Effect of Frying Conditions on Self-Heating Fried Spanish Mackerel Quality Attributes and Flavor Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Fried Fish Filets
2.3. Moisture Content Analysis
2.4. Textural Analysis
2.5. Color Measurement
2.6. Scanning Electron Microscopy (SEM)
2.7. HS-GC–IMS Analysis
2.8. Statistical Analysis
3. Results and Discussion
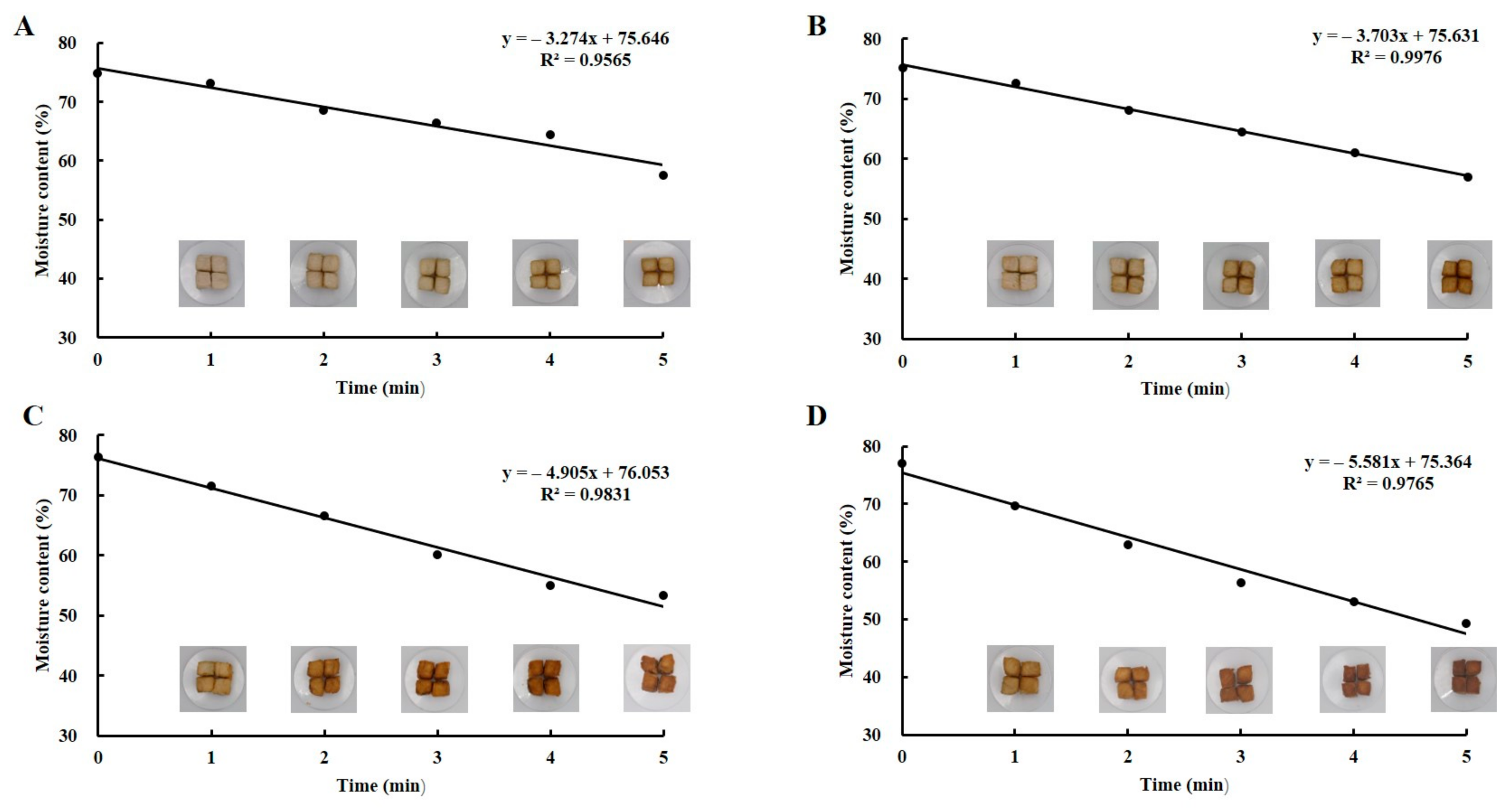
3.1. Fish Filet Moisture Content Determination under Different Conditions
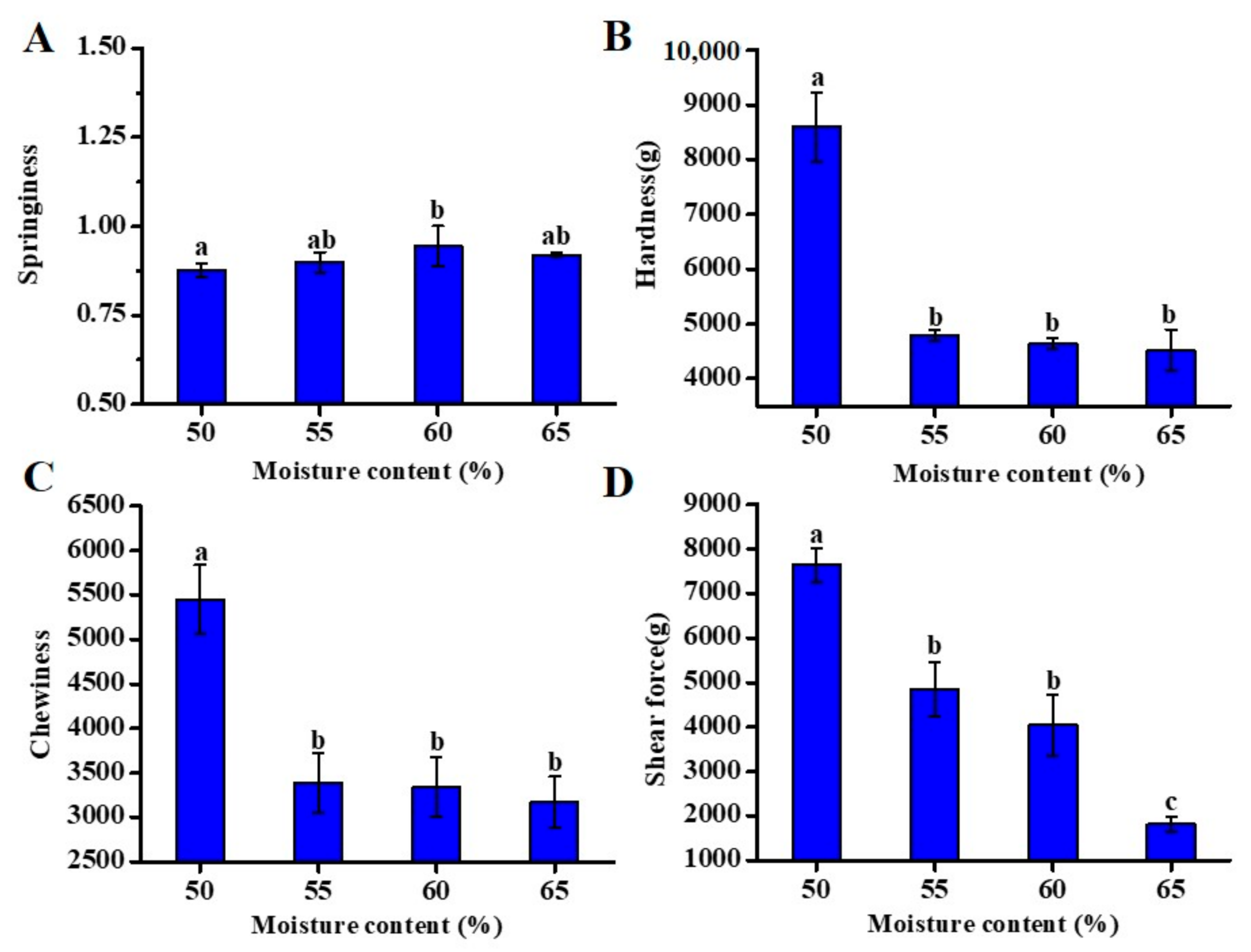
3.2. Effect of Different Moisture Content on Self-Heating Fried Fish Filet Texture
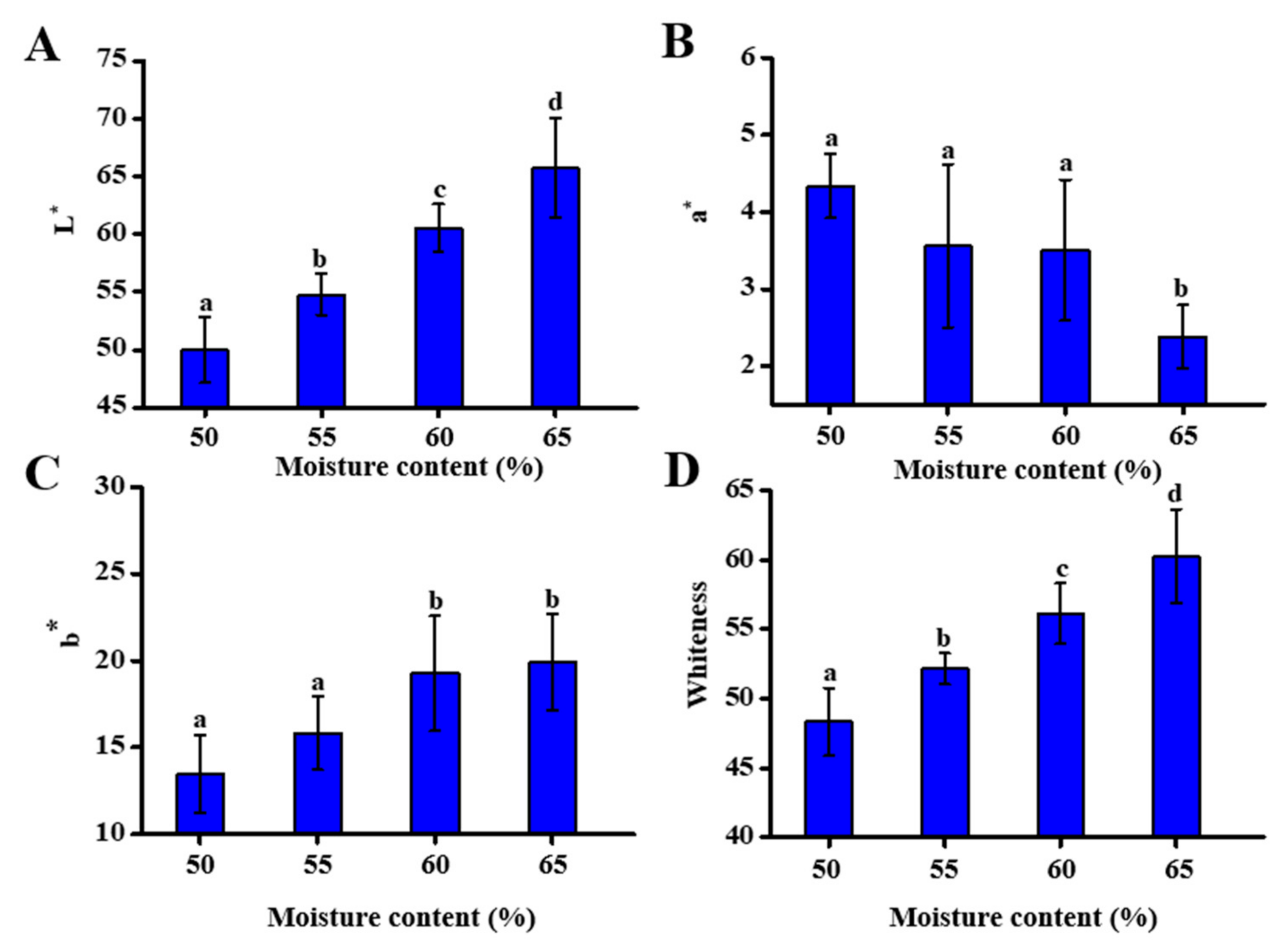
3.3. Effect of Different Moisture Content on Self-Heating Fried Fish Filet Color
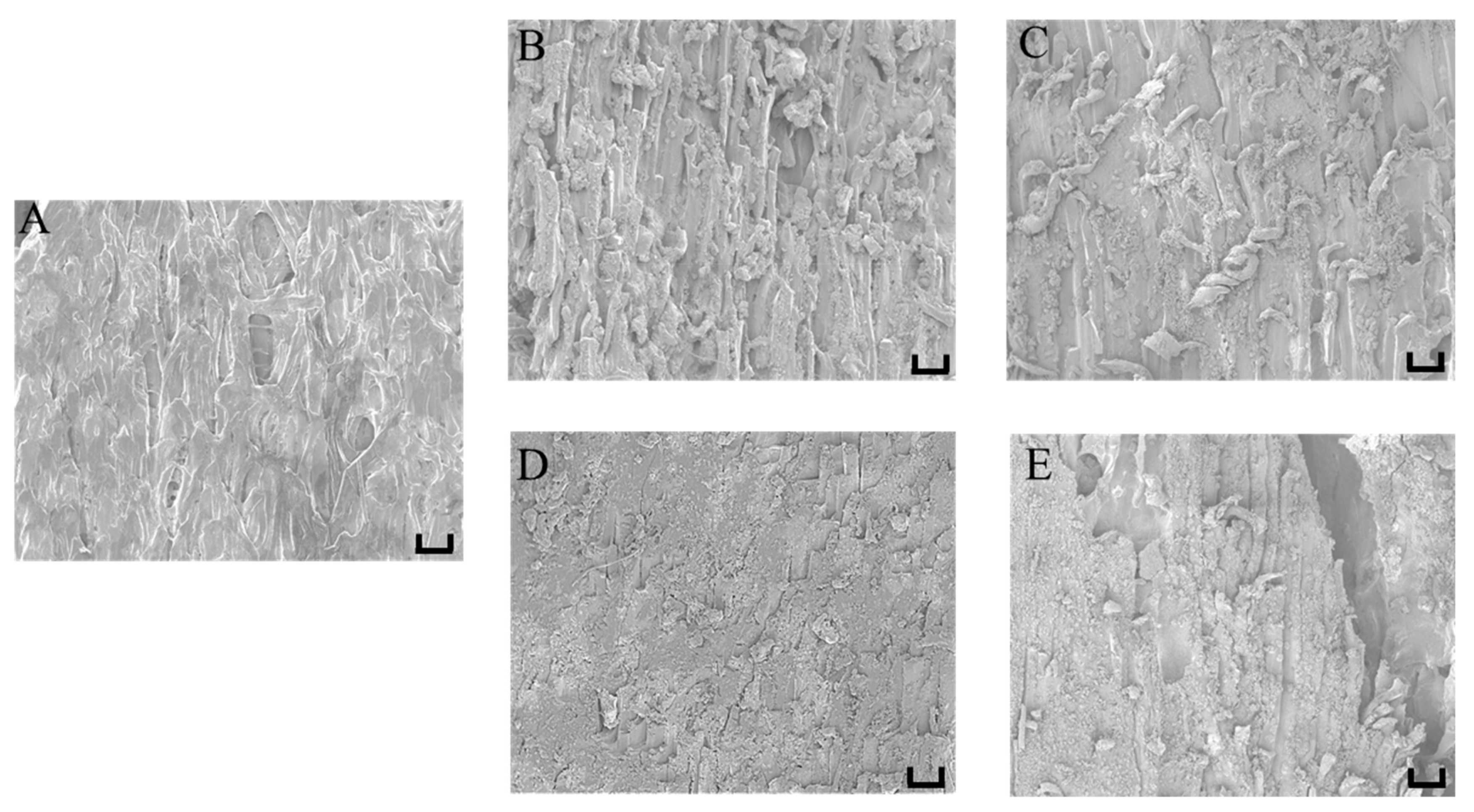
3.4. Effect of Different Moisture Content on Self-Heating Fried Fish Filet Microstructure
3.5. HS-GC–IMS Topographic Plots for Different Frying Temperatures of Self-Heating Fried Fish Filets
3.6. Differences of Volatile Compounds in Different Frying Temperatures of Self-Heating Fried Fish Filets
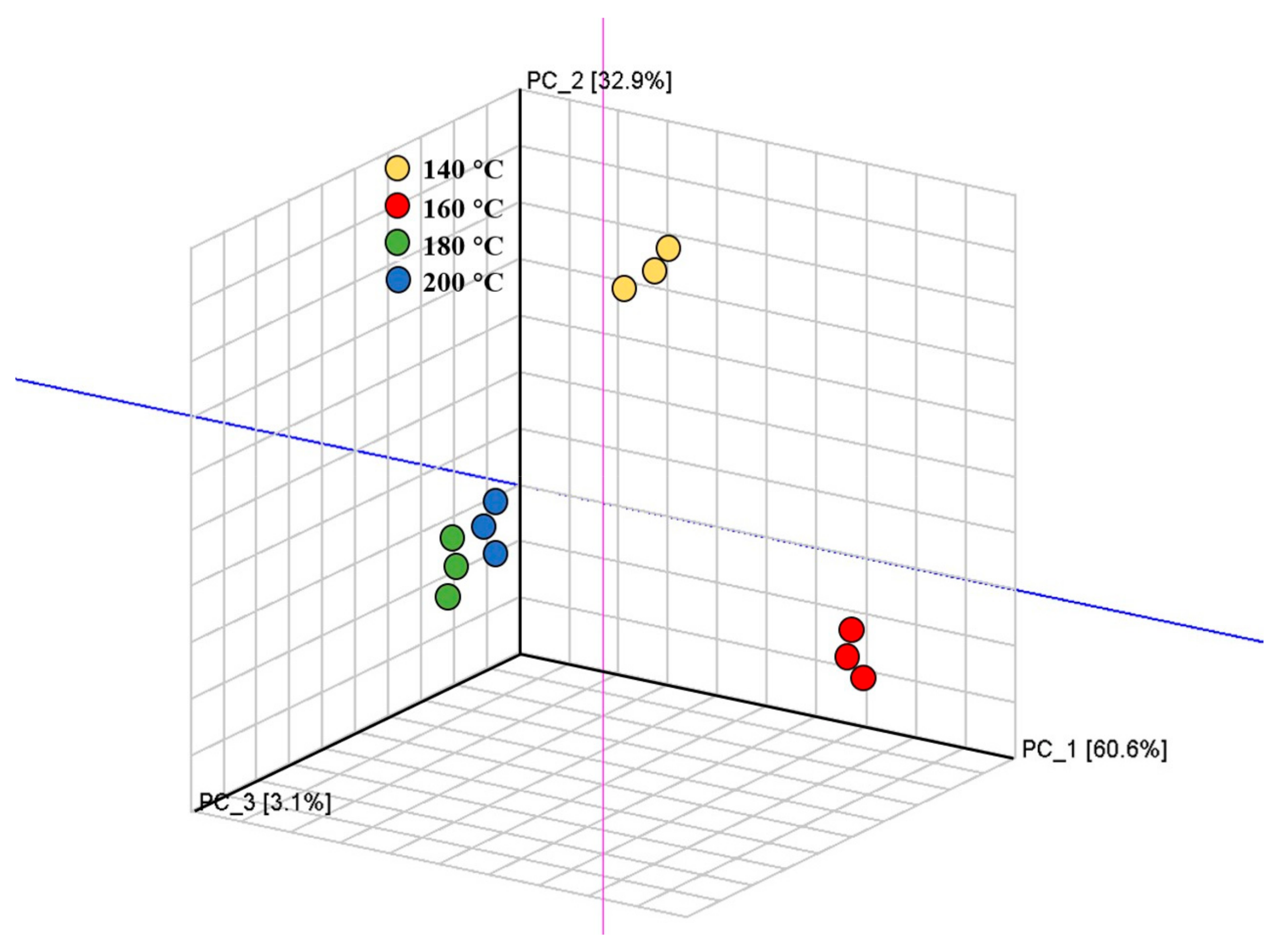
3.7. Principal Component Analysis (PCA)-Based Fingerprint Similarity Analysis
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silberbauer, A.; Schmid, M. Packaging Concepts for Ready-to-Eat Food: Recent Progress. J. Packag. Technol. Res. 2017, 1, 113–126. [Google Scholar] [CrossRef]
- Bindu, J.; Ravishankar, C.N.; Gopal, T.K.S. Shelf life evaluation of a ready-to-eat black clam (Villorita cyprinoides) product in indigenous retort pouches. J. Food Eng. 2007, 78, 995–1000. [Google Scholar] [CrossRef]
- Bou, R.; Claret, A.; Stamatakis, A.; Martnez, B.; Guerrero, L. Quality changes and shelf-life extension of ready-to-eat fish patties by adding encapsulated citric acid. J. Sci. Food Agric. 2017, 97, 5352–5360. [Google Scholar] [CrossRef] [PubMed]
- Ayadi, M.A.; Makni, I.; Attia, H. Thermal diffusivities and influence of cooking time on textural, microbiological and sensory characteristics of turkey meat prepared products. Food Bioprod. Process. 2009, 87, 327–333. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Zhou, G. Effects of ultrasound-assisted frying on the physiochemical properties and microstructure of fried meatballs. Int. J. Food Sci. Technol. 2019, 54, 2915–2926. [Google Scholar] [CrossRef]
- Zeng, P.; Zhang, Y.H.; Zhang, Y.S.; Ning, Z.X.; Chen, X.; Qin, Y.R. Effect of Cooking Methods on the Flavor and Microstructure of Salted-dried Fish. Mod. Food Sci. Technol. 2019, 35, 207–215. [Google Scholar]
- Barbut, S. Frying-Effect of coating on crust microstructure, color, and texture of lean meat portions. Meat Sci. 2013, 93, 269–274. [Google Scholar] [CrossRef]
- Yu, X.; Li, L.; Xue, J.; Wang, J.; Shen, Q. Effect of air-frying conditions on the quality attributes and lipidomic characteristics of surimi during processing. Innov. Food Sci. Emerg. Technol. 2020, 60, 102305. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Wang, Y.; Xing, L.; Zhang, W. Influences of ultrasonic-assisted frying on the flavor characteristics of fried meatballs. Innov. Food Sci. Emerg. Technol. 2020, 62, 102365. [Google Scholar] [CrossRef]
- Bordin, K.; Kunitake, M.T.; Aracava, K.K.; Trindade, C.S.F. Changes in food caused by deep fat frying—A review. Arch. Latinoam. Nutr. 2013, 63, 5–13. [Google Scholar]
- Zhang, Q.; Ding, Y.; Gu, S.; Zhu, S.; Ding, Y. Identification of changes in volatile compounds in dry-cured fish during storage using HS-GC-IMS. Food Res. Int. 2020, 137, 109339. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Q.; Yang, R.W.; Zhang, H.; Wang, S.L.; Chen, D.; Lin, S.Y. Development of a flavor fingerprint by HS-GC–IMS with PCA for volatile compounds of Tricholoma matsutake Singer. Food Chem. 2019, 290, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Li, X.R.; Wang, K.; Yang, R.E.; Dong, Y.F.; Lin, S.Y. Mechanism of aroma compounds changes from sea cucumber peptide powders (SCPPs) under different storage conditions. Food Res. Int. 2020, 128, 108757. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Chen, Y.; Ding, S.; Zhou, H.; Jiang, L.; Yi, Y.; Deng, F.; Wang, R. Changes in volatile flavor compounds of peppers during hot air drying process based on headspace-gas chromatography-ion mobility spectrometry (HS-GC-IMS). J. Sci. Food Agric. 2020, 100, 3087–3098. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, G.; Luo, Y.; Pu, Y.; Lia, G. Effect of natural spices on precursor substances and volatile flavor compounds of boiled Wuding chicken during processing. Flavour Fragr. J. 2020, 35, 570–583. [Google Scholar] [CrossRef]
- Kitada, S.; Nakajima, K.; Hamasaki, K. Population panmixia and demographic expansion of a highly piscivorous marine fish Scomberomorus niphonius. J. Fish. Biol. 2017, 91, 1435–1448. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1997. [Google Scholar]
- Dong, X.P.; Wu, Q.; Li, D.Y.; Wang, T.; Pan, J.F.; Zheng, J.J.; Fu, X.X.; Qi, L.B.; Chen, G.B. Physicochemical, micro-structural, and textural properties of different parts from farmed common carp (Cyprinus carpio). Int. J. Food Prop. 2017, 20, 946–955. [Google Scholar] [CrossRef]
- Chiou, T.K.; Tsai, C.Y.; Lan, H.L. Chemical, physical and sensory changes of small abalone meat during cooking. Fish. Sci. 2004, 70, 867–874. [Google Scholar] [CrossRef]
- Albertos, I.; Jaime, I.; Diez, A.M.; González-Arnaiz, L.; Rico, D. Carob seed peel as natural antioxidant in minced and refrigerated (4 °C) Atlantic horse mackerel (Trachurus trachurus). LWT Food Sci. Technol. 2015, 64, 650–656. [Google Scholar] [CrossRef]
- Raeisi, S.; Sharifi-Rad, M.; Quek, S.Y.; Shabanpour, B.; Sharifi-Rad, J. Evaluation of antioxidant and antimicrobial effects of shallot (Allium ascalonicum L.) fruit and ajwain (Trachyspermum ammi (L.) Sprague) seed extracts in semi-fried coated rainbow trout (Oncorhynchus mykiss) fillets for shelf-life extension. LWT Food Sci. Technol. 2016, 65, 112–121. [Google Scholar] [CrossRef]
- Etemadian, Y.; Shabanpour, B.; Mahoonak, A.R.S.; Shabani, A.; Alami, M. Cryoprotective effects of polyphosphates on Rutilus frisii kutum fillets during ice storage. Food Chem. 2011, 129, 1544–1551. [Google Scholar] [CrossRef]
- Singh, B.; Rachna, H.S.Z.; Sharma, S. Response surface analysis and process optimization of twin screw extrusion cooking of potato-based snacks. J. Food Process. Preserv. 2015, 39, 270–281. [Google Scholar] [CrossRef]
- Murphy, R.Y.; Marks, B.P. Effect of meat temperature on proteins, texture, and cook loss for ground chicken breast patties. Poult. Sci. 2000, 79, 99–104. [Google Scholar] [CrossRef]
- Garayo, J.; Moreira, R. Vacuum frying of potato chips. J. Food Eng. 2002, 55, 181–191. [Google Scholar] [CrossRef] [Green Version]
- Patras, A.; Tiwari, B.K.; Brunton, N.P.; Butler, F. Modelling the effect of different sterilisation treatments on antioxidant activity and colour of carrot slices during storage. Food Chem. 2009, 114, 484–491. [Google Scholar] [CrossRef]
- Herrero, A.M.; Carmona, P.; García, M.L.; Solas, M.T.; Careche, M. Ultrastructural changes and structure and mobility of myowater in frozen-stored hake (Merluccius merluccius L.) muscle: Relationship with functionality and texture. J. Agric. Food Chem. 2005, 53, 2558–2566. [Google Scholar] [CrossRef]
- Angel-Rendon, S.V.; Filomena-Ambrosio, A.; Hernandez-Carrion, M.; Llorca, E.; Hernand, I.; Quiles, A.; Sotelo-Diaz, I. Pork meat prepared by different cooking methods—A microstructural, sensorial and physicochemical approach. Meat Sci. 2020, 163, 108081–108089. [Google Scholar] [CrossRef]
- Yarmand, M.S.; Nikmaram, P.; Djomeh, Z.E.; Homayouni, A. Microstructural and mechanical properties of camel longissimus dorsi muscle during roasting, braising and microwave heating. Meat Sci. 2013, 95, 419–424. [Google Scholar] [CrossRef]
- Benjakul, S.; Visessanguan, W.; Kijroongrojana, K.; Sriket, P. Effect of heating on physical properties and microstructure of black tiger shrimp (Penaeus monodon) and white shrimp (Penaeus vannamei) meats. Int. J. Food Sci. Technol. 2008, 43, 1066–1072. [Google Scholar] [CrossRef]
- Arce, L.; Gallegos, J.; Garrido-Delgado, R.; Medina, L.M.; Sielemann, S.; Wortelmann, T. Ion mobility spectrometry a versatile analytical tool for metabolomics applications in food science. Curr. Metab. 2014, 2, 264–271. [Google Scholar] [CrossRef]
- Rodríguez-Maecker, R.; Vyhmeister, E.; Meisen, S.; Martinez, A.R.; Kuklya, A.; Telgheder, U. Identification of terpenes and essential oils by means of static headspace gas chromatography-ion mobility spectrometry. Anal. Bioanal. Chem. 2017, 409, 6595–6603. [Google Scholar] [CrossRef] [PubMed]
- Lantsuzskaya, E.V.; Krisilov, A.V.; Levina, A.M. Structure of aldehyde cluster ions in the gas phase, according to data from ion mobility spectrometry and ab initio calculations. Russ. J. Phys. Chem. A 2015, 89, 1590–1594. [Google Scholar] [CrossRef]
- Lund, M.N.; Ray, C.A. Control of Maillard Reactions in Foods: Strategies and Chemical Mechanisms. J. Agric. Food Chem. 2007, 65, 4537–4552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunton, N.P.; Cronin, D.A.; Monahan, F.J.; Durcan, R. A comparison of solid-phase microextraction (SPME) fibres for measurement of hexanal and pentanal in cooked turkey. Food Chem. 2000, 68, 339–345. [Google Scholar] [CrossRef]
- Alex, O.; Matthew, M. Purification of Alaskan walleye pollock (Gadus chalcogrammus) and New Zealand hoki (Macruronus novaezelandiae) liver oil using shortpath distillation. Nutrients 2014, 6, 2059–2076. [Google Scholar]
- Meinert, L.; Andersen, L.T.; Bredie, W.L.P.; Bjergegaard, C.; Aaslyng, M.D. Chemical and sensory characterisation of pan-fried pork flavour: Interactions between raw meat quality, ageing and frying temperature. Meat Sci. 2007, 75, 229–242. [Google Scholar] [CrossRef]
- Chung, H.Y.; Yeung, C.W.; Kim, J.S.; Chen, F. Static headspace analysis-olfactometry (SHA-O) of odor impact components in salted-dried white herring (Ilisha elongata). Food Chem. 2007, 104, 842–851. [Google Scholar] [CrossRef]
- Moretti, V.M.; Vasconi, M.; Caprino, F.; Bellagamba, F. Fatty Acid Profiles and Volatile Compounds Formation during Processing and Ripening of a Traditional Salted Dry Fish Product. J. Food Process. Preserv. 2017, 41, e13133. [Google Scholar] [CrossRef]
- Peinado, I.; Miles, W.; Koutsidis, G. Odour characteristics of seafood flavour formulations produced with fish by-products incorporating EPA, DHA and fish oil. Food Chem. 2016, 212, 612–619. [Google Scholar] [CrossRef]
- Guan, W.; Ren, X.; Li, Y.; Mao, L. The beneficial effects of grape seed, sage and oregano extracts on the quality and volatile flavor component of hairtail fish balls during cold storage at 4 °C. LWT 2019, 101, 25–31. [Google Scholar] [CrossRef]
- Fan, M.; Xiao, Q.; Xie, J.; Cheng, J.; Sun, B.; Du, W.; Wang, Y.; Wang, T. Aroma Compounds in Chicken Broths of Beijing Youji and Commercial Broilers. J. Agric. Food Chem. 2018, 66, 10242–10251. [Google Scholar] [CrossRef] [PubMed]
- Magagna, F.; Valverde-Som, L.; Ruíz-Samblás, C.; Cuadros-Rodríguez, L.; Cordero, C. Combined untargeted and targeted fingerprinting with comprehensive two-dimensional chromatography for volatiles and ripening indicators in olive oil. Anal. Chim. Acta 2016, 936, 245–258. [Google Scholar] [CrossRef]
- Azilawati, M.I.; Hashim, D.M.; Jamilah, B.; Amin, I. RP-HPLC method using 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate incorporated with normalization technique in principal component analysis to differentiate the bovine, porcine and fish gelatins. Food Chem. 2015, 172, 368–376. [Google Scholar] [CrossRef] [PubMed]
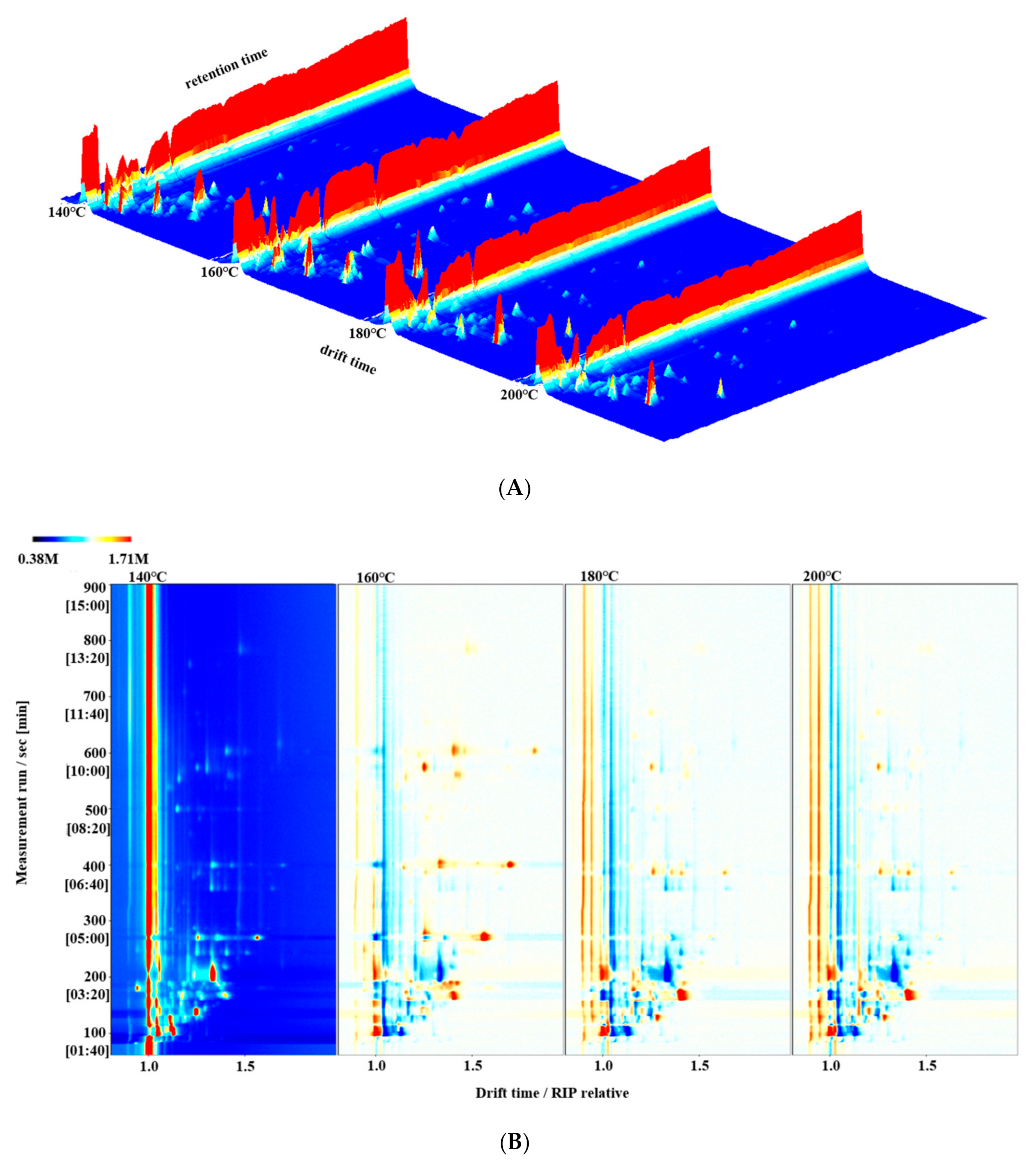

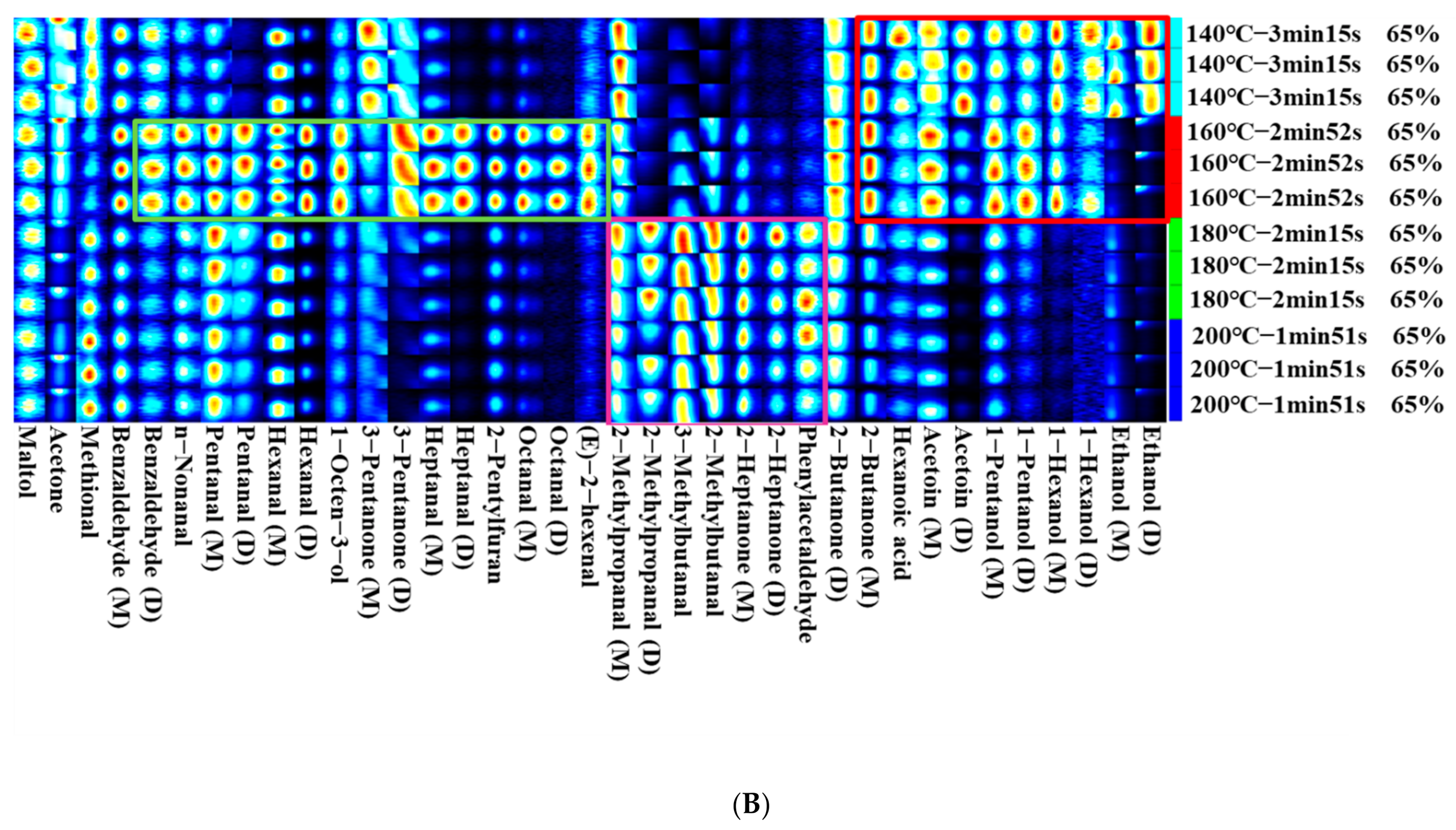
| Count | Compound | CAS# | Formula | MW | RI 1 | Rt 2 | Dt 3 |
|---|---|---|---|---|---|---|---|
| 1 | n-Nonanal | 124-19-6 | C9H18O | 142.2 | 1100.0 | 785.047 | 1.47752 |
| 2 | Octanal (Monomer) | 124-13-0 | C8H16O | 128.2 | 1010.7 | 604.389 | 1.40582 |
| 3 | Octanal (Dimer) | 124-13-0 | C8H16O | 128.2 | 1010.4 | 603.865 | 1.82482 |
| 4 | 2-Pentylfuran | 3777-69-3 | C9H14O | 138.2 | 994.0 | 574.541 | 1.25607 |
| 5 | Benzaldehyde (Monomer) | 100-52-7 | C7H6O | 106.1 | 956.9 | 500.183 | 1.14933 |
| 6 | Benzaldehyde (Dimer) | 100-52-7 | C7H6O | 106.1 | 957.2 | 500.707 | 1.47114 |
| 7 | Heptanal (Monomer) | 111-71-7 | C7H14O | 114.2 | 897.3 | 400.569 | 1.33497 |
| 8 | Heptanal (Dimer) | 111-71-7 | C7H14O | 114.2 | 897.6 | 400.906 | 1.69924 |
| 9 | 2-Heptanone (Monomer) | 110-43-0 | C7H14O | 114.2 | 888.4 | 387.755 | 1.26157 |
| 10 | 2-Heptanone (Dimer) | 110-43-0 | C7H14O | 114.2 | 887.0 | 385.731 | 1.63128 |
| 11 | 1-Hexanol (Monomer) | 111-27-3 | C6H14O | 102.2 | 865.7 | 357.743 | 1.33089 |
| 12 | 1-Hexanol (Dimer) | 111-27-3 | C6H14O | 102.2 | 866.8 | 359.092 | 1.63944 |
| 13 | (E)-2-hexenal | 6728-26-3 | C6H10O | 98.1 | 845.6 | 333.127 | 1.18137 |
| 14 | Hexanal (Monomer) | 66-25-1 | C6H12O | 100.2 | 790.5 | 274.022 | 1.25582 |
| 15 | Hexanal (Dimer) | 66-25-1 | C6H12O | 100.2 | 786.8 | 270.378 | 1.56279 |
| 16 | 1-Pentanol (Monomer) | 71-41-0 | C5H12O | 88.1 | 758.2 | 243.419 | 1.25456 |
| 17 | 1-Pentanol (Dimer) | 71-41-0 | C5H12O | 88.1 | 759.8 | 244.876 | 1.51247 |
| 18 | Acetoin (Monomer) | 513-86-0 | C4H8O2 | 88.1 | 713.8 | 206.623 | 1.05579 |
| 19 | Acetoin (Dimer) | 513-86-0 | C4H8O2 | 88.1 | 708.5 | 202.615 | 1.33634 |
| 20 | 2-Methylbutanal | 96-17-3 | C5H10O | 86.1 | 657.3 | 173.106 | 1.4005 |
| 21 | 3-Methylbutanal | 590-86-3 | C5H10O | 86.1 | 633.9 | 162.541 | 1.41182 |
| 22 | 2-Butanone (Monomer) | 78-93-3 | C4H8O | 72.1 | 577.1 | 139.589 | 1.06082 |
| 23 | 2-Butanone (Dimer) | 78-93-3 | C4H8O | 72.1 | 578.1 | 139.953 | 1.25456 |
| 24 | Ethanol (Monomer) | 64-17-5 | C2H6O | 46.1 | 450.9 | 99.514 | 1.04846 |
| 25 | Ethanol (Dimer) | 64-17-5 | C2H6O | 46.1 | 453.6 | 100.242 | 1.13192 |
| 26 | Pentanal (Monomer) | 110-62-3 | C5H10O | 86.1 | 690.4 | 189.5 | 1.1863 |
| 27 | Pentanal (Dimer) | 110-62-3 | C5H10O | 86.1 | 690.4 | 189.5 | 1.42404 |
| 28 | 1-Octen-3-ol | 3391-86-4 | C8H16O | 128.2 | 984.3 | 553.994 | 1.16002 |
| 29 | Hexanoic acid | 142-62-1 | C6H12O2 | 116.2 | 993.1 | 572.601 | 1.30267 |
| 30 | 3-Pentanone (Monomer) | 96-22-0 | C5H10O | 86.1 | 690.2 | 189.344 | 1.10834 |
| 31 | 3-Pentanone (Dimer) | 96-22-0 | C5H10O | 86.1 | 688.7 | 188.305 | 1.35869 |
| 32 | Acetone | 67-64-1 | C3H6O | 58.1 | 488.2 | 109.994 | 1.1234 |
| 33 | Methional | 3268-49-3 | C4H8OS | 104.2 | 901.1 | 406.224 | 1.09037 |
| 34 | 2-Methylpropanal (Monomer) | 78-84-2 | C4H8O | 72.1 | 540.9 | 126.679 | 1.11273 |
| 35 | 2-Methylpropanal (Dimer) | 78-84-2 | C4H8O | 72.1 | 543.7 | 127.642 | 1.28368 |
| 36 | Phenylacetaldehyde | 122-78-1 | C8H8O | 120.2 | 1046.3 | 670.867 | 1.25446 |
| 37 | Maltol | 118-71-8 | C6H6O3 | 126.1 | 1088.6 | 759.285 | 1.212 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, L.; Lin, S.; Zou, B.; Zheng, X.; Zhang, S.; Tang, Y. Effect of Frying Conditions on Self-Heating Fried Spanish Mackerel Quality Attributes and Flavor Characteristics. Foods 2021, 10, 98. https://doi.org/10.3390/foods10010098
Chang L, Lin S, Zou B, Zheng X, Zhang S, Tang Y. Effect of Frying Conditions on Self-Heating Fried Spanish Mackerel Quality Attributes and Flavor Characteristics. Foods. 2021; 10(1):98. https://doi.org/10.3390/foods10010098
Chicago/Turabian StyleChang, Lili, Songyi Lin, Bowen Zou, Xiaohan Zheng, Simin Zhang, and Yue Tang. 2021. "Effect of Frying Conditions on Self-Heating Fried Spanish Mackerel Quality Attributes and Flavor Characteristics" Foods 10, no. 1: 98. https://doi.org/10.3390/foods10010098





