier, E.; Almeida, M.; Quinquis, B.; Levenez, F.; Galleron, N. Dietary intervention impact on gut microbial gene richness. Nature 2013, 500, 585–588. [Google Scholar] [CrossRef]
- Friedman, M. Mushroom polysaccharides: Chemistry and antiobesity, antidiabetes, anticancer, and antibiotic properties in cells, rodents, and humans. Foods 2016, 5, 80. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.-L.; Deng, J.-C.; Pan, Y.-Y.; Xu, J.-X.; Hong, J.-L.; Shi, F.-F.; Liu, G.-L.; Qian, M.; Bai, W.-D.; Zhang, W.; et al. Hypoglycemic and hypolipidemic activities of Grifola frondosa polysaccharides and their relationships with the modulation of intestinal microflora in diabetic mice induced by high-fat diet and streptozotocin. Int. J. Biol. Macromol. 2020, 153, 1231–1240. [Google Scholar] [CrossRef]
- Gangarapu, V.; Yildiz, K.; İnce, A.T.; Baysal, B. Role of gut microbiota: Obesity and NAFLD. Turk. J. Gastroenterol. 2014, 25, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lee, Y.; Kim, Y.; Lee, S.; Ryu, S.; Fukuda, S.; Hase, K.; Yang, C.; Lim, H.; Kim, M. Gut commensal Bacteroides acidifaciens prevents obesity and improves insulin sensitivity in mice. Mucosal Immunol. 2017, 10, 104–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, W.-L.; Chen, M.; Pan, W.-L.; Zhang, Q.; Xu, J.-X.; Lin, Y.-C.; Li, L.; Liu, B.; Bai, W.-D.; Zhang, Y.-Y.; et al. Hypoglycemic and hypolipidemic mechanism of organic chromium derived from chelation of Grifola frondosa polysaccharide-chromium (III) and its modulation of intestinal microflora in high fat-diet and STZ-induced diabetic mice. Int. J. Biol. Macromol. 2020, 145, 1208–1218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Sun, W.; Yu, N.; Sun, J.; Yu, X.; Li, X.; Xing, Y.; Yan, D.; Ding, Q.; Xiu, Z. Anti-diabetic effect of baicalein is associated with the modulation of gut microbiota in streptozotocin and high-fat-diet induced diabetic rats. J. Funct. Foods 2018, 46, 256–267. [Google Scholar] [CrossRef]



| Components 1 (%) | Fruiting Body | Mycelium | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [8] * | [9] # | [12] # | [10] 2,# | [13] # | [11] 2,# | [7] 2,# | Average | [43] | [44] 2 | [11] 2 | Average | |
| Moisture | 83.1 | 89.1 | 90.9 | 86.1 | 90.4 | 95.6 | 95.2 | 90.1 ± 4.5 | 84.8 | 96.7 | 92.3 | 91.3 ± 6.0 |
| Dry matter 3 | 16.9 | 10.9 | 9.1 | 13.9 | 9.6 | 4.4 | 4.8 | 9.9 ± 4.5 | 15.2 | 3.3 | 7.7 | 8.7 ± 6.0 |
| Carbohydrate 4 | 70.4 | 74.9 | 72.3 | 68.8 | 71.8 | 66.3 | 70.3 | 70.7 ± 2.7 | 66.3 | 45.0 | 60.4 | 57.2 ± 11.0 |
| Crude ash | 6.5 | 4.8 | 6.6 | 7.0 | 7.1 | 6.2 | 4.9 | 6.1 ± 0.9 | 6.4 | 4.0 | 4.7 | 5.0 ± 1.3 |
| Crude fat | 4.5 | 1.5 | 3.3 | 3.1 | 2.4 | 6.5 | 5.6 | 3.8 ± 1.8 | 4.2 | 24.7 | 6.5 | 11.8 ± 11.2 |
| Crude protein | 18.6 | 18.9 | 17.8 | 21.1 | 18.8 | 21.0 | 19.2 | 19.3 ± 1.3 | 23.1 | 26.4 | 28.4 | 26.0 ± 2.7 |
| Component | Fruiting Body (mg/g Dry wt.) | Mycelium (mg/g Dry wt.) | ||||
|---|---|---|---|---|---|---|
| [9] # | [10] 1,# | [11] 1,# | [43] | [44] 1 | [11] 1 | |
| Arabinose | n.d. 2 | n.d. | n.d. | n.d. | n.d. | 5.37 |
| Arabitol | n.d. | n.d. | n.d. | n.d. | 12.65 | 2.01 |
| Fructose | n.d. | n.d. | n.d. | 1.00 | n.d. | 2.99 |
| Glucose | 59.30 | 14.02 | 2.42 | 8.00 | 19.72 | 2.18 |
| Lactose | n.d. | n.d. | n.d. | n.d. | n.d. | 0.93 |
| Mannitol | 7.20 | 9.36 | 1.00 | n.d. | 9.92 | 2.30 |
| Mannose | n.d. | n.d. | n.d. | n.d. | n.d. | 1.92 |
| Ribose | n.d. | n.d. | 8.34 | n.d. | n.d. | 4.04 |
| Trehalose | 45.80 | 161.83 | 99.94 | 65.00 | 41.60 | 65.32 |
| Total | 112.30 | 185.21 | 111.7 | 74.00 | 83.89 | 87.06 |
| Component (mg/g Dry wt.) | Fruiting Body | Mycelium | ||||
|---|---|---|---|---|---|---|
| [13] 1,# | [11] 1,# | [10] 1,# | [44] | [11] 1 | ||
| In Sawdust | In Log | |||||
| L-Alanine | 2.15 | 3.13 | 5.22 | 2.77 | 3.26 | 14.59 |
| L-Arginine | 3.02 | 3.21 | 1.66 | 0.64 | 0.97 | 12.39 |
| L-Aspartic acid | 1.61 | 1.25 | 1.88 | 0.42 | 2.75 | 19.40 |
| L-Glutamic acid | 8.01 | 9.10 | 12.62 | 0.67 | 3.76 | 2.10 |
| GABA | n.d. 2 | n.d. | 0.28 | n.d. | n.d. | 17.09 |
| Glycine | 1.53 | 1.53 | 2.46 | 0.57 | 1.93 | 7.81 |
| L-Histidine 3 | 1.53 | 0.94 | 19.50 | 0.59 | 4.10 | n.d. |
| L-Isoleucine 3 | 0.12 | 0.12 | 0.56 | 0.33 | 2.80 | 6.67 |
| L-Leucine 3 | 0.05 | 0.09 | 0.27 | 0.35 | 4.92 | 6.39 |
| L-Lysine 3 | 1.56 | 1.28 | 5.70 | 1.11 | 0.22 | 23.49 |
| L-Methionine 3 | n.d. | n.d. | 4.50 | 1.40 | 0.67 | n.d. |
| L-Phenylalanine 3 | 0.26 | 0.28 | 2.71 | 0.80 | 1.66 | 9.98 |
| L-Serine | 2.91 | 2.82 | 2.01 | 0.97 | 2.73 | 10.74 |
| L-Threonine 3 | 1.43 | 1.44 | n.d. | 4.40 | 8.23 | 10.85 |
| L-Tryptophan 3 | n.d. | n.d. | n.d. | 0.27 | n.d. | 12.01 |
| L-Tyrosine | 1.77 | 0.73 | 1.53 | n.d. | 2.15 | 17.99 |
| L-Valine 3 | 0.96 | 0.91 | 0.39 | 0.60 | 4.13 | 9.41 |
| Total | 29.26 | 29.38 | 61.29 | 15.9 | 44.28 | 180.91 |
| Name of Active Fractions/ Purified PS | MW | Structure/Composition | Monosaccharide Composition * | Extraction Solvent & Source | Reference |
|---|---|---|---|---|---|
| Grifolan-7N | 1200 kDa | (1→3)-linked β-D-glucan having a single β-D-glucopyranosyl group attached to position 6 of almost every 3rd backbone unit | Glc | Hot sodium hydroxide, Fruiting body | [18] |
| GRN | 500 kDa (Mw) | (1→6) –branched (1→3)-β-D-Glucan | Glc | 0.5% citrate buffer, Mycelium | [24] |
| X-fraction | - | β-1,6 glucan having alpha-1,4 branches | Glc | EtOEt-EtOH and then hot water, Fruiting body | [23] |
| D-fraction | 1000 kDa | Isolated beta-glucan polysaccharide compounds (beta-1,6 glucan and beta-1,3 glucan) with protein | Glc | Hot water, Fruiting body | [22,50] |
| MD-fraction | 1000 kDa | Purified D-fraction with the same main component where the glucan/protein ratio is in the range of 80:20 to 99:1 | Glc | Hot water, Fruiting body | [22] |
| MZ-fraction | 20 kDa (Mw) | β-1,6 main chain and a β-1,3 side chain | Glc | Hot water, Fruiting body | [25] |
| GFPS1b | 21 kDa | Backbone consisted of a-(1→4)-linked D-galacopyranosyl and a-(1→3)-linked D-glucopyranosyl residues substituted at O-6 with glycosyl residues composed of a-L-arabinose-(1→4)-a-D-glucose (1→linked residues | Glc: Gal: Ara = 4:2:1 | Hot water, Mycelium | [57] |
| EX-GF-Fr. III | 2.8 kDa | - | Glc: Rib: Man: Gal: Rha: Xylose = 3.98:1.44:1.34:1.00:0.41:0.15 | Mycelium | [58] |
| MZF | 23 kDa | →6)-α-d-Galp-(1→(36.2%),→3)-α-l-Fucp-(1→(14.5%),→6)-α-d-Manp-(1→(9.4%),→3)-β-d-Glcp-(1→(10.1%), α-d-Manp-(1→(23.2%), and →3,6)-β-d-Glcp-(1→(6.5%) | Gal: Man: Fuc: Glc = 1.24:1:0.95:0.88 | Hot water, Fruiting body | [59] |
| GFPBW1 | 300 kDa | β-D-(1-3)-linked glucan backbone with a single β-D-(1-6)-linked glucopyranosyl residue branched at C-6 on every third residue | Glc | Hot water then 5% NaOH solution, Fruiting body | [60] |
| GFPBW2 | 26.2 kDa | Backbone consisting of β-D-1,3- and β-D-1,4-linked glucopyranosyl residues, with branches attached to O-6 of β-D-1,3-linked glucopyranosyl residues | Glc | Hot water then 5% NaOH solution, Fruiting body | [61] |
| MT-α-glucan | 40–45 kDa | D-glucose with α-glucosidic bond | Glc | Hot water, fruiting body | [26,52,53] |
| GFPW | 15.7 kDa | Backbone of α-1,6-linked galactopyranosyl residues with branches attached to O-2 of α-1,3-linked fucose residues and terminal mannose | Man: Fuc: Gal = 0.41:0.44:1 | Hot water, fruiting body | [62] |
| GFPs-F2 and F3 | - | F2 with polysaccharide 62.5% and protein 37.5%; F3 with polysaccharide 78.3% and protein 21.7% | F2: Glc: Man: Gal: Xyl: Ara: Rha: Rib = 26.74:22.79:16.76: 16.02:14.29:2.05:1.35. F3: Ri: Ara: Xyl = 74.74:14.20:11.08 | Hot water, fruiting body | [63] |
| GP11 | 6.9 kDa | →1)-d-Manp-(6→,→1)-d-Glcp-(4→,→1)-d-Galp-(6→and→2,3,6)-d-Glcp-(1→, with branches attached at O-2,3 of 1,2,3,6-linked Glcp residues and terminal T-Glcp | Man: Glc: Gal = 1:5.04:2.61 | Hot water, fruiting body | [64] |
| GRP1 | 40.5 kDa | 1,6-β-D-glucan backbone with a single1,3-α-D-fucopyranosyl side-branching unit | Glc: Fuc = 2.3:0.5. | Hot water, mycelium | [27] |
| GFP-A | 848 kDa | Main chain consisted of (1→4)-linked and (1→6)-linked α-D-glucopyranosyl, and (1→3,6)-linked α-D-mannopyranosyl residues | Rha: Ara: Xyl: Gal: Man: Glc = 1.38:0.53:0.11:1.07:28.75:1.76 | Hot water, fruiting body | [54,65] |
| GFP-A | 2484 kDa | α-type rhamnopyranose, β-type mannopyranose and α-type galactopyranose | Rha: Xyl: Man: Glc: Gal = 25.98: 9.32: 11.73: 4.74: 48.22 | Ultrasound and hot water, fruiting body | [55] |
| Se-GP11 | 33 kDa | - | Man: Glc: Gal = 1:4.91:2.41 | Hot water, fruiting body | [66] |
| Se-GFP-22 | 4130 kDa | Backbone chain of 1,4-α-D-Glcp units with a branched point at C6 of both 1,3,6-β-D-Manp and 1,4,6-α-D-Galp units | Man: Glc: Gal = 3.3:23.3:1 | Hot water, fruiting body | [67] |
| GFP | 155 kDa | (1→4)-linked methylation backbone, Glcp residues were major structural polysaccharide GFP units, accounting of the polysaccharide backbone speculate GFP every 3)-Glcp-(1→and one 3,4)-Glcp-(1→connected interval with a small amount of 1→, 1→4,1→6 glycosidic linkage | Rha: Xyl: Man: Glc = 1.00:1.04:1.11:6.21 | Hot water, fruiting body | [68] |
| GFP30-2-a | 2040 kDa | Repeating unit of β D Glcp (1→[4) α D Glcp (1→4) α D Glcp (1]m→4) α D Glcp | Glc: Gal = 1:0.098 | Hot water, fruiting body | [69] |
| GFP-22 | 27.2 kDa | Backbone composed of 1,4-β-D-Glcp, 1,3-β-D-Glcp, 1,6-α-D-Glcp, 1,6-α-D-Galp, 1,4,6-α-D-Manp and 1,3,6-α-D-Manp units | Man: Glc: Gal = 2.8:15.2:1.0 | Hot water, fruiting body | [70] |
| GF70-F1 | 1260 kDa | (1→3),(1→6)-β-D-glucan &β-(1→4)- linked backbone and β-(1→6)-linked branches | Gal:Glc:Man = 1.24:56:1 | Hot water, fruiting body | [28] |
| LMw-GFP | 1790 Da | α-T-Glcp (28.26%), α-1→4-Glcp (50.24%) and α-1→3,4-Glcp (21.50%) | Glc | 65 °C water with ultrasound, fruiting body | [51] |
| GFAP | 644.9 kDa | (1→3)-β-D-Glcp and (1→3)-α-D-Manp | Gal: Glc:Man = 0.23:2.18:1 | Water with ultrasound, fruiting body | [56] |
| GFP-N | 1.26 × 107 Da | →2,6)-α-D-Manp-(1→4, α-L-Araf-C1→ and →3,6)-β-DGlcp-(1→ | Ara: Man: Glc = 3.79:1.00:49.70 | Hot water, fruiting body | [31] |
| Bioactive Protein/Peptide | MW | Composition *,#/Structure | Extraction Solvent & Source | Ref. |
|---|---|---|---|---|
| GFL | 30–52 kDa | Glycoprotein with 3.3% total sugar, amino acids with a high content of acidic and hydroxyl amino acids and a low content of Met and His | 2-Mercaptoethanol and Ethylenediaminetetraacetic acid (EDTA), fruiting body | [71] |
| Glyco-protein | 20 kDa | Protein to saccharide ratio from 75:25 to 90:10. Amino acid composition: Asn, Gln, Ser, Thr, Gly, Ala, Val, Cys, Met, Ile, Leu, Tyr, Phe, Lys. His, Arg and Pro. Monosaccharide composition: Gal, Man, Glc, N-acetylglucosamine and Fuc | Ethanol then hot water, fruiting body | [34] |
| GFAHP | 29.5 kDa | N-terminal sequence consisted of an 11-amino-acid peptide. | Hot water, fruiting body | [35] |
| GFG-3a | 88.01 kDa | Glycoprotein with O-glycosylation and 6.20% carbohydrate composed of Ara, Fru, Man and Glc in a molar ratio of 1.33:4.51:2.46:1.00; predominantly β-sheet glycoprotein with a relatively small α-helical content | Water, mycelium | [32] |
| GFPr | 83 kDa | Non-glucan heterodimeric protein that consists of two 41 kDa subunits | Buffer containing acetic acid, 2-mercaptoethanol, and sodium chloride, fruiting body | [33] |
| Name of Molecule/Fractions | Composition | Extraction Solvent & Source | Ref. |
|---|---|---|---|
| Fatty acid, Compounds 1,2,3 | Fatty acid composed of as palmitic, oleic, and linoleic acids; ergosterol (1), ergostra-4,6,8(14),22-tetraen-3-one (2), 1-oleoyl-2-linoleoyl-3-palmitoylglycerol (3) | Hexane, mycelium | [73] |
| HE-5-5 | o-orsellinaldehyde | Ethyl acetate, mycelium | [38] |
| Polyphenolics, flavonoids, ascorbic acid and α-tocopherol | - | Hot water/cold water/ethanol, fruiting body | [39] |
| AGF | - | Acetone, mycelium | [36] |
| Grifolaone A | (S)-methyl 2-(2-hydroxy-3,4-dimethyl-5-oxo-2,5-dihydrofuran-2-yl) acetate | Ethyl acetate, mycelium | [74] |
| GF-3 | Pyrrolefronine, seven pyrrole alkaloids and nine ergosterols | Ethanol, fruiting body | [37] |
| Ergosterol peroxide | - | Methanol, fruiting body | [75] |
| Bioactivity | Bioactive Components | Name of Fraction | Testing Method | Potency of Bioactivity | Ref. |
|---|---|---|---|---|---|
| Anti-tumor | PS | D-fraction | In vivo counting of the number of tumor foci metastasized using stereoscope wide field microscopy | 1 mg/kg/day for 17 days against MM46 liver carcinoma, 91.3% inhibition ratio | [80] |
| PS | GFP-A | In vitro testing of cancer cell viability using MTT assay | 150 μg/mL at 48 h, 50% inhibition (IC50) of human colon cancer cells | [65] | |
| PS | MD-fraction | In vivo assessing of inhibition rate by measuring tumor weight | 0.1 mg/kg at 10 times after transplanting MM46 carcinoma, 94.3% inhibition ratio | [22] | |
| PS | MZ-fraction | In vivo assessing of tumor inhibition by measuring tumor weight | 4 mg/kg/day against MM46 carcinoma, 70.3% inhibition ratio | [25] | |
| PS | GFP-A | In vivo assessing of tumor inhibition rate in mice inoculated with S180 sarcoma cells | Oral administration of 50, 100 and 200 mg/kg for 15 days, tumor inhibitory rates were 17.1%, 28.3% and 52.2% respectively | [55] | |
| PS | LMw-GFP | In vivo assessing of tumor inhibition rate in mice inoculated with H22 hepatoma cells | Oral administration of 200 mg/kg for 15 days, tumor inhibitory ratio was 40.1% | [51] | |
| PS | GFAP | In vivo assessing of tumor inhibition rate in H22 hepatoma cell-bearing mice | Intragastric administration of 100 and 200 mg/kg for 15 days, tumor inhibitory rate was 16.36% and 36.72% respectively | [56] | |
| Glycoprotein | GFG-3a | In vitro testing of cancer cell viability using MTT assay | 20 μg/mL against sarcoma 180 cells, 92% inhibition ratio; 60 μg/mL against BEL 7402 cells, 95% inhibition ratio | [32] | |
| Water soluble extract | - | In vitro counting under a phase-contrast microscope | 10% w/v Maitake extract against TMK-1 gastric cancer cell lines for 3 days, 90% inhibition ratio | [81] | |
| Immuno-modulatory | PS | D-fraction | In vitro evaluation of cytokine production using ELISA | 4.0 mg/kg/day, 3000 pg/mL IL-12 production | [82] |
| PS | GRN | In vitro evaluation of cytokine production and activity of macrophages using ELISA and MTT assay | 100 μg/mL, 11.050 ng/mL IL-6 production and 14.458 ng/mL TNF-α production by RAW264.7 cells | [24] | |
| PS | GP11 | In vitro evaluation of cytokine production and activity of macrophages using ELISA and MTT assay | 1000 μg/mL, 81.84 pg/mL TNF-α, 229.07 pg/mL IL-1β level | [64] | |
| PS | MZ-fraction | In vitro determination of TNF-α or IL-12 by ELISA | 500 μg/mL, around 85 pg/mL IL-12 and 50 ng/mL TNF-α by J774.1 macrophage | [25] | |
| PS | GFP | In vitro macrophage proliferation assessment using MTT assay; concentration of cytokine and chemokine measured by multiplex magnetic bead panel kit | 40 μg/mL, 150% cell viability for 36 h | [68] | |
| PS | Fr. I and II | In vitro human blood cytokine concentrations determined by ELISA | 0.1 mg/mL Fr. II, around 3700 pg/mL TNF-α, 360 pg/mL IFN-γ and 4400 pg/mL IL-6 | [83] | |
| Antiviral/ antibacterial | PS | D-fraction | In vivo determination of the survival rate of Listeria monocytogenes by estimating colony-forming units (CFUs); In vitro HBV DNA and viral antigen analysis using quantitative real-time polymerase chain reaction and end-point titration in radioimmunoassays, respectively | 10 mg/kg/d, survived rate of L. monocytogenes = 67%; IC50 for HBV DNA in cells = 0.59 mg/mL; IC50 for HBV polymerase = 1.38 mg/mL; | [84,85] |
| PS | GFP1 | In vitro EV71-infected cell inhibition rate determination using CCK-8 assay | 250 μg/mL extract, inhibition rate = 20% after 10 h | [27] | |
| Protein | GFAHP | In vitro HSV-1 virus quantity analysis using plaque reduction assay; In vivo HSV-1 virus measurement using plaque assay | IC50 for HSV-1 replication = 4.1 μg/mL; 150 μg/mL, mean virus titer 12.7% compared to control after 24 h | [35] | |
| Antidiabetic | PS | F2/F3 | In vivo fasting serum glucose (FSG) level measurement using glucose oxidase method in diabetes rat model | Intake 100 mg/kg/d F2 or 50 mg/kg/d F3 for two weeks, inhibits a rise in FSG level | [63] |
| PS | MT-α-glucan | In vivo glucose oxidase method using reflective glucometer on KK-Ay mice | Intake 150 mg/kg/d for two weeks, decrease around 23% FSG | [26] | |
| n-hexane extract | GF-H | In vitro α-amylase inhibition assay and α-glucosidase inhibition assay; In vivo glucose level measurement by the glucose oxidase method in high-fat-diet and streptozotocin (HFD + STZ)-induced hyperglycemic mice | IC50 of α-amylase and α-glucosidase: 3.75 mg/mL and 0.04 mg/mL respectively; Intake 600 mg/kg, blood glucose level decrease 28% | [86] | |
| Glycoprotein | SX-fraction | In vivo FBG measurement on type 2 diabetic patients | Intake 2–4 weeks, 30–63% decline in FBG | [87] | |
| Small molecules | Ergosterol peroxide | In vitro assessment of antidiabetic activity in palmitate-induced murine C2C12 skeletal muscle cells by measuring glucose uptake | At 5 μM, the increase in the glucose absorption rate was as good as that of the insulin-treated cells | [75] | |
| Lipid metabolism/ hypertension | Dry Maitake powder | - | In vitro testing using a commercial kit (cholesterol E-Test, Phospholipid B-Test Triglyceride E-Test) | Liver weight 0.68 times lower than control; Triglyceride, total cholesterol and free cholesterol reduced by 0.46 times, 0.54 times and 0.65 times in liver with diet containing 20% maitake for 11 d | [88] |
| Dry Maitake fiber | - | In vitro total cholesterol, HDL cholesterol and triglyceride concentrations in the serum, determined enzymatically by commercially available reagent kits | Serum total cholesterol concentration reduced by 11% than control by 50 g/kg maitake for 4 weeks | [89] | |
| Water extract | - | In vivo systolic blood pressure (SBP) level measurement using tail plethysmography in aging female rats | Intake 350 mg/kg for 120 d, significantly lower SBP level | [90] | |
| Antioxidant | PS | IZPS | In vitro access of hydroxyl radical, DPPH radical, superoxide radical and hydrogen peroxide scavenging ability, reducing power and Fe2+chelating activity by chemical methods | EC50 scavenging •OH, DPPH• and O2− are 204 mg/L; 211 mg/L and 525 mg/L; At 1000 mg/L, H2O2 scavenging rate 95%; reducing power (abs at 700 nm) 0.38; Fe2+chelating activity 51% | [91] |
| PS | G-2/G-3 | In vitro assessment of the superoxide scavenging activity by chemical assay; In vitro assessment of free radical scavenging activity after UV irradiation in HDF cells | At 0.2% w/v, inhibit 90% (G2) and 75% (G3) O2−; decreased free radicals (formed after UV irradiation) by 20% for both G2 and G3 | [92] | |
| PS | GFP-1, GFP-2/GFP-3 | In vitro assessment of of hydroxyl radical, DPPH radical and superoxide radical scavenging ability and Fe2+chelating activity by chemical methods | At 3.0 mg/mL, the scavenging rate of DPPH•, •OH, O2−•: 49, 48 and 45% (GFP-1); 78, 53 & 53% (GFP-2) & 66, 93 & 83% (GFP-3); At 5 mg/L, Fe2+chelating rate: 91% (GFP-1); 98% (GFP-2) and 80% (GFP-3) | [93] | |
| PS | Se-GFP-22 | In vitro assessment of DPPH radical scavenging ability | At 1000 μg/mL, 46% scavenging rate | [67] | |
| Protein | GFHT-4 | In vitro assessment of DPPH radical scavenging ability, Fe2+chelating activity, reducing power and inhibition of linoleic acid autoxidation power by chemical methods | At 2.5 mg/mL, inhibits 90% DPPH•, chelate 80% Fe2+, reducing power close to 1.5 mg ascorbic acid/mL; At 0.5 mg/mL, inhibition of linoleic acid autoxidation power equivalent to BHA (0.5 mg/mL) | [94] | |
| Small molecule | Ergosterol, ergostra-4,6,8(14), 22-tetraen-3-one, & 1-oleoyl-2-linoleoyl-3-palmitoylglycerol | In vitro assessment of antioxidants by liposome oxidation model | At 100 μg/mL, 79, 48% and 42% inhibition rate, respectively | [73] | |
| Microbiota regulation | PS | GFP | In vivo measurement of gut microbiota in high-fat-diet-fed rats by high-throughput sequencing | For GFP (400 mg/kg day)-treated group, significant increase in the relative abundance of Helicobater, Intestinimonas, Barnesiella, Parasutterella, Ruminococcus and Flavonifracter, and decrease in Clostridium-XVIII, Butyricicoccus and Turicibacter. Similar gut microbiota composition to that of the normal group. | [95] |
| PS | GFP-N | In vivo determination of intestinal microflora in a diabetes rat model using single-molecule real-time sequencing | For GFP-N-fed group (75 and 150 mg/kg day), significant increase of the relative abundances of Porphyromonas gingivalis, Akkermansia muciniphila, Lactobacillus acidophilus, Tannerella forsythia, Bacteroides acidifaciens and Roseburia intestinalis. Similar gut microbiota composition to that of the normal group. | [31] | |
| PS | GFP | In vivo access of gut microbiota in high-fat diet-fed and streptozotocin-treated mice by high throughput sequencing. | For GFP-treated (900 mg/kg day) group, significant increase in the relative abundance of Alistipes and Bacteroides, and decrease in Enterococcus. | [96] | |
| PS | GFP | In vivo evaluation of gut microbiota in high-fat-diet-fed rats by high-throughput next-generation 16S rRNA gene sequencing. | For GFP-treated (150 mg/kg day) group, significant increase in the relative abundance of Allobaculum, Bacteroides, Bifidobacterium and other cecal microbiota compared with the HFD-fed group. | [30] | |
| PS | GFWE | In vivo access of gut microbiota in high-sucrose- and high-fat-diet-fed rats by real-time sequencing. | For GFWE-treated (150 mg/kg day) group, increase in the relative abundance of caecal bacteria Oscillibacter and Barnesiella. | [97] | |
| Small molecule | GF95 (mainly 4-hydroxyhippuric acid, flavone derivatives, luteolin, luteolin 6,7-dimethoxy & jaceosidin or 5,7,4-trihydroxy-3) | In vivo access of gut microbiota in high-fat-diet-fed rats by real-time sequencing | For GF95 (150 mg/kg day)-fed group, a higher relative abundance of Intestinimonas and Butyricimonas than that fed with HFD only. | [98] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.-Y.; Siu, K.-C.; Geng, P. Bioactive Ingredients and Medicinal Values of Grifola frondosa (Maitake). Foods 2021, 10, 95. https://doi.org/10.3390/foods10010095
Wu J-Y, Siu K-C, Geng P. Bioactive Ingredients and Medicinal Values of Grifola frondosa (Maitake). Foods. 2021; 10(1):95. https://doi.org/10.3390/foods10010095
Chicago/Turabian StyleWu, Jian-Yong, Ka-Chai Siu, and Ping Geng. 2021. "Bioactive Ingredients and Medicinal Values of Grifola frondosa (Maitake)" Foods 10, no. 1: 95. https://doi.org/10.3390/foods10010095
APA StyleWu, J.-Y., Siu, K.-C., & Geng, P. (2021). Bioactive Ingredients and Medicinal Values of Grifola frondosa (Maitake). Foods, 10(1), 95. https://doi.org/10.3390/foods10010095







