Global Proteomic Analysis of Listeria monocytogenes’ Response to Linalool
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Linalool
2.3. Treatment of LM by Linalool
2.4. iTRAQ-Based Quantitative Proteomics Analysis
2.4.1. Protein Extraction, Digestion, and Labeling with iTRAQ Reagents
2.4.2. Chromatographic Separation and LC-MS/MS Analysis
2.4.3. Proteomic Analysis
2.5. Flow Cytometry Analysis
2.5.1. Membrane Permeability
2.5.2. Membrane Potential
2.5.3. Efflux Activity
2.5.4. Respiratory Activity
2.6. Statistical Analysis
3. Results
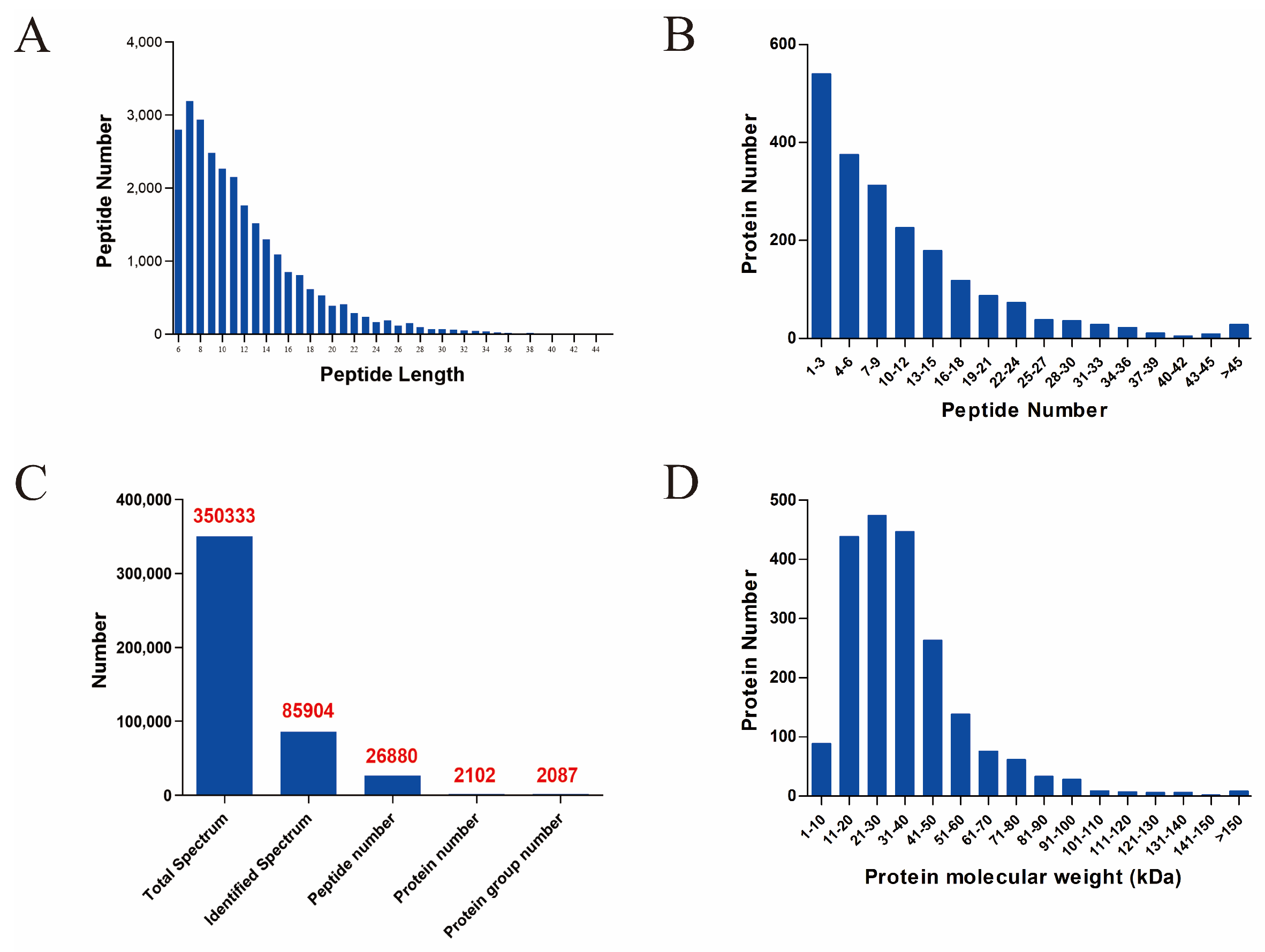
3.1. Quality Assessment of Proteomics Sequencing
3.2. Functional Annotation and Analysis of Proteins
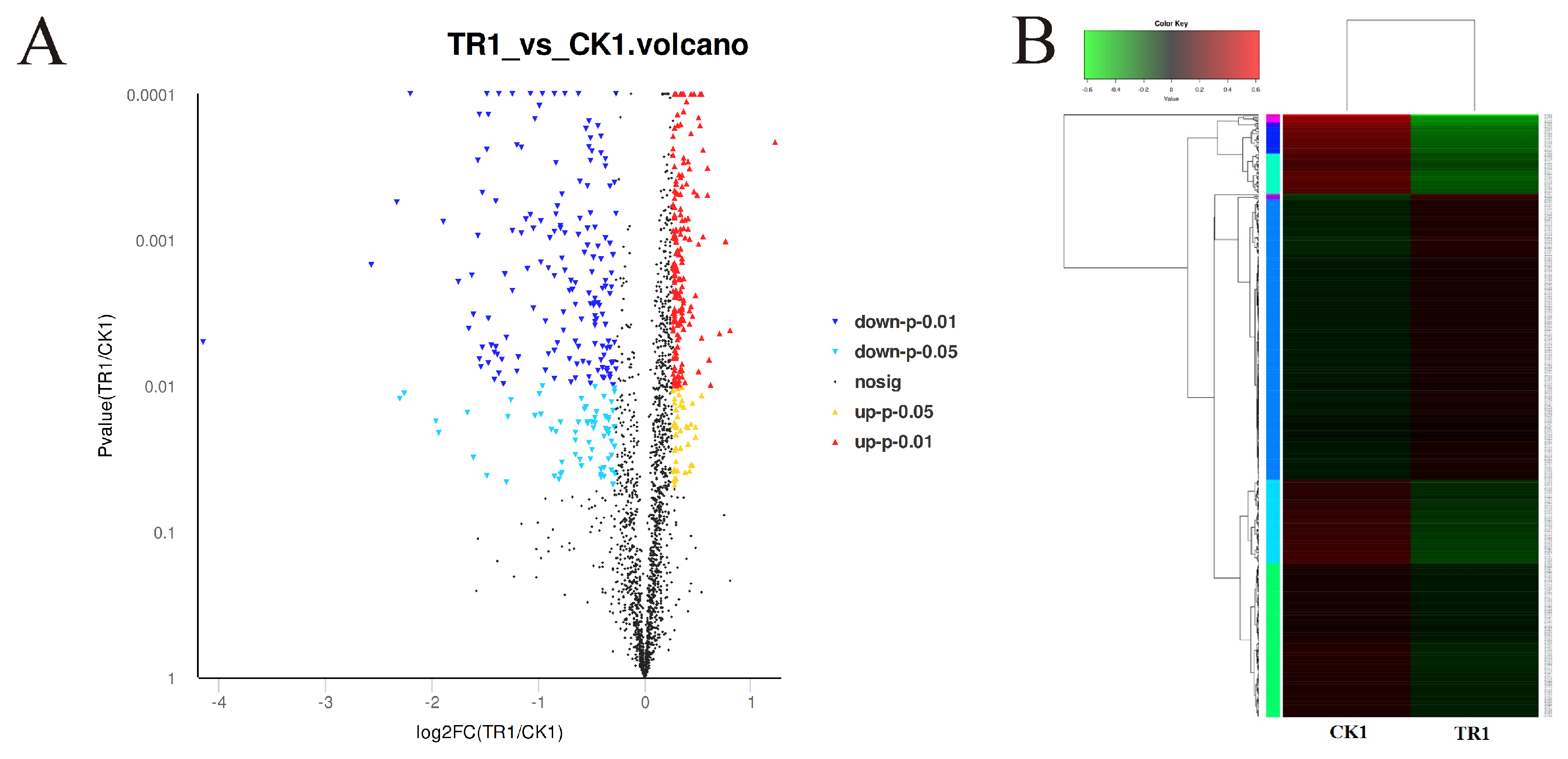
3.3. Analysis of DEPs
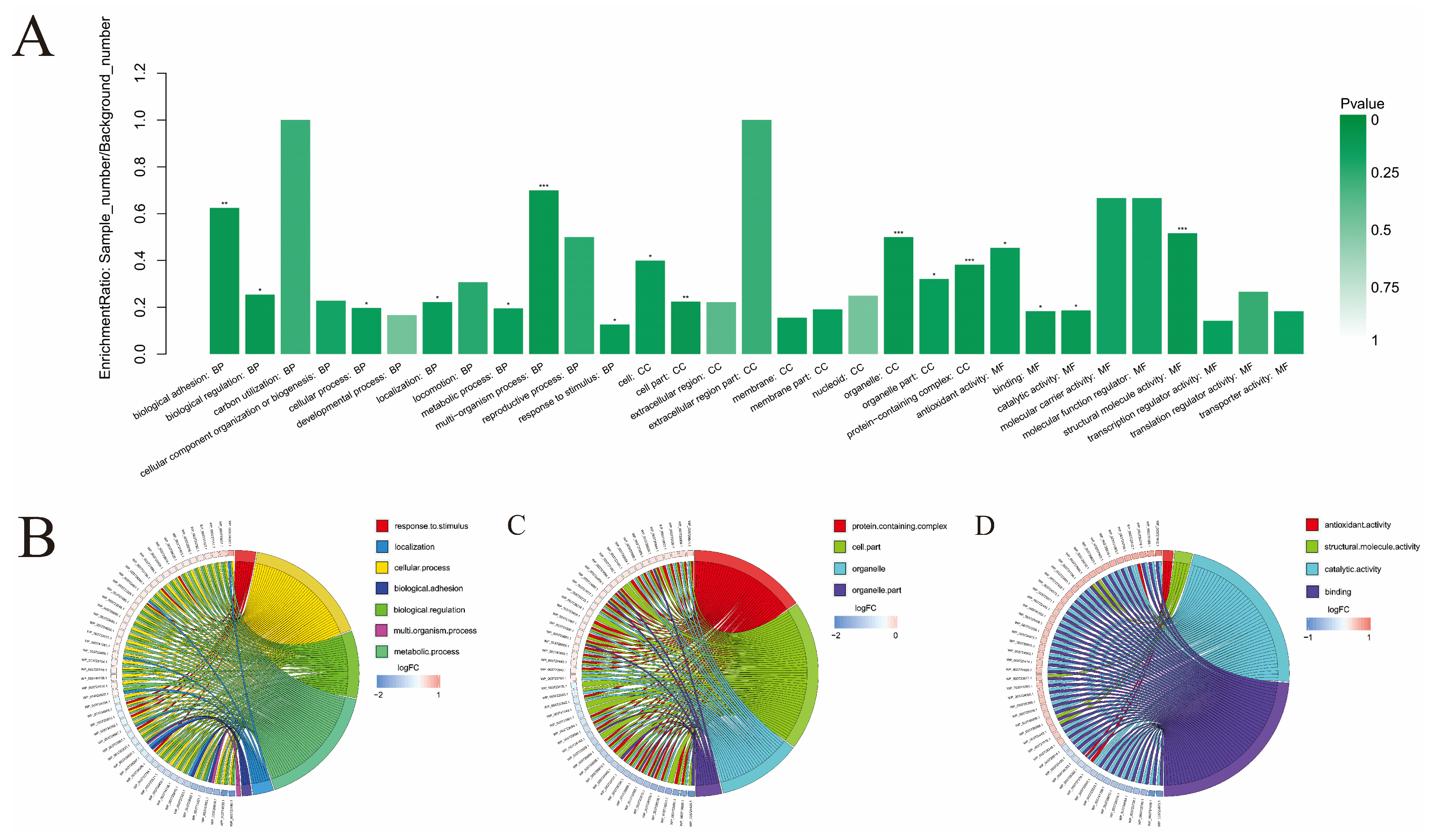
3.4. The GO Enrichment Analysis
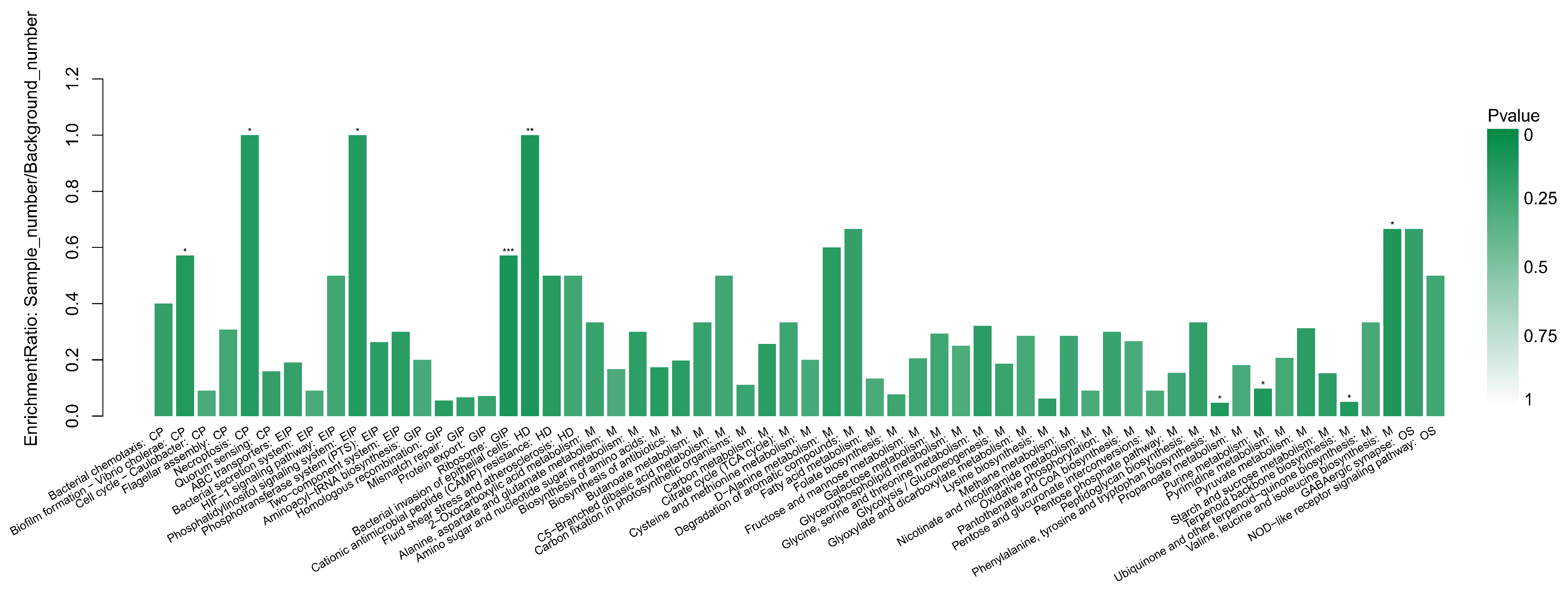
3.5. The KEGG Enrichment Analysis
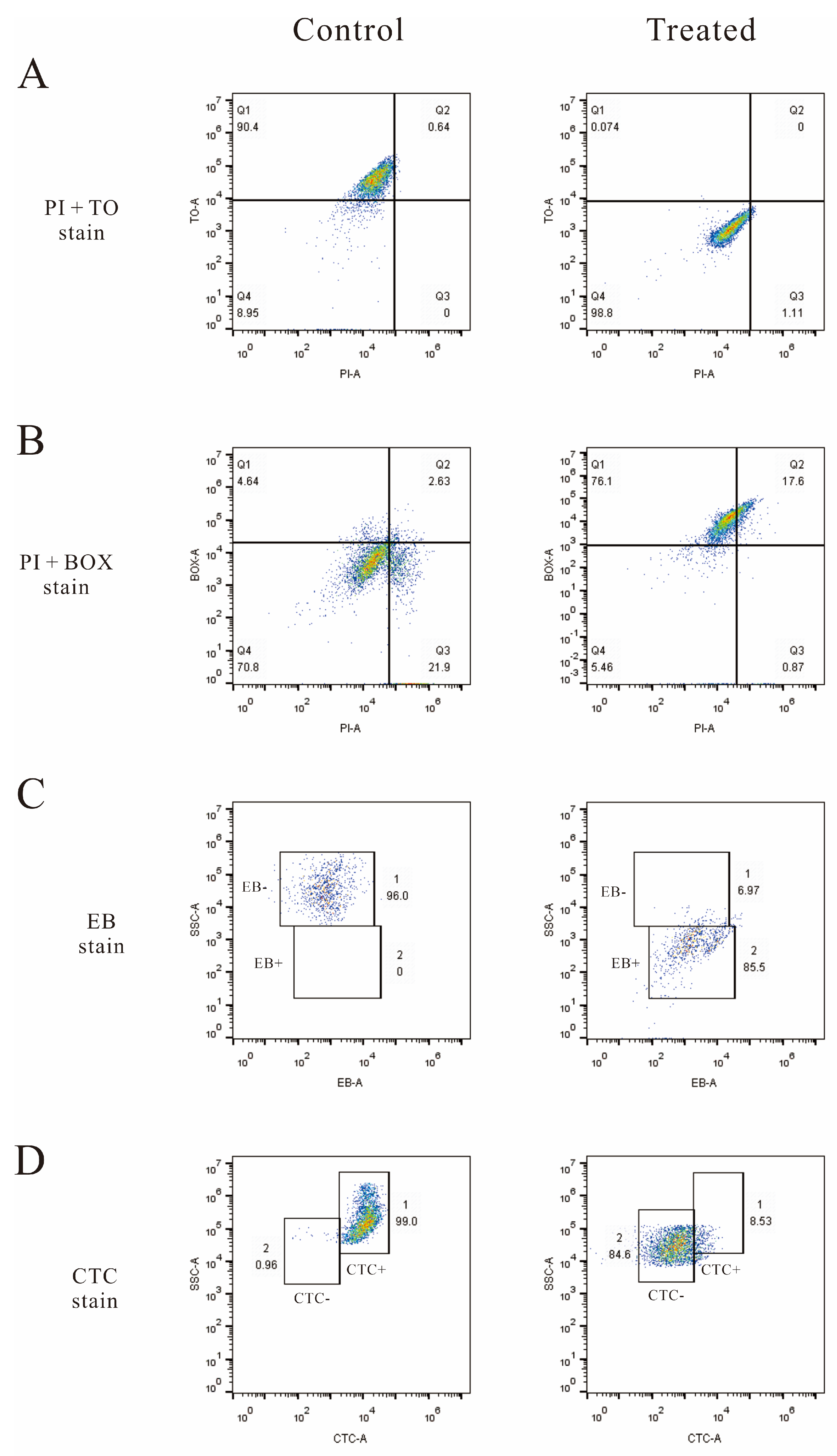
3.6. Flow Cytometry Analysis
4. Discussion
4.1. Proteomic Technology Used in the Antimicrobial Research Field
4.2. Further Analysis of Important Functions in GO Enrichment Analysis
4.3. Further Analysis of Important Pathways in KEGG Enrichment Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- King, T.; Cole, M.; Farber, J.M.; Eisenbrand, G.; Zabaras, D.; Fox, E.M.; Hill, J.P. Food safety for food security: Relationship between global megatrends and developments in food safety. Trends Food Sci. Technol. 2017, 68, 160–175. [Google Scholar] [CrossRef]
- Radoshevich, L.; Cossart, P. Listeria monocytogenes: Towards a complete picture of its physiology and pathogenesis. Nat. Rev. Microbiol. 2018, 16, 32–46. [Google Scholar] [CrossRef]
- Freitag, N.E.; Port, G.C.; Miner, M.D. Listeria monocytogenes—From saprophyte to intracellular pathogen. Nat. Rev. Microbiol. 2009, 7, 623–628. [Google Scholar] [CrossRef]
- Pizarro-Cerda, J.; Cossart, P. Listeria monocytogenes: Cell biology of invasion and intracellular growth. Microbiol. Spectr. 2018, 6, 6.6. 05. [Google Scholar] [CrossRef]
- European Food Safety Authority, European Centre for Disease Prevention and Control. The European Union One Health 2019 Zoonoses Report. EFSA J. 2021, 19, e06406. [Google Scholar]
- Maury, M.M.; Bracq-Dieye, H.; Huang, L.; Vales, G.; Lavina, M.; Thouvenot, P.; Disson, O.; Leclercq, A.; Brisse, S.; Lecuit, M. Hypervirulent Listeria monocytogenes clones’ adaption to mammalian gut accounts for their association with dairy products. Nat. Commun. 2019, 10, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Fan, Z.; Xie, J.; Li, Y.; Wang, H. Listeriosis in mainland China: A systematic review. Int. J. Infect. Dis. 2019, 81, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.M.; Tau, N.P.; Smouse, S.L.; Allam, M.; Ismail, A.; Ramalwa, N.R.; Disenyeng, B.; Ngomane, M.; Thomas, J. Outbreak of Listeria monocytogenes in South Africa, 2017–2018: Laboratory activities and experiences associated with whole-genome sequencing analysis of isolates. Foodborne Pathog. Dis. 2019, 16, 524–530. [Google Scholar] [CrossRef] [Green Version]
- Charlier, C.; Disson, O.; Lecuit, M. Maternal-neonatal listeriosis. Virulence 2020, 11, 391–397. [Google Scholar] [CrossRef]
- Guo, J.; Gao, Z.; Li, G.; Fu, F.; Liang, Z.; Zhu, H.; Shan, Y. Antimicrobial and antibiofilm efficacy and mechanism of essential oil from Citrus Changshan-huyou YB chang against Listeria monocytogenes. Food Control 2019, 105, 256–264. [Google Scholar] [CrossRef]
- Awad, A.H.; Parmar, A.; Ali, M.R.; El-Mogy, M.M.; Abdelgawad, K.F. Extending the shelf-life of fresh-cut green bean pods by ethanol, ascorbic acid, and essential oils. Foods 2021, 10, 1103. [Google Scholar] [CrossRef]
- Guo, J.; Hu, X.; Gao, Z.; Li, G.; Fu, F.; Shang, X.; Liang, Z.; Shan, Y. Global transcriptomic response of Listeria monocytogenes exposed to Fingered Citron (Citrus medica L. var. sarcodactylis Swingle) essential oil. Food Res. Int. 2021, 143, 110274. [Google Scholar] [CrossRef]
- Yang, S.-K.; Yusoff, K.; Thomas, W.; Akseer, R.; Alhosani, M.S.; Abushelaibi, A.; Lai, K.-S. Lavender essential oil induces oxidative stress which modifies the bacterial membrane permeability of carbapenemase producing Klebsiella pneumoniae. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Drug Administration Code of Federal Regulations Title 21; 21CFR20157; Department of Health and Human Services, Ed.; US Food and Drug Administration: Washington, DC, USA, 2014. [Google Scholar]
- Gao, Z.; Van Nostrand, J.D.; Zhou, J.; Zhong, W.; Chen, K.; Guo, J. Anti-Listeria activities of linalool and its mechanism revealed by comparative transcriptome analysis. Front. Microbiol. 2019, 10, 2947. [Google Scholar] [CrossRef]
- Guo, J.-J.; Gao, Z.-P.; Xia, J.-L.; Ritenour, M.A.; Li, G.-Y.; Shan, Y. Comparative analysis of chemical composition, antimicrobial and antioxidant activity of citrus essential oils from the main cultivated varieties in China. Lwt 2018, 97, 825–839. [Google Scholar] [CrossRef]
- Gao, Z.; Zhong, W.; Chen, K.; Tang, P.; Guo, J. Chemical composition and anti-biofilm activity of essential oil from Citrus medica L. var. sarcodactylis Swingle against Listeria monocytogenes. Ind. Crop. Prod. 2020, 144, 112036. [Google Scholar] [CrossRef]
- Xu, Y.-J. Foodomics: A novel approach for food microbiology. TrAC Trends Anal. Chem. 2017, 96, 14–21. [Google Scholar] [CrossRef]
- Misra, B.B.; Langefeld, C.; Olivier, M.; Cox, L.A. Integrated omics: Tools, advances and future approaches. J. Mol. Endocrinol. 2019, 62, R21–R45. [Google Scholar] [CrossRef] [Green Version]
- Walsh, A.M.; Crispie, F.; Claesson, M.J.; Cotter, P.D. Translating omics to food microbiology. Ann. Rev. Food Sci. Technol. 2017, 8, 113–134. [Google Scholar] [CrossRef]
- Gajdošik, M.Š.; Andjelković, U.; Gašo-Sokač, D.; Pavlović, H.; Shevchuk, O.; Martinović, T.; Clifton, J.; Josić, D. Proteomic analysis of food borne pathogens following the mode of action of the disinfectants based on pyridoxal oxime derivatives. Food Res. Int. 2017, 99, 560–570. [Google Scholar] [CrossRef]
- Zhao, Y.-L.; Zhou, Y.-H.; Chen, J.-Q.; Huang, Q.-Y.; Han, Q.; Liu, B.; Cheng, G.-D.; Li, Y.-H. Quantitative proteomic analysis of sub-MIC erythromycin inhibiting biofilm formation of S. suis in vitro. J. Proteom. 2015, 116, 1–14. [Google Scholar] [CrossRef]
- Hesketh, A.; Deery, M.J.; Hong, H.-J. High-resolution mass spectrometry based proteomic analysis of the response to vancomycin-induced cell wall stress in Streptomyces coelicolor A3(2). J. Proteome Res. 2015, 14, 2915–2928. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.; Zhang, D.; Li, G.; Liu, J.; He, G.; Zhang, P.; Yang, L.; Zhu, H.; Xu, N.; Liang, S. Antibacterial mechanism of daptomycin antibiotic against Staphylococcus aureus based on a quantitative bacterial proteome analysis. J. Proteom. 2017, 150, 242–251. [Google Scholar] [CrossRef] [Green Version]
- Yuan, P.; He, L.; Chen, D.; Sun, Y.; Ge, Z.; Shen, D.; Lu, Y. Proteomic characterization of Mycobacterium tuberculosis reveals potential targets of bostrycin. J. Proteom. 2020, 212, 103576. [Google Scholar] [CrossRef]
- Li, L.; Tian, Y.; Yu, J.; Song, X.; Jia, R.; Cui, Q.; Tong, W.; Zou, Y.; Li, L.; Yin, L. iTRAQ-based quantitative proteomic analysis reveals multiple effects of Emodin to Haemophilus parasuis. J. Proteom. 2017, 166, 39–47. [Google Scholar] [CrossRef]
- Sun, L.; Chen, H.; Lin, W.; Lin, X. Quantitative proteomic analysis of Edwardsiella tarda in response to oxytetracycline stress in biofilm. J. Proteom. 2017, 150, 141–148. [Google Scholar] [CrossRef]
- Zieske, L.R. A perspective on the use of iTRAQ™ reagent technology for protein complex and profiling studies. J. Exp. Bot. 2006, 57, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, D.W.; Therneau, T.M.; Heppelmann, C.J.; Higgins, L.; Benson, L.M.; Zenka, R.M.; Jagtap, P.; Nelsestuen, G.L.; Bergen, H.R., III; Oberg, A.L. Relative quantification: Characterization of bias, variability and fold changes in mass spectrometry data from iTRAQ-labeled peptides. J. Proteome Res. 2011, 10, 4325–4333. [Google Scholar] [CrossRef] [Green Version]
- De Sousa Guedes, J.P.; de Souza, E.L. Investigation of damage to Escherichia coli, Listeria monocytogenes and Salmonella Enteritidis exposed to Mentha arvensis L. and M. piperita L. essential oils in pineapple and mango juice by flow cytometry. Food Microbiol. 2018, 76, 564–571. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, X.; Xia, X.; Li, B.; Hung, Y.-C. Viability assay of E. coli O157: H7 treated with electrolyzed oxidizing water using flow cytometry. Food Control 2018, 88, 47–53. [Google Scholar] [CrossRef]
- Xu, J.; Shao, X.; Wei, Y.; Xu, F.; Wang, H. iTRAQ proteomic analysis reveals that metabolic pathways involving energy metabolism are affected by tea tree oil in Botrytis cinerea. Front. Microbiol. 2017, 8, 1989. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.; Feng, K.; Xiu, Z.; Jiang, A.; Lao, Y. Tandem mass tag-based quantitative proteomic analysis reveal the inhibition mechanism of thyme essential oil against flagellum of Listeria monocytogenes. Food Res. Int. 2019, 125, 108508. [Google Scholar]
- Phelps, C.C.; Vadia, S.; Arnett, E.; Tan, Y.; Zhang, X.; Pathak-Sharma, S.; Gavrilin, M.A.; Seveau, S. Relative roles of listeriolysin O, InlA, and InlB in Listeria monocytogenes uptake by host cells. Infect. Immun. 2018, 86, e00555-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, B.-Y.; Kim, T.-J.; Silva, J.L.; Jung, Y.-S. Positive correlation between the expression of inlA and inlB genes of Listeria monocytogenes and its attachment strength on glass surface. Food Biophys. 2009, 4, 304–311. [Google Scholar] [CrossRef]
- Taguchi, A.; Welsh, M.A.; Marmont, L.S.; Lee, W.; Sjodt, M.; Kruse, A.C.; Kahne, D.; Bernhardt, T.G.; Walker, S. FtsW is a peptidoglycan polymerase that is functional only in complex with its cognate penicillin-binding protein. Nat. Microbiol. 2019, 4, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Gautam, A.; Vyas, R.; Tewari, R. Peptidoglycan biosynthesis machinery: A rich source of drug targets. Crit. Rev. Biotechnol. 2011, 31, 295–336. [Google Scholar] [CrossRef]
- Strehl, E.; Kees, F. Pharmacological Properties of Parenteral Cephalosporins. Drugs 2000, 59, 9–18. [Google Scholar] [CrossRef]
- Heidrich, C.; Templin, M.F.; Ursinus, A.; Merdanovic, M.; Berger, J.; Schwarz, H.; De Pedro, M.A.; Höltje, J.V. Involvement of N-acetylmuramyl-L-alanine amidases in cell separation and antibiotic-induced autolysis of Escherichia coli. Mol. Microbiol. 2001, 41, 167–178. [Google Scholar] [CrossRef]
- Stohl, E.A.; Lenz, J.D.; Dillard, J.P.; Seifert, H.S. The gonococcal NlpD protein facilitates cell separation by activating peptidoglycan cleavage by AmiC. J. Bacteriol. 2016, 198, 615–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Z.; Zhong, W.; Liu, T.; Zhao, T.; Guo, J. Global Proteomic Analysis of Listeria monocytogenes’ Response to Linalool. Foods 2021, 10, 2449. https://doi.org/10.3390/foods10102449
Gao Z, Zhong W, Liu T, Zhao T, Guo J. Global Proteomic Analysis of Listeria monocytogenes’ Response to Linalool. Foods. 2021; 10(10):2449. https://doi.org/10.3390/foods10102449
Chicago/Turabian StyleGao, Zhipeng, Weiming Zhong, Ting Liu, Tianyu Zhao, and Jiajing Guo. 2021. "Global Proteomic Analysis of Listeria monocytogenes’ Response to Linalool" Foods 10, no. 10: 2449. https://doi.org/10.3390/foods10102449




