Anti-Helicobacter pylori Activity of a Lactobacillus sp. PW-7 Exopolysaccharide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Screening of LAB
2.3. Morphology of Strains
2.4. 16S rDNA Gene Identification
2.5. Analysis of Components of Strain PW-7 Inhibiting H. pylori
2.5.1. Hydrogen Peroxide Detection and Elimination
2.5.2. Detection and Elimination of Organic Acids
2.5.3. Bacteriostatic Test of Bacteriocin
2.5.4. Bacteriostatic Test of EPS
2.6. Structure Characterization of EPS
2.6.1. Monosaccharide Composition Analysis
2.6.2. Determination of Molecular Weight
2.7. Minimum Inhibitory Concentration (MIC)
2.8. Antibacterial Mechanism
2.8.1. Cell Membrane Permeability
2.8.2. Cell Membrane Integrity
2.8.3. NPN Uptake
2.8.4. Scanning Electron Microscopy
2.9. Antioxidant Activity of Exopolysaccharides In Vitro
2.9.1. DPPH Free Radical Scavenging Experiment
2.9.2. Hydroxyl Radical Scavenging Experiment
2.9.3. Superoxide Anion Radical Scavenging Experiment
2.9.4. Reducing Power
2.10. Statistical Significance
3. Results and Discussions
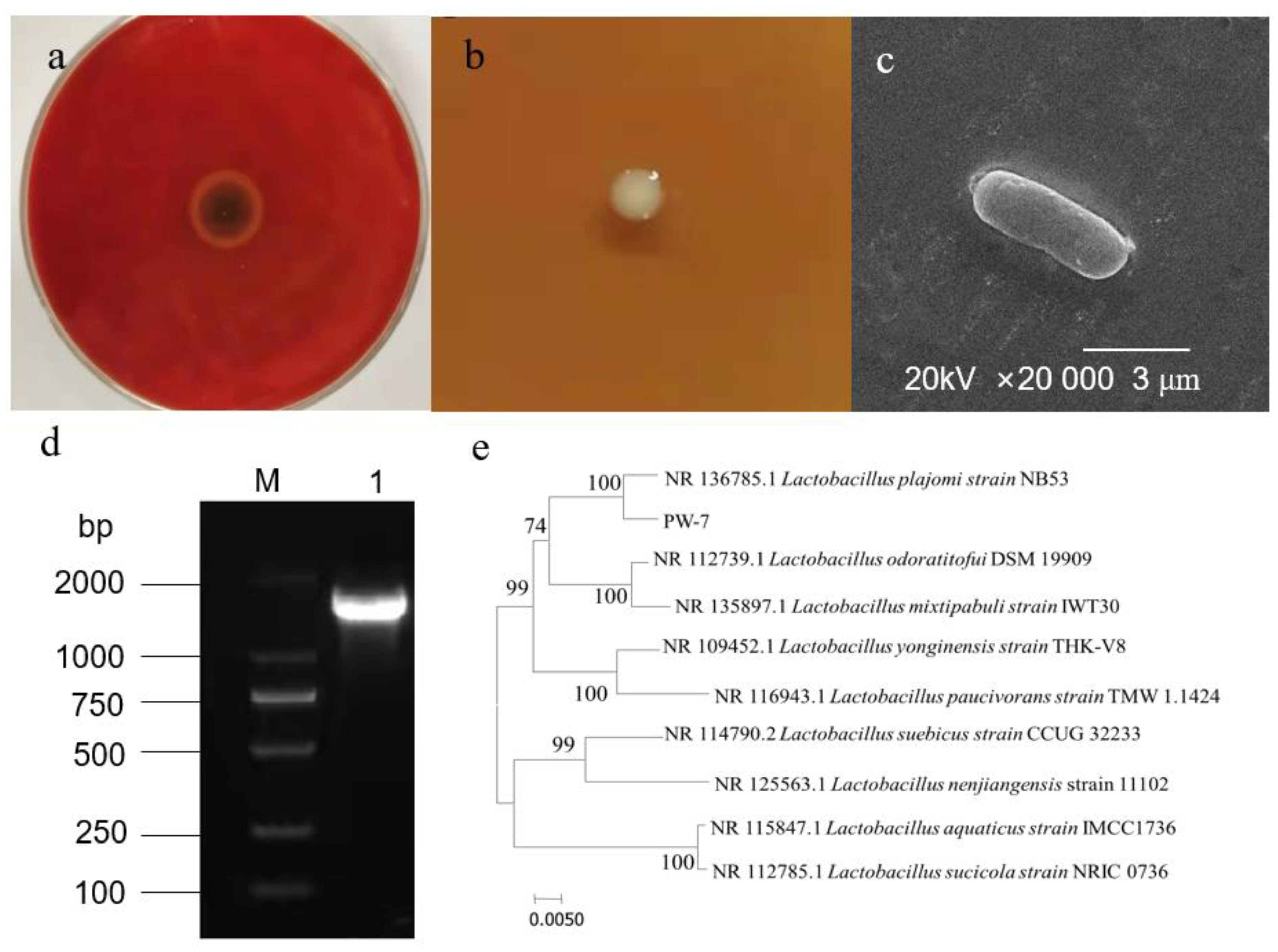
3.1. Screening and Identification of Strain
3.1.1. Screening of Strain
3.1.2. Morphological Characteristics of Strain PW-7
3.1.3. Sequence Analysis of 16S rDNA of Strain PW-7
3.2. Analysis of Components of Strain PW-7 Inhibiting H. pylori
3.3. Structure Analysis of PW-7 EPS
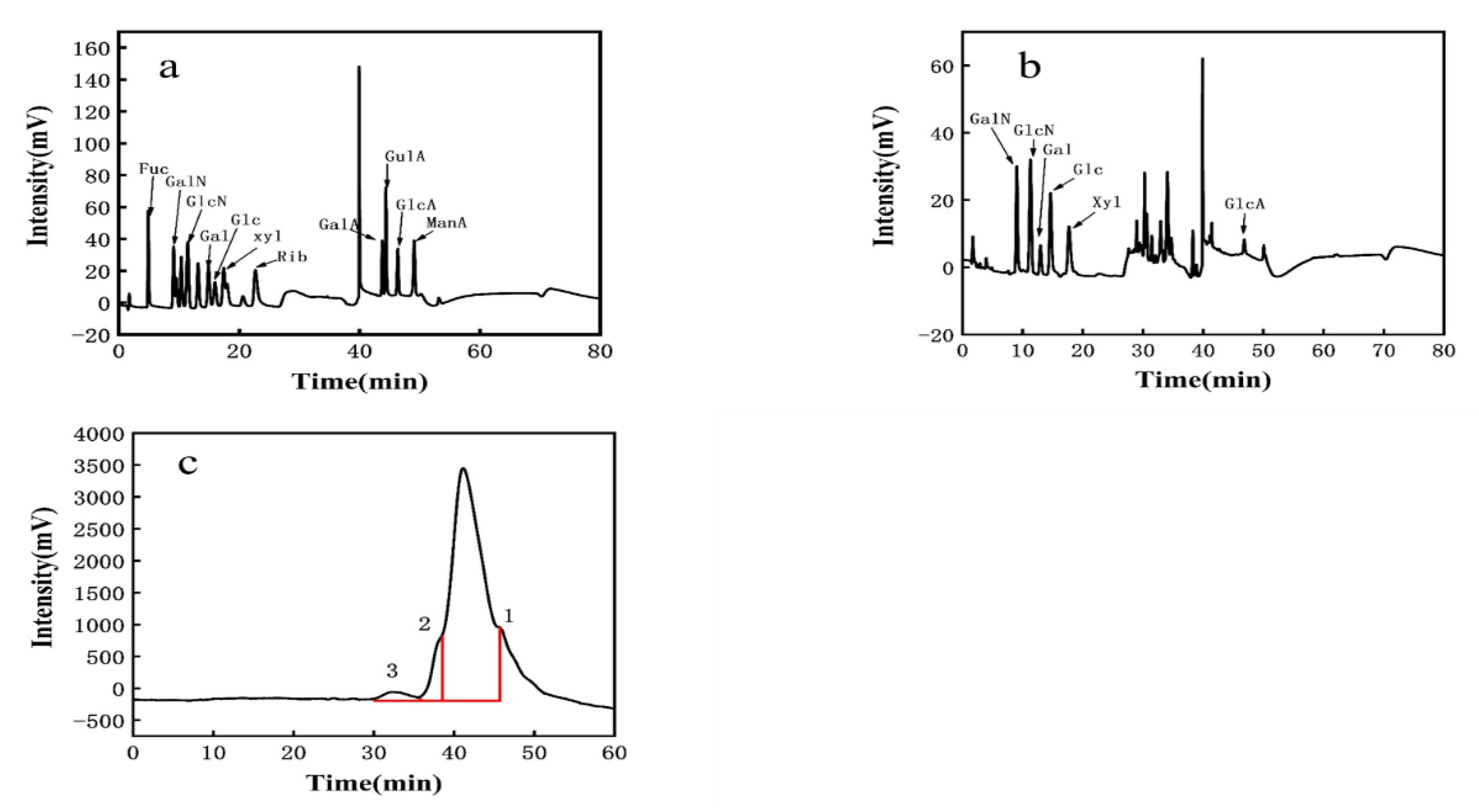
3.3.1. Monosaccharide Composition of EPS
3.3.2. Molecular Weight of EPS
3.4. Minimum Inhibitory Concentration (MIC)
3.5. Antibacterial Mechanism
3.5.1. Cell Membrane Permeability
3.5.2. Integrity of Cell Membranes
3.5.3. Cell Membrane NPN Uptake
3.5.4. Electron Microscopic Observations
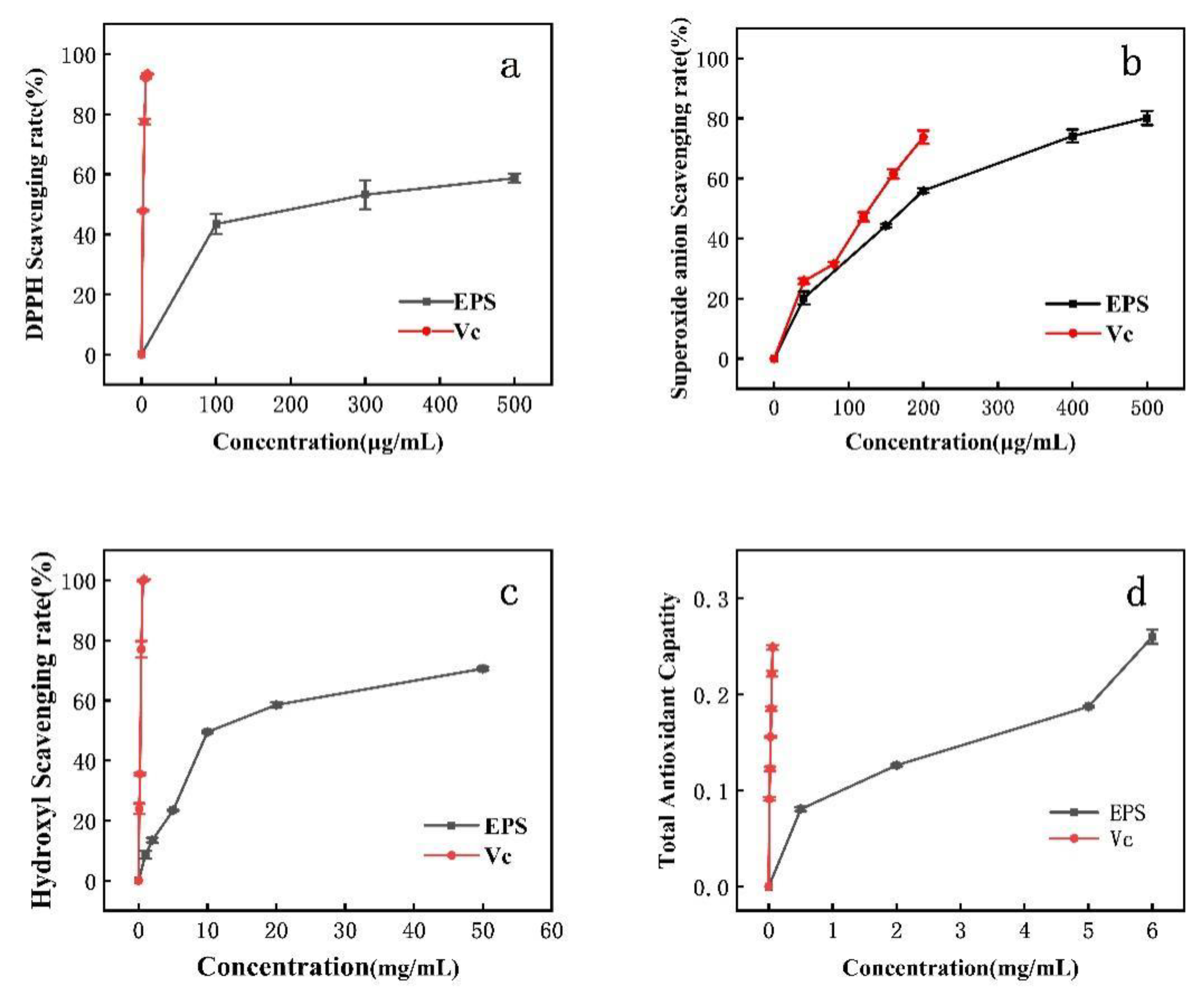
3.6. Antioxidant Activity of EPS
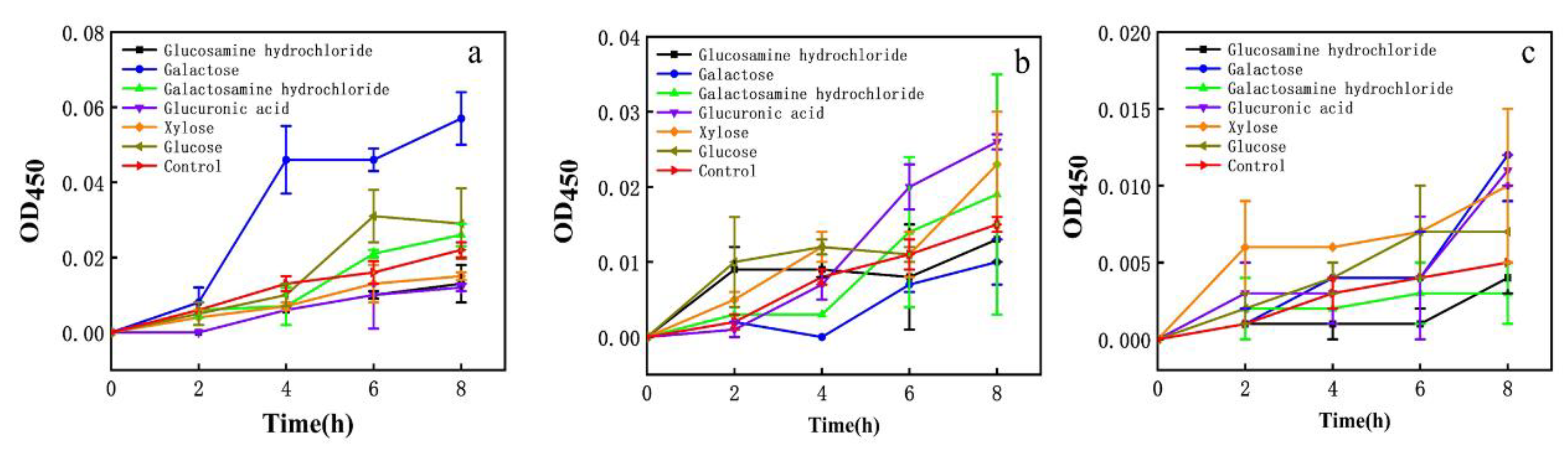
3.7. Effect of Monosaccharide on Cell Membrane Permeability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Juvale, K.; Purushothaman, G.; Singh, V.; Shaik, A.; Ravi, S.; Thiruvenkatam, V.; Kirubakaran, S. Identification of selective inhibitors of Helicobacter pylori IMPDH as a targeted therapy for the infection. Sci. Rep. 2019, 9, 190. [Google Scholar] [CrossRef] [Green Version]
- Miqueleiz-Zapatero, A.; Alba-Rubio, C.; Domingo-Garcia, D.; Canton, R.; Gomez-Garcia de la Pedrosa, E.; Aznar-Cano, E.; Leiva, J.; Montes, M.; Sanchez-Romero, I.; Rodriguez-Diaz, J.C.; et al. First national survey of the diagnosis of Helicobacter pylori infection in Clinical Microbiology Laboratories in Spain. Enferm. Infecc. Microbiol. Clin. (Engl. Ed.) 2020, 38, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Ghobadi, E.; Ghanbarimasir, Z.; Emami, S. A review on the structures and biological activities of anti-Helicobacter pylori agents. Eur. J. Med. Chem. 2021, 223, 113669. [Google Scholar] [CrossRef]
- Gutierrez-Zamorano, C.; Gonzalez-Avila, M.; Diaz-Blas, G.; Smith, C.T.; Gonzalez-Correa, C.; Garcia-Cancino, A. Increased anti-Helicobacter pylori effect of the probiotic Lactobacillus fermentum UCO-979C strain encapsulated in carrageenan evaluated in gastric simulations under fasting conditions. Food Res. Int. 2019, 121, 812–816. [Google Scholar] [CrossRef]
- Karbalaei, M.; Keikha, M. Rescue effects of Lactobacillus-containing bismuth regimens after Helicobacter pylori treatment failure. New Microbes New Infect 2021, 42, 100904. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Tian, F.; Liu, X.; Zhao, J.; Zhang, H.P.; Zhang, H.; Chen, W. In vitro screening of lactobacilli with antagonistic activity against Helicobacter pylori from traditionally fermented foods. J. Dairy Sci. 2010, 93, 5627–5634. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Hormazabal, P.; Arenas, A.; Serrano, C.; Pizarro, M.; Fuentes-Lopez, E.; Arnold, J.; Berger, Z.; Musleh, M.; Valladares, H.; Lanzarini, E.; et al. Prevalence of Helicobacter pylori Antimicrobial Resistance Among Chilean Patients. Arch. Med. Res. 2021, 52, 529–534. [Google Scholar] [CrossRef]
- Azizian, K.; Osquee, H.O.; Pourlak, T.; Hosseinpour, R.; Asgharzadeh, M.; Ganvarov, K.; Kafil, H.S. Genetic diversity of Lactobacillus spp. isolates from oral cavity and their probiotic and antimicrobial properties. Gene Rep. 2021, 24, 101231. [Google Scholar] [CrossRef]
- Jiang, B.; Tian, L.; Huang, X.; Liu, Z.; Jia, K.; Wei, H.; Tao, X. Characterization and antitumor activity of novel exopolysaccharide APS of Lactobacillus plantarum WLPL09 from human breast milk. Int. J. Biol. Macromol. 2020, 163, 985–995. [Google Scholar] [CrossRef]
- Xu, Y.; Cui, Y.; Yue, F.; Liu, L.; Shan, Y.; Liu, B.; Zhou, Y.; Lu, X. Exopolysaccharides produced by Lactic Acid Bacteria and Bifidobacteria: Structures, Physiochemical Functions and Applications in the Food Industry. Food Hydrocoll. 2019, 94, 475–499. [Google Scholar] [CrossRef]
- Sauer, M.; Russmayer, H.; Grabherr, R.; Peterbauer, C.K.; Marx, H. The Efficient Clade: Lactic Acid Bacteria for Industrial Chemical Production. Trends Biotechnol. 2017, 35, 756–769. [Google Scholar] [CrossRef]
- Abdullah, A.; Winaningsih, I.; Hadiyarto, A. Lactic Acid Fermentation from Durian Seeds (Durio zibethinus Murr.) Using Lactobacillus plantarum. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2021; Volume 1053, p. 012032. [Google Scholar]
- Yu, M.; Zhang, R.; Ni, P.; Chen, S.; Duan, G. Efficacy of Lactobacillus-supplemented triple therapy for H. pylori eradication: A meta-analysis of randomized controlled trials. PLoS ONE 2019, 14, e0223309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poonyam, P.; Chotivitayatarakorn, P.; Vilaichone, R.K. High Effective of 14-Day High-Dose PPI- Bismuth-Containing Quadruple Therapy with Probiotics Supplement for Helicobacter Pylori Eradication: A Double Blinded-Randomized Placebo-Controlled Study. Asian Pac. J. Cancer Prev. 2019, 20, 2859–2864. [Google Scholar] [CrossRef] [PubMed]
- García, A.; Navarro, K.; Sanhueza, E.; Pineda, S.; Pastene, E.; Quezada, M.; Henríquez, K.; Karlyshev, A.; Villena, J.; González, C. Characterization of Lactobacillus fermentum UCO-979C, a probiotic strain with a potent anti-Helicobacter pylori activity. Electron. J. Biotechnol. 2017, 25, 75–83. [Google Scholar] [CrossRef]
- Wu, D.; Cao, M.; Zhou, J.; Yan, S.; Peng, J.; Yu, Z.; Zhang, A.; Wu, J.; Yan, X.; Zhao, J. Lactobacillus casei T1 from kurut against Helicobacter pylori-induced inflammation and the gut microbial disorder. J. Funct. Foods 2021, 85, 104611. [Google Scholar] [CrossRef]
- Zhang, X.; Ali Esmail, G.; Fahad Alzeer, A.; Valan Arasu, M.; Vijayaraghavan, P.; Choon Choi, K.; Abdullah Al-Dhabi, N. Probiotic characteristics of Lactobacillus strains isolated from cheese and their antibacterial properties against gastrointestinal tract pathogens. Saudi J. Biol. Sci. 2020, 27, 3505–3513. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.X.; Fang, H.Y.; Yang, H.B.; Tien, N.Y.; Wang, M.C.; Wu, J.J. Lactobacillus pentosus strain LPS16 produces lactic acid, inhibiting multidrug-resistant Helicobacter pylori. J. Microbiol. Immunol. Infect. 2016, 49, 168–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.W.; Xiang, Y.Z.; Zhang, M.; Jiang, Y.H.; Zhang, Q.L. A novel bacteriocin from Lactobacillus salivarius against Staphylococcus aureus: Isolation, purification, identification, antibacterial and antibiofilm activity. LWT-Food Sci. Technol. 2020, 140, 110826. [Google Scholar] [CrossRef]
- Ale, E.C.; Rojas, M.F.; Reinheimer, J.A.; Binetti, A.G. Lactobacillus fermentum: Could EPS production ability be responsible for functional properties? Food Microbiol. 2020, 90, 103465. [Google Scholar] [CrossRef]
- Rahbar Saadat, Y.; Yari Khosroushahi, A.; Pourghassem Gargari, B. A comprehensive review of anticancer, immunomodulatory and health beneficial effects of the lactic acid bacteria exopolysaccharides. Carbohydr. Polym. 2019, 217, 79–89. [Google Scholar] [CrossRef]
- Ayyash, M.; Abu-Jdayil, B.; Itsaranuwat, P.; Galiwango, E.; Tamiello-Rosa, C.; Abdullah, H.; Esposito, G.; Hunashal, Y.; Obaid, R.S.; Hamed, F. Characterization, bioactivities, and rheological properties of exopolysaccharide produced by novel probiotic Lactobacillus plantarum C70 isolated from camel milk. Int. J. Biol. Macromol. 2020, 144, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Ryan, K.A.; Daly, P.; Li, Y.; Hooton, C.; O’Toole, P.W. Strain-specific inhibition of Helicobacter pylori by Lactobacillus salivarius and other lactobacilli. J. Antimicrob. Chemother. 2008, 61, 831–834. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Luo, J.; Xue, R.Y.; Guo, L.; Nie, L.; Li, S.; Ji, L.; Ma, C.J.; Chen, D.Q.; Miao, K.; et al. The mucosal adjuvant effect of plant polysaccharides for induction of protective immunity against Helicobacter pylori infection. Vaccine 2019, 37, 1053–1061. [Google Scholar] [CrossRef]
- Garcia-Castillo, V.; Marcial, G.; Albarracin, L.; Tomokiyo, M.; Clua, P.; Takahashi, H.; Kitazawa, H.; Garcia-Cancino, A.; Villena, J. The Exopolysaccharide of Lactobacillus fermentum UCO-979C Is Partially Involved in Its Immunomodulatory Effect and Its Ability to Improve the Resistance against Helicobacter pylori Infection. Microorganisms 2020, 8, 479. [Google Scholar] [CrossRef] [Green Version]
- Kšonžeková, P.; Bystrický, P.; Vlčková, S.; Pätoprstý, V.; Pulzová, L.; Mudroňová, D.; Kubašková, T.; Csank, T.; Tkáčiková, Ľ. Exopolysaccharides of Lactobacillus reuteri: Their influence on adherence of E. coli to epithelial cells and inflammatory response. Carbohydr. Polym. 2016, 141, 10–19. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, X.; Jiang, Y.; Zhao, W.; Guo, T.; Cao, Y.; Teng, J.; Hao, X.; Zhao, J.; Yang, Z. Antioxidant status and gut microbiota change in an aging mouse model as influenced by exopolysaccharide produced by Lactobacillus plantarum YW11 isolated from Tibetan kefir. J. Dairy Sci. 2017, 100, 6025–6041. [Google Scholar] [CrossRef] [PubMed]
- Chen, O.; Hong, Y.; Ma, J.; Deng, L.; Yi, L.; Zeng, K. Screening lactic acid bacteria from pickle and cured meat as biocontrol agents of Penicillium digitatum on citrus fruit. Biol. Control 2021, 158, 104606. [Google Scholar] [CrossRef]
- Pan, M.; Wan, C.; Xie, Q.; Huang, R.; Tao, X.; Shah, N.P.; Wei, H. Changes in gastric microbiota induced by Helicobacter pylori infection and preventive effects of Lactobacillus plantarum ZDY 2013 against such infection. J. Dairy Sci. 2016, 99, 970–981. [Google Scholar] [CrossRef]
- Lorn, D.; Nguyen, T.K.; Ho, P.H.; Tan, R.; Licandro, H.; Wache, Y. Screening of lactic acid bacteria for their potential use as aromatic starters in fermented vegetables. Int. J. Food Microbiol. 2021, 350, 109242. [Google Scholar] [CrossRef]
- Snow, M.J.; Manley-Harris, M. On the nature of non-peroxide antibacterial activity in New Zealand manuka honey. Food Chem. 2004, 84, 145–147. [Google Scholar] [CrossRef]
- Le, V.T.; Leelakriangsak, M.; Lee, S.W.; Panphon, S.; Utispan, K.; Koontongkaew, S. Characterization and safety evaluation of partially purified bacteriocin produced by Escherichia coli E isolated from fermented pineapple Ananas comosus (L.) Merr. Braz. J. Microbiol. 2019, 50, 33–42. [Google Scholar] [CrossRef]
- Ndlovu, B.; Schoeman, H.; Franz, C.M.; du Toit, M. Screening, identification and characterization of bacteriocins produced by wine-isolated LAB strains. J. Appl. Microbiol. 2015, 118, 1007–1022. [Google Scholar] [CrossRef]
- Mahdhi, A.; Leban, N.; Chakroun, I.; Chaouch, M.A.; Hafsa, J.; Fdhila, K.; Mahdouani, K.; Majdoub, H. Extracellular polysaccharide derived from potential probiotic strain with antioxidant and antibacterial activities as a prebiotic agent to control pathogenic bacterial biofilm formation. Microb. Pathog. 2017, 109, 214–220. [Google Scholar] [CrossRef]
- Bomfim, V.B.; Pereira Lopes Neto, J.H.; Leite, K.S.; de Andrade Vieira, É.; Iacomini, M.; Silva, C.M.; Olbrich dos Santos, K.M.; Cardarelli, H.R. Partial characterization and antioxidant activity of exopolysaccharides produced by Lactobacillus plantarum CNPC003. LWT 2020, 127, 109349. [Google Scholar] [CrossRef]
- Xu, Y.; Cui, Y.; Wang, X.; Yue, F.; Shan, Y.; Liu, B.; Zhou, Y.; Yi, Y.; Lü, X. Purification, characterization and bioactivity of exopolysaccharides produced by Lactobacillus plantarum KX041. Int. J. Biol. Macromol. 2019, 128, 480–492. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.J.; Kim, J.H.; Lee, N.K.; Paik, H.D. Inhibitory effects of Lactobacillus brevis KU15153 against Streptococcus mutans KCTC 5316 causing dental caries. Microb. Pathog. 2021, 157, 104938. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.; Chen, J.; Zhou, Q.; Wang, X.; Shan, Y.; Yi, Y.; Liu, B.; Zhou, Y.; Lu, X. Purification, characterization, and mode of action of a novel bacteriocin BM173 from Lactobacillus crustorum MN047 and its effect on biofilm formation of Escherichia coli and Staphylococcus aureus. J. Dairy Sci. 2021, 104, 1474–1483. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.; Chen, H.; Xiang, A.; Wang, Y.; Yue, T.; Yuan, Y. Identification and characterization of Lactobacillus paracasei strain MRS-4 antibacterial activity against Alicyclobacillus acidoterrestris. LWT 2021, 150, 111991. [Google Scholar] [CrossRef]
- Zhang, L.-L.; Zhang, L.-F.; Hu, Q.-P.; Hao, D.-L.; Xu, J.-G. Chemical composition, antibacterial activity of Cyperus rotundus rhizomes essential oil against Staphylococcus aureus via membrane disruption and apoptosis pathway. Food Control 2017, 80, 290–296. [Google Scholar] [CrossRef]
- Xing, K.; Chen, X.G.; Kong, M.; Liu, C.S.; Cha, D.S.; Park, H.J. Effect of oleoyl-chitosan nanoparticles as a novel antibacterial dispersion system on viability, membrane permeability and cell morphology of Escherichia coli and Staphylococcus aureus. Carbohydr. Polym. 2009, 76, 17–22. [Google Scholar] [CrossRef]
- Dong, D.; Wang, X.; Deng, T.; Ning, Z.; Tian, X.; Zu, H.; Ding, Y.; Wang, C.; Wang, S.; Lyu, M. A novel dextranase gene from the marine bacterium Bacillus aquimaris S5 and its expression and characteristics. FEMS Microbiol. Lett. 2021, 368, fnab007. [Google Scholar] [CrossRef] [PubMed]
- Riaz Rajoka, M.S.; Jin, M.; Haobin, Z.; Li, Q.; Shao, D.; Jiang, C.; Huang, Q.; Yang, H.; Shi, J.; Hussain, N. Functional characterization and biotechnological potential of exopolysaccharide produced by Lactobacillus rhamnosus strains isolated from human breast milk. LWT 2018, 89, 638–647. [Google Scholar] [CrossRef]
- Zhang, N.; Zhao, Y.; Shi, Y.; Chen, R.; Fu, X.; Zhao, Y. Polypeptides extracted from Eupolyphaga sinensis walker via enzymic digestion alleviate UV radiation-induced skin photoaging. Biomed. Pharmacother. 2019, 112, 108636. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, Z.; Qiu, L.; Zhang, F.; Xu, X.; Wei, H.; Tao, X. Characterization and bioactivities of the exopolysaccharide from a probiotic strain of Lactobacillus plantarum WLPL04. J. Dairy Sci. 2017, 100, 6895–6905. [Google Scholar] [CrossRef]
- Caggianiello, G.; Kleerebezem, M.; Spano, G. Exopolysaccharides produced by lactic acid bacteria: From health-promoting benefits to stress tolerance mechanisms. Appl. Microbiol. Biotechnol. 2016, 100, 3877–3886. [Google Scholar] [CrossRef]
- Das, D.; Baruah, R.; Goyal, A. A food additive with prebiotic properties of an alpha-d-glucan from lactobacillus plantarum DM5. Int. J. Biol. Macromol. 2014, 69, 20–26. [Google Scholar] [CrossRef]
- Do, T.B.T.; Tran, T.A.L.; Tran, T.V.T.; Le, T.H.; Jayasena, V.; Nguyen, T.H.C.; Nguyen, C.C.; Kim, S.Y.; Le, Q.V. Novel Exopolysaccharide Produced from Fermented Bamboo Shoot-Isolated Lactobacillus Fermentum. Polymers 2020, 12, 1531. [Google Scholar] [CrossRef]
- You, X.; Li, Z.; Ma, K.; Zhang, C.; Chen, X.; Wang, G.; Yang, L.; Dong, M.; Rui, X.; Zhang, Q.; et al. Structural characterization and immunomodulatory activity of an exopolysaccharide produced by Lactobacillus helveticus LZ-R-5. Carbohydr. Polym. 2020, 235, 115977. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, X.; Tian, Z.; Yang, Y.; Yang, Z. Characterization of an exopolysaccharide produced by Lactobacillus plantarum YW11 isolated from Tibet Kefir. Carbohydr. Polym. 2015, 125, 16–25. [Google Scholar] [CrossRef]
- Lynch, K.M.; Zannini, E.; Coffey, A.; Arendt, E.K. Lactic Acid Bacteria Exopolysaccharides in Foods and Beverages: Isolation, Properties, Characterization, and Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 155–176. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Wang, Y.; Jiang, P.; Quek, S. Antibacterial activity and mechanism of cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Food Control 2016, 59, 282–289. [Google Scholar] [CrossRef]
- Pradeepa; Shetty, A.D.; Matthews, K.; Hegde, A.R.; Akshatha, B.; Mathias, A.B.; Mutalik, S.; Vidya, S.M. Multidrug resistant pathogenic bacterial biofilm inhibition by Lactobacillus plantarum exopolysaccharide. Bioact. Carbohydr. Diet. Fibre 2016, 8, 7–14. [Google Scholar] [CrossRef]
- Adebayo-Tayo, B.; Fashogbon, R. In vitro antioxidant, antibacterial, in vivo immunomodulatory, antitumor and hematological potential of exopolysaccharide produced by wild type and mutant Lactobacillus delbureckii subsp. bulgaricus. Heliyon 2020, 6, e03268. [Google Scholar] [CrossRef] [PubMed]


| Indicator Bacteria | Diameter of Inhibition Zone (mm) |
|---|---|
| A. salmonicida | 31.953 ± 0.497 |
| H. pylori | 30.613 ± 0.588 |
| V. parahaemolyticus | 28.727 ± 0.454 |
| P. aeruginosa | 26.107 ± 0.555 |
| E. coli | 25.623 ± 0.015 |
| A. hydrophila | 24.817 ± 0.617 |
| S. aureus | 24.637 ± 0.791 |
| B. subtilis | 21.820 ± 0.562 |
| Name | RT (min) | Mole Ratio | Area |
|---|---|---|---|
| Galactosamine hydrochloride | 9.025 | 0.129 | 10.077 |
| Glucosamine hydrochloride | 11.300 | 0.194 | 13.538 |
| Galactose | 12.959 | 0.091 | 3.265 |
| Glucose | 14.642 | 0.290 | 10.148 |
| Xylose | 17.709 | 0.250 | 7.630 |
| Glucuronic acid | 46.825 | 0.046 | 1.404 |
| Indicator Bacteria | MIC (mg/mL) |
|---|---|
| E. coli | 40 |
| S. aureus | 50 |
| H. pylori | 50 |
| Indicator Bacteria | Experience Group | Cell Constituents’ Release | ||
|---|---|---|---|---|
| Cell Constituents (OD260nm) | Soluble Protein (mg/mL) | Reducing Sugar (mg/mL) | ||
| H. pylori | Control | 0.024 ± 0.007 d | 0.185 ± 0.005 d | 0.043 ± 0.001 e |
| 1× MIC | 0.057 ± 0.006 b | 2.043 ± 0.057 c | 0.400 ± 0.016 c | |
| 2× MIC | 0.115 ± 0.008 a | 3.312 ± 0.059 a | 0.858 ± 0.009 d | |
| E. coli | Control | 0.037 ± 0.008 cd | 0.252 ± 0.023 d | 0.039 ± 0.002 e |
| 1× MIC | 0.066 ± 0.009 b | 2.260 ± 0.017 b | 1.148 ± 0.050 b | |
| 2× MIC | 0.123 ± 0.007 a | 3.385 ± 0.054 a | 1.408 ± 0.057 a | |
| S. aureus | Control | 0.019 ± 0.001 d | 0.169 ± 0.001 d | 0.039 ± 0.001 e |
| 1× MIC | 0.052 ± 0.004 bc | 1.960 ± 0.048 c | 1.098 ± 0.149 b | |
| 2× MIC | 0.107 ± 0.002 a | 3.295 ± 0.017 a | 1.429 ± 0.011 a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Tian, X.; Wei, T.; Wu, H.; Lu, J.; Lyu, M.; Wang, S. Anti-Helicobacter pylori Activity of a Lactobacillus sp. PW-7 Exopolysaccharide. Foods 2021, 10, 2453. https://doi.org/10.3390/foods10102453
Hu J, Tian X, Wei T, Wu H, Lu J, Lyu M, Wang S. Anti-Helicobacter pylori Activity of a Lactobacillus sp. PW-7 Exopolysaccharide. Foods. 2021; 10(10):2453. https://doi.org/10.3390/foods10102453
Chicago/Turabian StyleHu, Jingfei, Xueqing Tian, Tong Wei, Hangjie Wu, Jing Lu, Mingsheng Lyu, and Shujun Wang. 2021. "Anti-Helicobacter pylori Activity of a Lactobacillus sp. PW-7 Exopolysaccharide" Foods 10, no. 10: 2453. https://doi.org/10.3390/foods10102453
APA StyleHu, J., Tian, X., Wei, T., Wu, H., Lu, J., Lyu, M., & Wang, S. (2021). Anti-Helicobacter pylori Activity of a Lactobacillus sp. PW-7 Exopolysaccharide. Foods, 10(10), 2453. https://doi.org/10.3390/foods10102453