Ultrasonic-Assisted Extraction and Structural Characterization of Chondroitin Sulfate Derived from Jumbo Squid Cartilage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pretreatment of the Jumbo Squid Cartilage
2.3. Conventional Shaking Extraction
2.4. Ultrasonic-Assisted Extraction
2.5. Determination of Mass Transfer Coefficients
2.6. Experimental Design
2.7. Purification of ChS by Ethanol Fractionation
2.8. Analysis
2.9. SEM, FTIR and NMR Spectroscopy
3. Results
3.1. Comparison of Conventional Shaking Extraction and Ultrasonic-Assisted Extraction
3.2. Mass Transfer Coefficients during the Extraction of ChS
3.3. ChS Extraction Based on the Box-Behnken Design and RSM Model
+0.01306X3X1−0.00094X3X2−0.00076X32
3.4. Response Surface Analysis
3.5. Attaining Optimum Conditions
3.6. Purification of ChS by Ethanol Fractionation
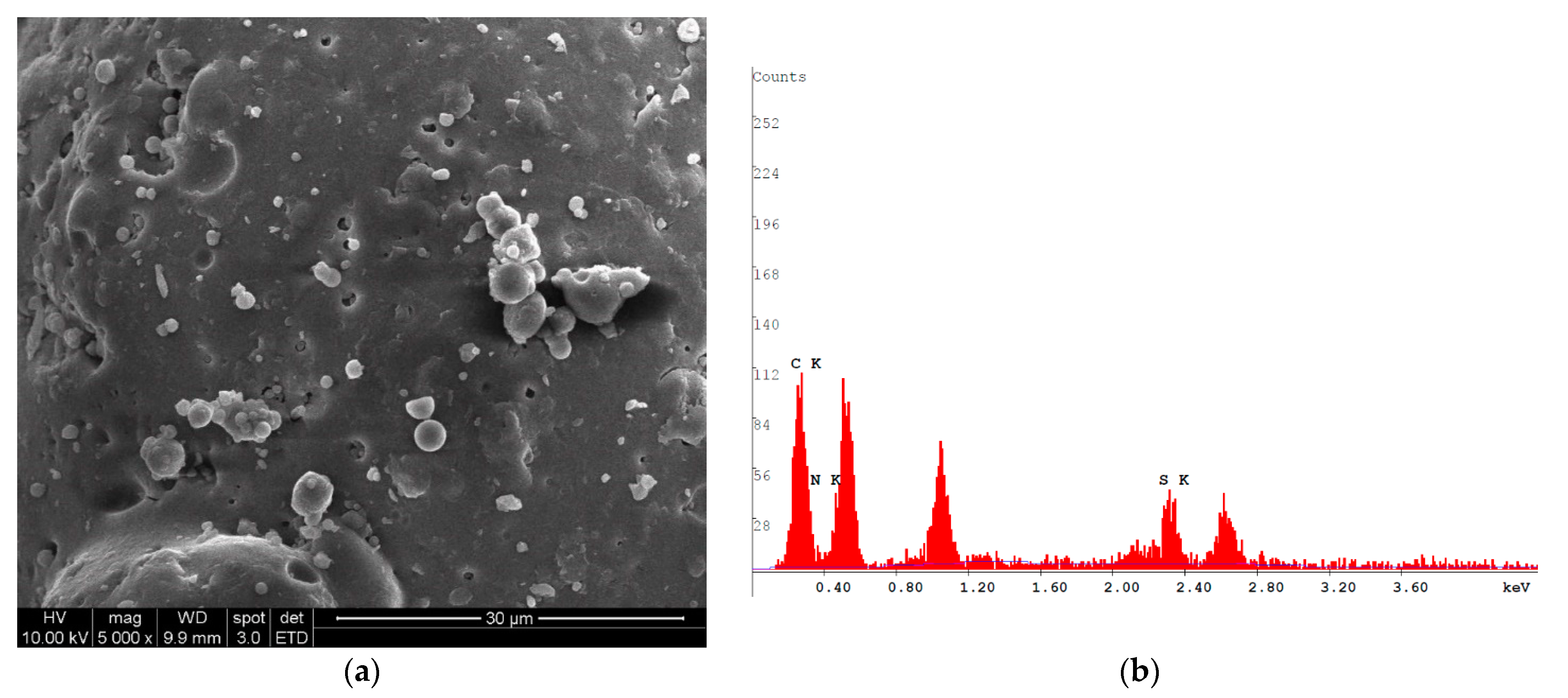
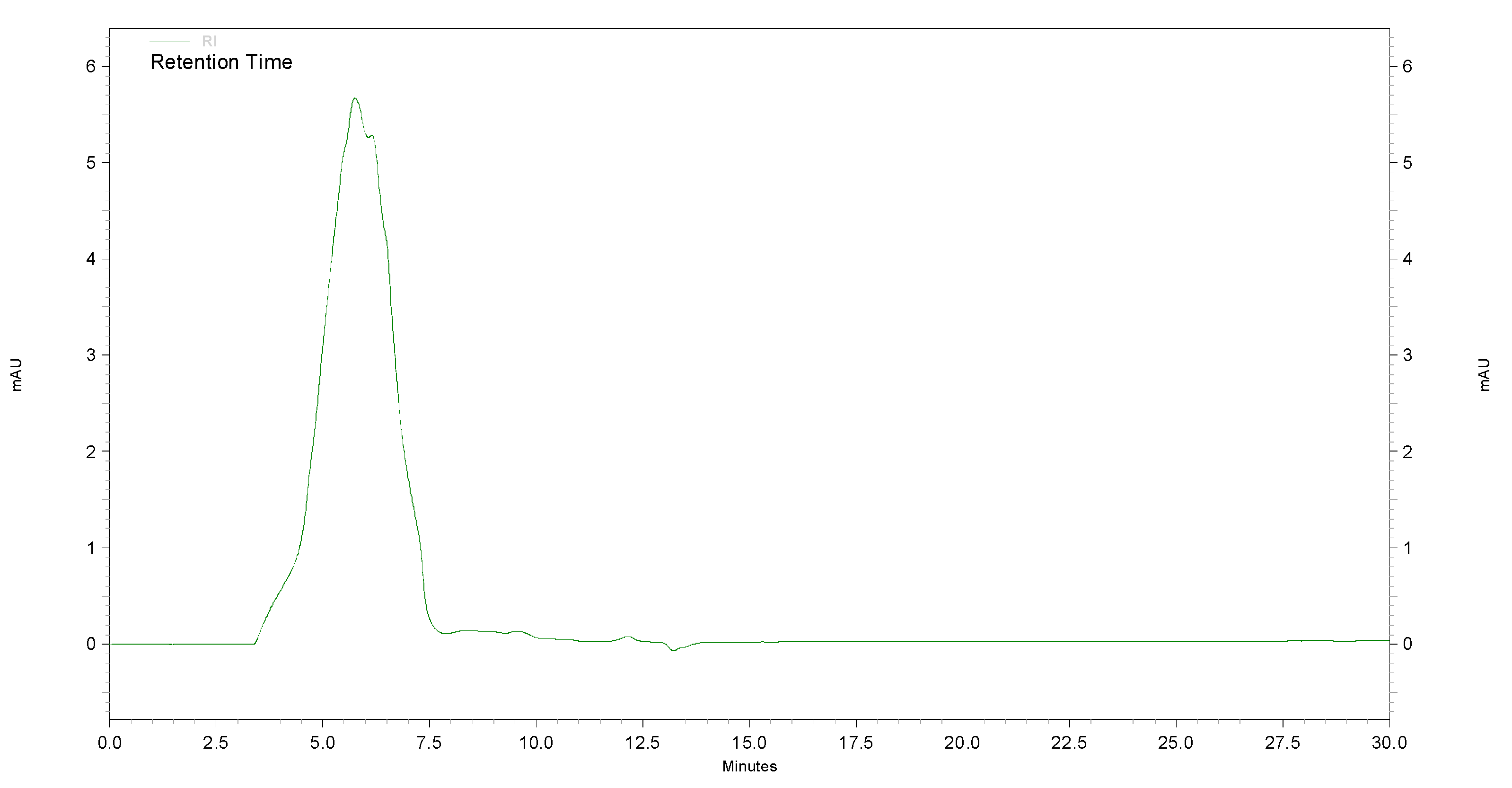
3.7. Characterization of Purified ChS
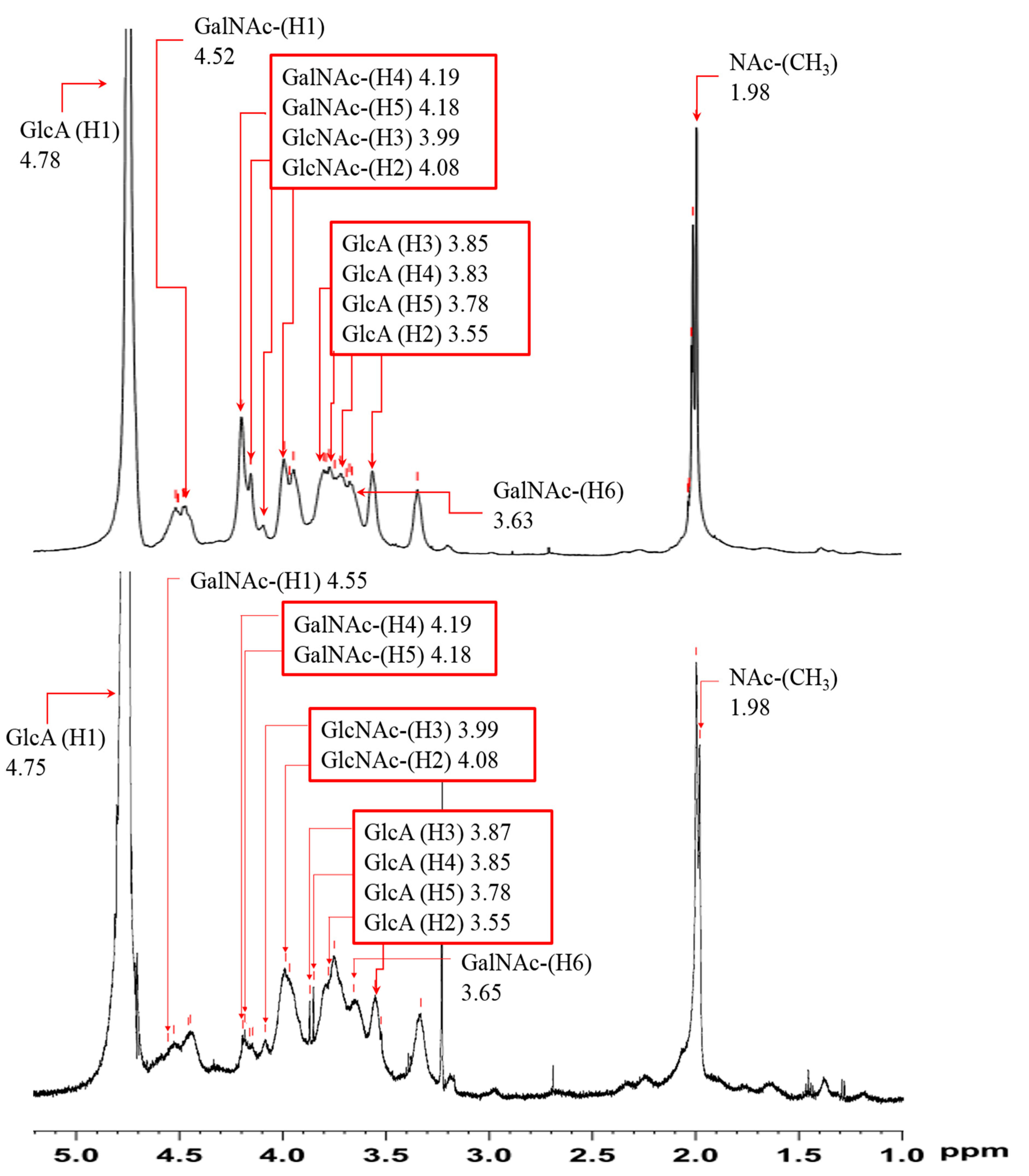
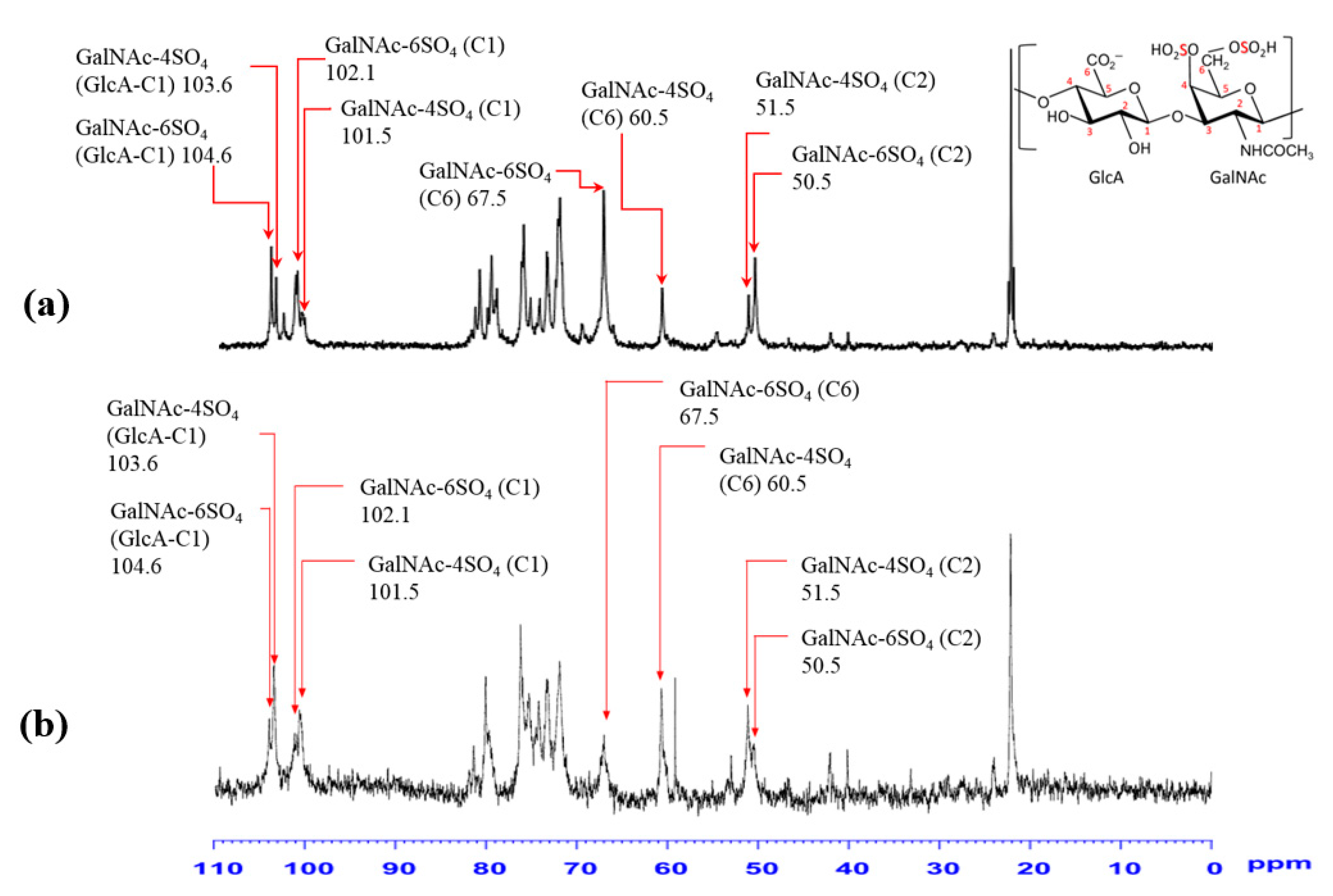
3.8. Structural Features of Purified ChS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Volpi, N. Chondroitin sulfate safety and quality. Molecules 2019, 24, 1447. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.g.; Meng, Y.c.; Li, J.r.; Chen, J.; Liu, Y.h.; Bai, X. Chondroitin sulfate: Extraction, purification, microbial and chemical synthesis. J. Chem. Technol. Biotech. 2014, 89, 1445–1465. [Google Scholar] [CrossRef]
- Maccari, F.; Ferrarini, F.; Volpi, N. Structural characterization of chondroitin sulfate from sturgeon bone. Carbohydr. Res. 2010, 345, 1575–1580. [Google Scholar] [CrossRef]
- López-Senra, E.; Casal-Beiroa, P.; López-Álvarez, M.; Serra, J.; González, P.; Valcarcel, J.; Vázquez, J.A.; Burguera, E.F.; Blanco, F.J.; Magalhães, J. Impact of prevalence ratios of chondroitin sulfate (CS)-4 and-6 isomers derived from marine sources in cell proliferation and chondrogenic differentiation processes. Mar. Drugs 2020, 18, 94. [Google Scholar] [CrossRef] [Green Version]
- Vázquez, J.A.; Rodríguez-Amado, I.; Montemayor, M.I.; Fraguas, J.; González, M.D.P.; Murado, M.A. Chondroitin sulfate, hyaluronic acid and chitin/chitosan production using marine waste sources: Characteristics, applications and eco-friendly processes: A review. Mar. Drugs 2013, 11, 747–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volpi, N. Quality of different chondroitin sulfate preparations in relation to their therapeutic activity. J. Pharm. Pharmacol. 2009, 61, 1271–1280. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Braschi, V.; Gasparri, C.; Nichetti, M.; Faliva, M.A.; Peroni, G.; Naso, M.; Iannello, G.; Spadaccini, D.; Miraglia, N. Effectiveness of non-animal chondroitin sulfate supplementation in the treatment of moderate knee osteoarthritis in a group of overweight subjects: A randomized, double-blind, placebo-controlled pilot study. Nutrients 2019, 11, 2027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martel-Pelletier, J.; Farran, A.; Montell, E.; Vergés, J.; Pelletier, J.-P. Discrepancies in composition and biological effects of different formulations of chondroitin sulfate. Molecules 2015, 20, 4277–4289. [Google Scholar] [CrossRef] [Green Version]
- Rnjak-Kovacina, J.; Tang, F.; Whitelock, J.M.; Lord, M.S. Glycosaminoglycan and proteoglycan-based biomaterials: Current trends and future perspectives. Adv. Healthc. Mater. 2018, 7, 1701042. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Pietrasik, Z.; Ozimek, L.; Betti, M. Extraction, isolation and analysis of chondroitin sulfate from broiler chicken biomass. Process Biochem. 2012, 47, 1909–1918. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Fraguas, J.; Novoa-Carvallal, R.; Reis, R.L.; Antelo, L.T.; Pérez-Martín, R.I.; Valcarcel, J. Isolation and chemical characterization of chondroitin sulfate from cartilage by-products of blackmouth catshark (Galeus melastomus). Mar. Drugs 2018, 16, 344. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, M.J.; Costa, R.R.; Mano, J.F. Marine origin polysaccharides in drug delivery systems. Mar. Drugs 2016, 14, 34. [Google Scholar] [CrossRef] [Green Version]
- Food and Agriculture Organization of the United Nations. Species Fact Sheets Dosidicus gigas (Orbigny, 1835). Available online: http://www.fao.org/fishery/species/2721/en (accessed on 3 September 2021).
- Wang, C.H.; Doan, C.T.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. Reclamation of fishery processing waste: A mini-review. Molecules 2019, 24, 2234. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.-Y.; Kuo, C.-H.; Wu, C.-H.; Ku, M.-W.; Chen, P.-W. Extraction of crude chitosans from squid (Illex argentinus) pen by a compressional puffing-pretreatment process and evaluation of their antibacterial activity. Food Chem. 2018, 254, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.L.; Tsai, Y.H. Extraction of chitosan from squid pen waste by high hydrostatic pressure: Effects on physicochemical properties and antioxidant activities of chitosan. Int. J. Biol. Macromol. 2020, 160, 677–687. [Google Scholar] [CrossRef]
- Al Khawli, F.; Pateiro, M.; Domínguez, R.; Lorenzo, J.M.; Gullón, P.; Kousoulaki, K.; Ferrer, E.; Berrada, H.; Barba, F.J. Innovative green technologies of intensification for valorization of seafood and their by-products. Mar. Drugs 2019, 17, 689. [Google Scholar] [CrossRef] [Green Version]
- Hwang, L.S.; Liang, J.H. Fractionation of urea-pretreated squid visceral oil ethyl esters. J. Am. Oil Chem. Soc. 2001, 78, 473–476. [Google Scholar] [CrossRef]
- Carrera, M.; Ezquerra-Brauer, J.M.; Aubourg, S.P. Characterization of the jumbo squid (Dosidicus gigas) skin by-product by shotgun proteomics and protein-based bioinformatics. Mar. Drugs 2020, 18, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krichen, F.; Bougatef, H.; Sayari, N.; Capitani, F.; Amor, I.B.; Koubaa, I.; Maccari, F.; Mantovani, V.; Galeotti, F.; Volpi, N. Isolation, purification and structural characterestics of chondroitin sulfate from smooth hound cartilage: In vitro anticoagulant and antiproliferative properties. Carbohydr. Polym. 2018, 197, 451–459. [Google Scholar] [CrossRef]
- Sundaresan, G.; Abraham, R.J.; Rao, V.A.; Babu, R.N.; Govind, V.; Meti, M.F. Established method of chondroitin sulphate extraction from buffalo (Bubalus bubalis) cartilages and its identification by FTIR. J. Food Sci. Technol. 2018, 55, 3439–3445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gargiulo, V.; Lanzetta, R.; Parrilli, M.; De Castro, C. Structural analysis of chondroitin sulfate from Scyliorhinus canicula: A useful source of this polysaccharide. Glycobiology 2009, 19, 1485–1491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garnjanagoonchorn, W.; Wongekalak, L.; Engkagul, A. Determination of chondroitin sulfate from different sources of cartilage. Chem. Eng. Process. Process. Intensif. 2007, 46, 465–471. [Google Scholar] [CrossRef]
- He, G.; Yin, Y.; Yan, X.; Yu, Q. Optimisation extraction of chondroitin sulfate from fish bone by high intensity pulsed electric fields. Food Chem. 2014, 164, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-H.; Chen, B.-Y.; Liu, Y.-C.; Chen, J.-H.; Shieh, C.-J. Production of resveratrol by piceid deglycosylation using cellulase. Catalysts 2016, 6, 32. [Google Scholar] [CrossRef]
- Zou, T.-B.; Jia, Q.; Li, H.-W.; Wang, C.-X.; Wu, H.-F. Response surface methodology for ultrasound-assisted extraction of astaxanthin from Haematococcus pluvialis. Mar. Drugs 2013, 11, 1644–1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.-M.; Wu, P.-Y.; Chen, J.-H.; Kuo, C.-H.; Shieh, C.-J. Developing a high-temperature solvent-free system for efficient biocatalysis of octyl ferulate. Catalysts 2018, 8, 338. [Google Scholar] [CrossRef] [Green Version]
- Kuo, C.-H.; Chen, B.-Y.; Liu, Y.-C.; Chang, C.-M.J.; Deng, T.-S.; Chen, J.-H.; Shieh, C.-J. Optimized ultrasound-assisted extraction of phenolic compounds from Polygonum cuspidatum. Molecules 2014, 19, 67–77. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.-Y.; Kuo, C.-H.; Liu, Y.-C.; Ye, L.-Y.; Chen, J.-H.; Shieh, C.-J. Ultrasonic-assisted extraction of the botanical dietary supplement resveratrol and other constituents of Polygonum cuspidatum. J. Nat. Prod. 2012, 75, 1810–1813. [Google Scholar] [CrossRef]
- Giacometti, J.; Žauhar, G.; Žuvić, M. Optimization of ultrasonic-assisted extraction of major phenolic compounds from olive leaves (Olea europaea L.) using response surface methodology. Foods 2018, 7, 149. [Google Scholar] [CrossRef] [Green Version]
- Montero-Calderon, A.; Cortes, C.; Zulueta, A.; Frigola, A.; Esteve, M.J. Green solvents and ultrasound-assisted extraction of bioactive orange (Citrus sinensis) peel compounds. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef]
- Anticona, M.; Blesa, J.; Lopez-Malo, D.; Frigola, A.; Esteve, M.J. Effects of ultrasound-assisted extraction on physicochemical properties, bioactive compounds, and antioxidant capacity for the valorization of hybrid mandarin peels. Food Biosci. 2021, 42, 101185. [Google Scholar] [CrossRef]
- Kuo, C.H.; Liao, H.Z.; Wang, Y.H.; Wang, H.M.D.; Shieh, C.J.; Tseng, C.Y. Highly efficient extraction of EPA/DHA-enriched oil from cobia liver using homogenization plus sonication. Eur. J. Lipid Sci. Technol. 2017, 119, 1600466. [Google Scholar] [CrossRef]
- Gulzar, S.; Benjakul, S. Effect of pre-treatments on yield and properties of lipid extracted from cephalothorax of Pacific white shrimp (Litopenaeus vannamei) by ultrasonic assisted process. LWT - Food Science and Technology. 2019, 100, 106–113. [Google Scholar] [CrossRef]
- Association of Official Agricultural Chemists. Official Methods of Analysis of AOAC International; AOAC Int.: Gaithersburg, MD, USA, 2019. [Google Scholar]
- Farndale, R.W.; Buttle, D.J.; Barrett, A.J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim. Biophys. Acta Gen. Subj. 1986, 883, 173–177. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Qiu, F.-P.; Zhang, L.; Yu, J. Determination of the content of sulfate group in pilose antler polysaccarides by barium sulfate turbidity. J. Changchun Univ. Technol. 2005, 4, 268–270. [Google Scholar]
- Fu, Y.; Lin, S.; Lu, M.; Wei, S.-Y.; Zhou, J.; Zhao, L.; Zhang, Q.; Lin, D.-R.; Liu, Y.-T.; Chen, H. Quantitative evaluation of ultrasound-assisted extraction of 1, 3-β-glucans from Dictyophora indusiata using an improved fluorometric assay. Polymers 2019, 11, 864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jalili Safaryan, M.; Ganjloo, A.; Bimakr, M.; Zarringhalami, S. Optimization of ultrasound-assisted extraction, preliminary characterization and in vitro antioxidant activity of polysaccharides from green pea pods. Foods 2016, 5, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Essa, H.L.; Guirguis, H.A.; El-Sayed, M.M.; Rifaat, D.; Abdelfattah, M.S. Ultrasonically-extracted marine polysaccharides as potential green antioxidant alternatives. Proceedings 2020, 67, 23. [Google Scholar] [CrossRef]
- Nie, X.-R.; Fu, Y.; Wu, D.-T.; Huang, T.-T.; Jiang, Q.; Zhao, L.; Zhang, Q.; Lin, D.-R.; Chen, H.; Qin, W. Ultrasonic-assisted extraction, structural characterization, chain conformation, and biological activities of a pectic-polysaccharide from okra (Abelmoschus esculentus). Molecules 2020, 25, 1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Cheng, L.; Liu, F.; Li, T.; Yu, Z.; Xu, Y.; Yang, Y. Optimization of ultrasound-assisted extraction and structural characterization of the polysaccharide from pumpkin (Cucurbita moschata) seeds. Molecules 2018, 23, 1207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, F.-J.; Qian, L.-S.; Sun, W.-J.; Zhang, J.-S.; Yang, Y.; Li, N.; Zhuang, H.-N.; Wu, D. Ultrasound-assisted extraction of polysaccharides from Volvariella volvacea: Process optimization and structural characterization. Molecules 2018, 23, 1706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo, Y.-J.; Kim, J.-H. Effective diffusivity and mass transfer coefficient during the extraction of paclitaxel from Taxus chinensis using methanol. Biotechnol. Bioprocess. Eng. 2019, 24, 818–823. [Google Scholar] [CrossRef]
- Gradov, O.M.; Zakhodyaeva, Y.A.; Zinov’eva, I.V.; Voshkin, A.A. Ultrasonic intensification of mass transfer in organic acid extraction. Processes 2021, 9, 15. [Google Scholar] [CrossRef]
- Al-Dhabi, N.A.; Ponmurugan, K.; Jeganathan, P.M. Development and validation of ultrasound-assisted solid-liquid extraction of phenolic compounds from waste spent coffee grounds. Ultrasonics Sonochem. 2017, 34, 206–213. [Google Scholar] [CrossRef]
- Zhao, T.; Zhou, Y.; Mao, G.; Zou, Y.; Zhao, J.; Bai, S.; Yang, L.; Wu, X. Extraction, purification and characterisation of chondroitin sulfate in Chinese sturgeon cartilage. J. Sci. Food Agric. 2013, 93, 1633–1640. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Blanco, M.; Fraguas, J.; Pastrana, L.; Pérez-Martín, R. Optimisation of the extraction and purification of chondroitin sulphate from head by-products of Prionace glauca by environmental friendly processes. Food Chem. 2016, 198, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Vázquez, J.A.; Fraguas, J.; Novoa-Carballal, R.; Reis, R.L.; Pérez-Martín, R.I.; Valcarcel, J. Optimal isolation and characterisation of chondroitin sulfate from rabbit fish (Chimaera monstrosa). Carbohydr. Polym. 2019, 210, 302–313. [Google Scholar] [CrossRef] [Green Version]
- Ben Mansour, M.; Balti, R.; Ollivier, V.; Ben Jannet, H.; Chaubet, F.; Maaroufi, R.M. Characterization and anticoagulant activity of a fucosylated chondroitin sulfate with unusually procoagulant effect from sea cucumber. Carbohydr. Polym. 2017, 174, 760–771. [Google Scholar] [CrossRef]
- Li, S.; Ma, F.; Pang, X.; Tang, B.; Lin, L. Synthesis of chondroitin sulfate magnesium for osteoarthritis treatment. Carbohydr. Polym. 2019, 212, 387–394. [Google Scholar] [CrossRef]
- Zou, Z.; Wei, M.; Fang, J.; Dai, W.; Sun, T.; Liu, Q.; Gong, G.; Liu, Y.; Song, S.; Ma, F. Preparation of chondroitin sulfates with different molecular weights from bovine nasal cartilage and their antioxidant activities. Int. J. of Biol. Macromol. 2020, 152, 1047–1055. [Google Scholar] [CrossRef]
- Novoa-Carballal, R.; Pérez-Martín, R.; Blanco, M.; Sotelo, C.G.; Fassini, D.; Nunes, C.; Coimbra, M.A.; Silva, T.H.; Reis, R.L.; Vázquez, J.A. By-products of Scyliorhinus canicula, Prionace glauca and Raja clavata: A valuable source of predominantly 6S sulfated chondroitin sulfate. Carbohydr. Polym. 2017, 157, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Rani, A.; Baruah, R.; Goyal, A. Physicochemical, antioxidant and biocompatible properties of chondroitin sulphate isolated from chicken keel bone for potential biomedical applications. Carbohydr. Polym. 2017, 159, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Mucci, A.; Schenetti, L.; Volpi, N. H-1 and C-13 nuclear magnetic resonance identification and characterization of components of chondroitin sulfates of various origin. Carbohydr. Polym. 2000, 41, 37–45. [Google Scholar] [CrossRef]
- Kinoshita-Toyoda, A.; Yamada, S.; Haslam, S.M.; Khoo, K.H.; Sugiura, M.; Morris, H.R.; Dell, A.; Sugahara, K. Structural determination of five novel tetrasaccharides containing 3-O-sulfated D-glucuronic acid and two rare oligosaccharides containing a beta-D-glucose branch isolated from squid cartilage chondroitin sulfate E. Biochemisrty 2004, 43, 11063–11074. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Wang, Q.; Jiao, R.; Xu, Y.; Han, N.; Wang, W.; Zhu, C.; Li, F. A novel chondroitin sulfate E from Dosidicus gigas cartilage and its antitumor metastatic activity. Carbohydr. Polym. 2021, 262, 117971. [Google Scholar] [CrossRef] [PubMed]






| Parameters | Symbol | Coded Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| NaOH (%) | X1 | 2 | 4 | 6 |
| Temp (°C) | X2 | 30 | 40 | 50 |
| Time (min) | X3 | 20 | 40 | 60 |
| Group | X1 NaOH (%) | X2 Temp (°C) | X3 Time (min) | Response a ChS (mg mL−1) |
|---|---|---|---|---|
| 1 | 2 (−1) b | 40 (0) | 20 (−1) | 11.2 ± 0.3 |
| 2 | 2 (−1) | 30 (−1) | 40 (0) | 10.4 ± 1.3 |
| 3 | 2 (−1) | 50 (1) | 40 (0) | 12.5 ± 0.7 |
| 4 | 2 (−1) | 40 (0) | 60 (1) | 11.3 ± 0.8 |
| 5 | 4 (0) | 30 (−1) | 20 (−1) | 10.2 ± 0.6 |
| 6 | 4 (0) | 50 (1) | 20 (−1) | 12.3 ± 0.1 |
| 7 | 4 (0) | 40 (0) | 40 (0) | 12.2 ± 0.6 |
| 8 | 4 (0) | 40 (0) | 40 (0) | 12.1 ± 0.9 |
| 9 | 4 (0) | 40 (0) | 40 (0) | 12.1 ± 0.7 |
| 10 | 4 (0) | 30 (−1) | 60 (1) | 11.7 ± 0.3 |
| 11 | 4 (0) | 50 (1) | 60 (1) | 13.1 ± 0.4 |
| 12 | 6 (1) | 40 (0) | 20 (−1) | 10.5 ± 0.5 |
| 13 | 6 (1) | 30 (−1) | 40 (0) | 11.5 ± 0.9 |
| 14 | 6 (1) | 50 (1) | 40 (0) | 12.6 ± 0.9 |
| 15 | 6 (1) | 40 (0) | 60 (1) | 12.7 ± 0.5 |
| Coded Radius | NaOH (%) | Temp (°C) | Time (min) | Estimated (mg mL−1) | Actual (mg mL−1) |
|---|---|---|---|---|---|
| 0 | 4.00 | 40 | 40 | 12.1 | 11.8 ± 0.6 |
| 0.6 | 4.20 | 45 | 45 | 12.7 | 12.7 ± 0.9 |
| 1.2 | 4.15 | 52 | 46 | 13.2 | 13.1 ± 0.4 |
| 1.8 | 3.80 | 58 | 43 | 13.7 | 13.1 ± 0.3 |
| 2.4 | 3.36 | 64 | 39 | 14.2 | 13.0 ± 0.4 |
| 3.0 | 2.90 | 69 | 35 | 14.8 | 13.0 ± 0.2 |
| Ethanol (%) | Yield (%) | Purity (%) | Soluble protein (%) | Uronic Acid (mg g−1) | Sulfate (mg g−1) |
|---|---|---|---|---|---|
| 80 | 37.8 ± 0.3 e* | 40.7 ± 1.8 a | 12.8 ± 0.1 bc | 134.5 ± 10.3 a | 49.3 ± 3.9 a |
| 75 | 31.4 ± 0.1 d | 64.6 ± 0.0 b | 12.9 ± 0.7 bc | 170.1 ± 13.4 b | 60.9 ± 3.3 b |
| 70 | 28.1 ± 0.5 c | 68.0 ± 1.9 b,c | 14.4 ± 0.9 c | 204.4 ± 3.2 c | 73.1 ± 1.0 c |
| 60 | 25.6 ± 0.1 b | 70.8 ± 4.9 c | 10.4 ± 0.1 b | 216.0 ± 5.3 cd | 75.0 ± 0.3 c |
| 50 | 23.7 ± 0.0 a | 82.3 ± 2.7 d | 5.8 ± 0.2 a | 241.7 ± 9.0 d | 80.1 ± 3.3 c |
| Residue | Proton | Current 1H Chemical Shift (ppm) |
|---|---|---|
| GlcA | H-1 | 4.75 |
| H-2 | 3.55 | |
| H-3 | 3.87 | |
| H-4 | 3.85 | |
| H-5 | 3.78 | |
| GalNAc | H-1 | 4.55 |
| H-2 | 4.08 | |
| H-3 | 3.99 | |
| H-4 | 4.19 | |
| H-5 | 4.18 | |
| H-6 | 3.65 | |
| NAc (CH3) | 1.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, K.-R.; Tsai, M.-F.; Shieh, C.-J.; Arakawa, O.; Dong, C.-D.; Huang, C.-Y.; Kuo, C.-H. Ultrasonic-Assisted Extraction and Structural Characterization of Chondroitin Sulfate Derived from Jumbo Squid Cartilage. Foods 2021, 10, 2363. https://doi.org/10.3390/foods10102363
Yang K-R, Tsai M-F, Shieh C-J, Arakawa O, Dong C-D, Huang C-Y, Kuo C-H. Ultrasonic-Assisted Extraction and Structural Characterization of Chondroitin Sulfate Derived from Jumbo Squid Cartilage. Foods. 2021; 10(10):2363. https://doi.org/10.3390/foods10102363
Chicago/Turabian StyleYang, Kai-Ruei, Ming-Fong Tsai, Chwen-Jen Shieh, Osamu Arakawa, Cheng-Di Dong, Chun-Yung Huang, and Chia-Hung Kuo. 2021. "Ultrasonic-Assisted Extraction and Structural Characterization of Chondroitin Sulfate Derived from Jumbo Squid Cartilage" Foods 10, no. 10: 2363. https://doi.org/10.3390/foods10102363
APA StyleYang, K.-R., Tsai, M.-F., Shieh, C.-J., Arakawa, O., Dong, C.-D., Huang, C.-Y., & Kuo, C.-H. (2021). Ultrasonic-Assisted Extraction and Structural Characterization of Chondroitin Sulfate Derived from Jumbo Squid Cartilage. Foods, 10(10), 2363. https://doi.org/10.3390/foods10102363