The Use of Selenium Yeast and Phytobiotic in Improving the Quality of Broiler Chicken Meat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals, Diets and Housing
2.2. Sample Collection and Muscles Analysis
2.3. Statistical Analysis
- -
- pH, TBA: analysis of diatomic variance, where differences between groups were assessed using the Tukey test;
- -
- WHC and selenium content in muscles results by analysis of univariate variability and the significance of differences between groups using the Tukey test.
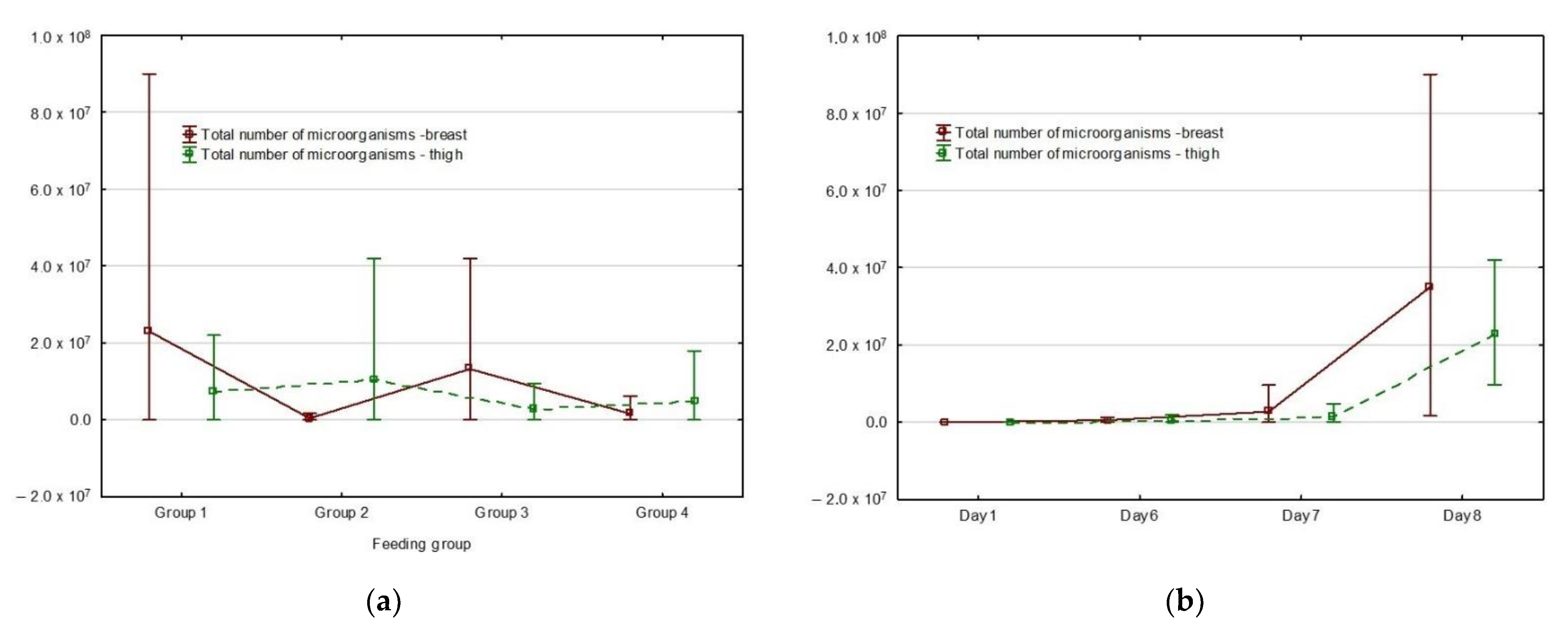
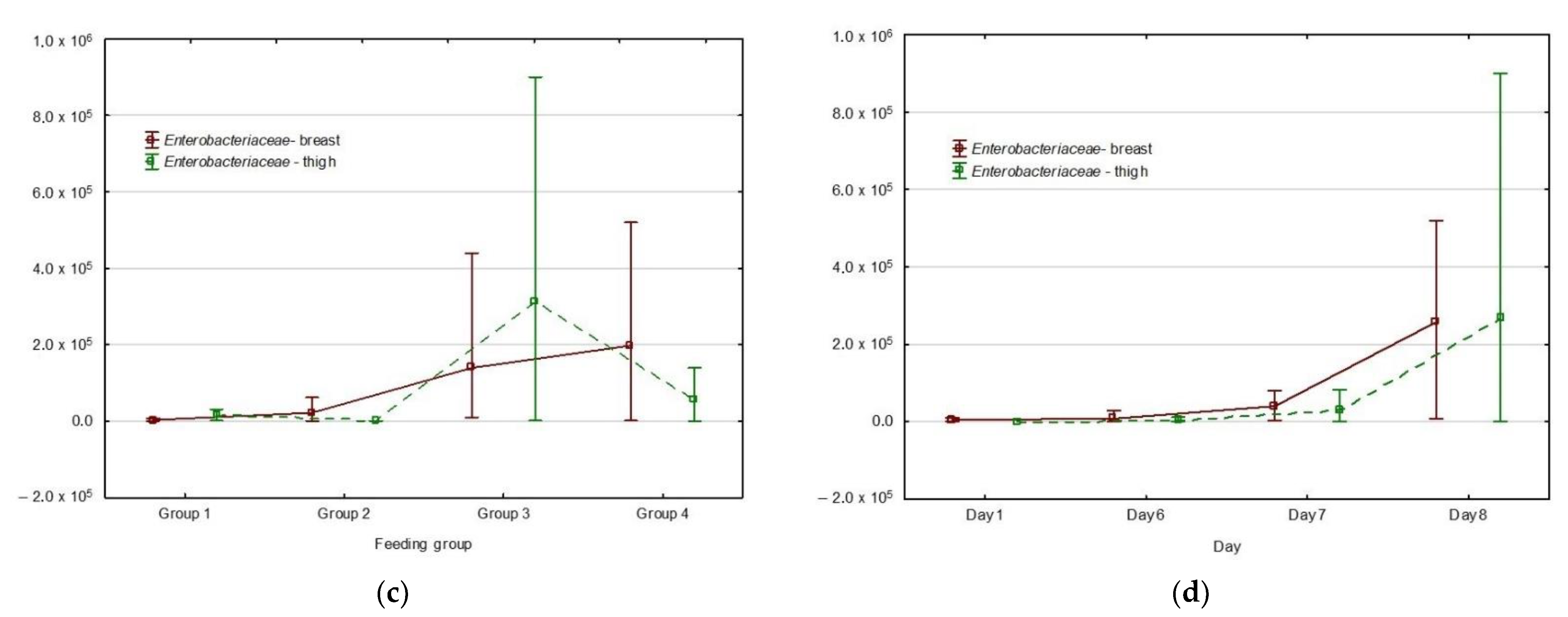
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Surai, P.F.; Fisinin, V.I. Selenium in poultry breeder nutrition: An update. Anim. Feed Sci. Technol. 2014, 191, 1–15. [Google Scholar] [CrossRef]
- Hariharan, S.; Dharmaraj, S. Selenium and selenoproteins: It’s role in regulation of inflammation. Inflammopharmacology 2020, 28, 667–695. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, H.M.; Cao, W.; Li, Y.B. Effect of selenium supplementation on pigeon reproductive performance, selenium concentration and antioxidant status. Poult. Sci. 2017, 96, 3407–3413. [Google Scholar] [CrossRef] [PubMed]
- Emamverdi, M.; Zare-Shahneh, A.; Zhandi, M.; Zaghari, M.; Minai-Tehrani, D.; Khodaei-Motlagh, M. An improvement in productive and reproductive performance of aged broiler breeder hens by dietary supplementation of organic selenium. Theriogenology 2018, 126, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Xing, L.; Bao, J.; Sun, H.; Bi, Y.; Liu, H.; Li, J. Selenium supplementation can protect from enhanced risk of keel bone damage in laying hens exposed to cadmium. RSC Adv. 2017, 7, 7170–7178. [Google Scholar] [CrossRef] [Green Version]
- Marković, R.; Ćirić, J.; Starčević, M.; Šefer, D.; Baltić, M. Effect of selenium source and level in diet on glutathione peroxidase activity, tissue selenium distribution, and growth performance in poultry. Anim. Health Res. Rev. 2018, 19, 166–176. [Google Scholar] [CrossRef]
- Wang, Y.X.; Zhan, X.A.; Yuan, D.; Zhang, X.W.; Wu, R.J. Effects of selenomethionine and sodium selenite supplementation on meat quality, selenium distribution and antioxidant status in broilers. Czech. J. Anim. Sci. 2011, 56, 305–313. [Google Scholar] [CrossRef] [Green Version]
- Marković, R.; Ćirić, J.; Drljačić, A.; Šefer, D.; Jovanović, I.; Jovanović, D.; Milanović, S.; Trbović, D.; Radulović, S.; Baltić, M.Ž.; et al. The effects of dietary Selenium-yeast level on glutathione peroxidase activity, tissue Selenium content, growth performance, and carcass and meat quality of broilers. Poult. Sci. 2018, 97, 2861–2870. [Google Scholar] [CrossRef]
- Mohapatra, P.; Swain, R.K.; Mishra, S.K.; Behera, T.; Swain, P.; Mishra, S.S.; Behura, N.C.; Sabat, S.C.; Sethy, K.; Dhama, K.; et al. Effects of dietary nano-Se on tissue Se deposition antioxidant status and immune functions in layer chicks. Int. J. Pharmacol. 2014, 10, 160–167. [Google Scholar] [CrossRef]
- Shi, L.; Xun, W.; Yue, W.; Zhang, C.; Ren, Y.; Shi, L.; Wang, Q.; Yang, R.; Lei, F. Effect of sodium selenite, Se-yeast, and nano-elemental selenium on growth performance, Se concentration and antioxidants status in growing male goats. Small Rumin. Res. 2011, 96, 49–52. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Xu, T. Elemental selenium and nano size (nano-Se) as a potential chemopreventive agent with reduced risk of selenium toxicity: Comparison with Se-methylselenocysteine in mice. Toxicol. Sci. 2008, 101, 22–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhama, K.; Latheef, S.K.; Saminathan, M.; Abdul Samad, H.; Karthik, K.; Tiwari, R.; Khan, R.U.; Alagawany, M.; Farag, M.R.; Alam, G.M.; et al. Multiple beneficial applications and modes of action of herbs in poultry health and production—A review. Int. J. Pharmacol. 2015, 11, 152–176. [Google Scholar] [CrossRef] [Green Version]
- Alagawany, M.; El-Hack, M.A.; Farag, M.R.; Shaheen, H.M.; Abdel-Latif, M.A.; Nreldin, A.E.; Patra, A.K. The usefulness of oregano and its derivatives in poultry nutrition. World’s Poult. Sci. J. 2018, 74, 463–474. [Google Scholar] [CrossRef]
- Jamroz, D.; Wertelecki, T.; Houszka, M.; Kamel, C. Influence of diet type on the inclusion of plant origin active substances on morphological and histochemical characteristics of the stomach and jejunum walls in chicken. J. Anim. Physiol. Anim. Nutr. 2006, 90, 255–268. [Google Scholar] [CrossRef]
- Hall, J.A.; Van Saun, R.J.; Bobe, G.; Stewart, W.C.; Vorachek, V.R.; Mosher, W.D.; Nichols, T.; Forsberg, N.E.; Pirelli, G.J. Organic and inorganic selenium: I. Oral bioavailability in ewes. J. Anim. Sci. 2012, 90, 568–576. [Google Scholar] [CrossRef]
- Wang, Y.; Zhan, X.; Zhang, X.; Wu, R.; Yuan, D. Comparison of different forms of dietary selenium supplementation on growth performance, meat quality, selenium deposition, and antioxidant property in broilers. Biol. Trace Elem. Res. 2011, 143, 261–273. [Google Scholar] [CrossRef]
- Risdianto, D.; Suthama, N.; Suprijanta, E.; Sunarso, S. Inclusion effect of ginger and turmeric mixture combined with Lactobacillus spp. Isolated from rumen fluid of cattle on health status and growth of broiler. J. Indones. Trop. Anim. 2019, 44, 324–433. [Google Scholar] [CrossRef]
- Rychen, G.; Aquilina, G.; Azimonti, G.; Bampidis, V.; de Lourdes Bastos, M.; Bories, G.; Chesson, A.; Cocconcelli, P.S.; Flachowsky, G.; Gropp, J.; et al. Safety and efficacy of selenium-enriched yeast (Saccharomyces cerevisiae NCYC R397) for all animal species. EFSA J. 2016, 14, e04624. [Google Scholar]
- Smulikowska, S.; Rutkowski, A. Poultry Nutritional Standards; IFiŻZ PAN: Warsaw, Poland, 2005. [Google Scholar]
- AOAC. Official Method of Analysis, 20th ed.; Publisher: Gaithersburg, MD, USA, 2016. [Google Scholar]
- European Parliament. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. Book Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. 2010, pp. 33–78. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32010L0063 (accessed on 1 December 2020).
- Szmańko, T. Urządzenie do Pomiaru Zdolności Utrzymywania Wody. Polish Patent No. 40767; Polish Patent and Trademark Office: Warsaw, Poland, 1986. [Google Scholar]
- McDonald, R.E.; Hultin, H.O. Some characteristics of the enzymic lipid peroxidation system in the microsomal fraction of flounder skeletal muscle. J. Food Sci. 1987, 52, 15–21. [Google Scholar] [CrossRef]
- Díaz-Alarcón, J.P.; Navarro-Alarcón, M.; López-García de la Serrana, H.; López-Martínez, M.C. Determination of selenium in meat products by hydride generation atomic absorption spectrometry selenium levels in meat, organ meats, and sausages in Spain. J. Agri. Food Chem. 1996, 44, 1494–1497. [Google Scholar] [CrossRef]
- PN-EN ISO 4833-1:2013-12. Microbiology of the Food Chain. 2013. Available online: https://www.iso.org/standard/53728.html (accessed on 8 September 2021).
- PN ISO 21528-2:2005. Microbiology of Food and Animal Feeding Stuffs—Horizontal Methods for the Detection and Enumeration of Enterobacteriaceae—Part 2: Colony-Count Method. 2005. Available online: https://www.iso.org/standard/34566.html (accessed on 8 September 2021).
- PKN ISO/TS 10272-2:2008. Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Detection and Enumeration of Campylobacter spp.—Part 2: Colony-Count Method. 2008. Available online: https://www.iso.org/standard/37092.html (accessed on 8 September 2021).
- Berri, C.; Le, B.D.E.; Debut, M.; Santé-Lhoutellier, V.; Baéza, E.; Gigaud, V.; Jego, Y.; Duclos, M.J. Consequence of muscle hypertrophy on characteristics of pectoralis major muscle and breast meat quality of broiler chickens. J. Anim. Sci. 2007, 85, 2005–2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bardzardi, M.M.; Ghazanfari, S.; Salehi, A.; Sharidi, S.D. Effect of dietary myrtle essential oil on iron-induced lipid oxidation of breast, thigh and abdominal fat muscles and serum biochemical parameters in broiler chickens. Eur. Poult. Sci. 2014, 78, 1–11. [Google Scholar]
- Li, J.L.; Zhang, L.; Yang, Z.Y.; Zhang, Z.Y.; Jiang, Y.; Gao, F.; Zhou, G.H. Effects of different selenium sources on growth performance, antioxidant capacity and meat quality of local chinese subei chickens. Biol. Trace Elem. Res. 2018, 181, 340–346. [Google Scholar] [CrossRef]
- Mohammadi, A.; Ghazanfari, S.; Sharifi, S.D. Comparative effects of dietary organic, inorganic, and Nano-selenium complexes and rosemary essential oil on performance, meat quality and selenium deposition in muscles of broiler chickens. Livest. Sci. 2019, 226, 21–30. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, C.; Zheng, W.; Teng, X.; Li, S. The functions of antioxidants and heat shock proteins are altered in the immune organs of selenium-deficient in broiler chickens. Biol. Trace Elem. Res. 2017, 169, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Rozbicka-Wieczorek, A.J.; Szarpak, E.; Brzóska, F.; Śliwiński, B.; Kowalczyk, J.; Czauderna, M. Dietary lycopenes, selenium compounds and fish oil affect the profile of fatty acids and axidative stress in chicken breast muscle. J. Anim. Feed Sci. 2012, 21, 705–724. [Google Scholar] [CrossRef]
- Zhan, X.A.; Wang, H.F.; Yuan, D.; Wang, Y.X.; Zhu, F. Comparison of different forms of dietary selenium supplementation on gene expression of cytoplasmic thioredoxin reductase, selenoprotein P, and selenoprotein W in broilers. Czech. J. Anim. Sci. 2014, 59, 571–578. [Google Scholar] [CrossRef] [Green Version]
- Chantiratikul, A.; Pakmaruek, P.; Chinrasri, O.; Aengwanich, W.; Chookhampaeng, S.; Maneetong, S.; Chantiratikul, P. Efficacy of selenium from hydroponically produced selenium-enriched kale sprout (Brassica oleracea var. alboglabra L.) in broilers. Biol. Trace Elem. Res. 2015, 165, 96–102. [Google Scholar] [CrossRef]
- Bakhshalinejad, R.; Hassanabadi, A.; Swick, R.A. Dietary sources and levels of selenium supplements affect growth, performance, carcass yield, meat quality and tissue selenium deposition in broilers. Anim. Nutr. 2019, 5, 256–263. [Google Scholar] [CrossRef]
- Youssef, S.F.; Selim, N.A.; Abdel-Salam, A.F.; Nada, S.A. Evaluations of some natural antioxidants sources in broiler diets: 3-effect of different ginger extract forms and levels on broiler performance, immune response and quality of chilled and frozen meat. Egypt. Poult. Sci. J. 2016, 36, 299–317. [Google Scholar]
- Kamboh, A.A.; Memon, A.M.; Mughal, M.J.; Memon, J.; Bakhetgul, M. Dietary effects of soy and citrus flavonoid on antioxidation and microbial quality of meat in broilers. J. Anim. Physiol. Anim. Nutr. 2018, 102, 235–240. [Google Scholar] [CrossRef] [PubMed]
| Starter | Grower | ||
|---|---|---|---|
| Component | Content (%) | Component | Content (%) |
| Wheat | 44.681 | Wheat below 11% TP | 42.948 |
| Soybean meal | 22.560 | Soybean meal | 21.860 |
| Maize | 20 | Maize | 15 |
| Fishmeal total protein | 0.500 | Wheat full of grain | 5 |
| Soybean oil | 1.380 | Soybean oil | 1.5 |
| Rapeseed oilcake | 4 | Rapeseed oilcake | 6.6 |
| Calcium phosphate | 0.850 | Calcium phosphate | 0.4 |
| Feed salt | 0.204 | Feed salt | 0.2 |
| Acidic calcium carbonate | 0.096 | Acidic calcium carbonate | 0.174 |
| Choline chloride 60% | 0.100 | Choline chloride 60% | 0.1 |
| Sacox 210 | 0.053 | Sacox 210 | 0.053 |
| L-lysine HCl | 0.174 | L-lysine HCl | 0.156 |
| L-threonine | 0.126 | L-threonine | 0.132 |
| RHODIMET AT88 | 0.336 | RHODIMET AT88 | 0.312 |
| Fodder meal | 0.780 | Fodder meal | 0.74 |
| Sodium sulfate | 0.100 | Lysine sulfate (55% L-lysine) | 0.4 |
| Lysine sulfate (55% L-lysine) | 0.300 | Pork fat | 2.5 |
| Hemoglobin-pork blood product | 2.060 | Medium-chain fatty acids | 1.6 |
| Medium-chain fatty acids | 1.300 | Rovabio Excel LC2 | 0.01 |
| Rovabio Excel LC2 | 0.010 | Quantum Blue 5L | 0.02 |
| Quantum Blue 5L | 0.020 | Max-Vit 0.25% Prestige * | 0.295 |
| Max-Vit 0.25% Prestige * | 0.370 | ||
| Group | Dry Matter (%) | Ash (%) | Crude Protein (%) | Crude Fiber (%) | Crude Fat (%) | Selenium (mg/kg) | Metabolic Energy (kcal) | |
|---|---|---|---|---|---|---|---|---|
| G1 | Starter | 89.90 | 4.38 | 20.85 | 2.68 | 3.49 | 0.102 | 3000.78 |
| G2 | 90.26 | 4.57 | 19.75 | 3.36 | 4.80 | 0.375 | 3000.02 | |
| G3 | 91.20 | 4.20 | 20.90 | 2.85 | 4.17 | 0.357 | 2999.85 | |
| G4 | 91.03 | 4.47 | 21.42 | 2.75 | 4.45 | 0.282 | 2999.48 | |
| G1 | Grower | 88.86 | 3.92 | 18.47 | 3.67 | 7.55 | 0.140 | 3148.75 |
| G2 | 88.78 | 3.99 | 19.01 | 3.41 | 7.58 | 0.327 | 3148.91 | |
| G3 | 89.09 | 3.79 | 18.37 | 3.68 | 7.23 | 0.363 | 3149.01 | |
| G4 | 89.96 | 3.82 | 18.09 | 3.53 | 7.74 | 0.425 | 3149.32 |
| Group of Active Compounds | Minimum Content in 1 kg of Preparation |
|---|---|
| Glucosinolates | 2600 mg |
| Curcuminoids | 1900 mg |
| Essential oils | 14,950 mg |
| Phenols and polyphenols | 4950 mg |
| Description | Breast Muscles | SEM | p-Value | Thigh Muscles | SEM | p-Value | ||
|---|---|---|---|---|---|---|---|---|
| Group | G1 | 6.19 ab | 0.011 | <0.050 | 6.33 a | 0.020 | <0.050 | |
| G2 | 6.22 b | 0.010 | 6.33 a | 0.021 | ||||
| G3 | 6.16 a | 0.011 | 6.27 b | 0.011 | ||||
| G4 | 6.19 ab | 0.001 | 6.31 ab | 0.010 | ||||
| Day | Day 1 | 6.16 a | 0.012 | <0.050 | 6.28 a | 0.012 | <0.050 | |
| Day 5 | 6.18 a | 0.011 | 6.28 a | 0.010 | ||||
| Day 7 | 6.23 b | 0.013 | 6.33 b | 0.011 | ||||
| Interaction | G1 | Day 1 | 6.17 | 0.020 | 0.470 | 6.30 | 0.031 | 0.810 |
| G1 | Day 5 | 6.19 | 0.022 | 6.31 | 0.030 | |||
| G1 | Day 7 | 6.22 | 0.021 | 6.37 | 0.021 | |||
| G2 | Day 1 | 6.18 | 0.031 | 6.31 | 0.032 | |||
| G2 | Day 5 | 6.20 | 0.020 | 6.31 | 0.030 | |||
| G2 | Day 7 | 6.28 | 0.012 | 6.37 | 0.021 | |||
| G3 | Day 1 | 6.14 | 0.021 | 6.26 | 0.020 | |||
| G3 | Day 5 | 6.15 | 0.021 | 6.26 | 0.023 | |||
| G3 | Day 7 | 6.18 | 0.022 | 6.28 | 0.020 | |||
| G4 | Day 1 | 6.18 | 0.010 | 6.30 | 0.021 | |||
| G4 | Day 5 | 6.19 | 0.010 | 6.31 | 0.022 | |||
| G4 | Day 7 | 6.21 | 0.001 | 6.32 | 0.020 | |||
| Description | Breast Muscles | SEM | p-Value | Thigh Muscles | SEM | p-Value | ||
|---|---|---|---|---|---|---|---|---|
| Group | G1 | 3.00 ab | 0.151 | <0.050 | 3.28 a | 0.192 | <0.050 | |
| G2 | 2.87 a | 0.140 | 3.23 a | 0.163 | ||||
| G3 | 2.83 a | 0.122 | 3.37 a | 0.181 | ||||
| G4 | 3.17 b | 0.101 | 2.75 b | 0.120 | ||||
| Day | Day 1 | 2.04 a | 0.040 | <0.050 | 1.84 a | 0.040 | <0.050 | |
| Day 5 | 3.05 b | 0.052 | 3.59 b | 0.062 | ||||
| Day 7 | 3.88 c | 0.051 | 3.77 c | 0.061 | ||||
| Interaction | G1 | Day 1 | 1.89 a | 0.081 | <0.050 | 1.71 a | 0.060 | <0.050 |
| G1 | Day 5 | 2.97 bcd | 0.143 | 4.12 def | 0.173 | |||
| G1 | Day 7 | 4.16 g | 0.092 | 4.02 ef | 0.181 | |||
| G2 | Day 1 | 1.92 a | 0.060 | 1.90 a | 0.122 | |||
| G2 | Day 5 | 2.65 bc | 0.071 | 3.92 cdef | 0.130 | |||
| G2 | Day 7 | 4.04 g | 0.081 | 3.86 cdef | 0.171 | |||
| G3 | Day 1 | 1.86 a | 0.090 | 1.85 a | 0.070 | |||
| G3 | Day 5 | 3.25 def | 0.113 | 4.01 def | 0.183 | |||
| G3 | Day 7 | 3.38 f | 0.121 | 4.25 f | 0.141 | |||
| G4 | Day 1 | 2.47 a | 0.062 | 1.87 a | 0.150 | |||
| G4 | Day 5 | 3.30 b | 0.110 | 3.10 b | 0.091 | |||
| G4 | Day 7 | 3.76 bc | 0.121 | 3.28 bc | 0.172 | |||
| Group | Selenium Content (μg/kg) | Water-Holding Capacity (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Thigh Muscles | Breast Muscles | Breast | SEM | p-Value | Thigh | SEM | p-Value | |
| G1 | 248 A | 360 A | 57.6 | 0.06 | 0.100 | 57.6 | 0.07 | 0.100 |
| G2 | 461 B | 406 A | 57.4 | 0.07 | 57.5 | 0.06 | ||
| G3 | 896 C | 708 B | 57.8 | 0.06 | 57.4 | 0.05 | ||
| G4 | 763 D | 733 C | 57.7 | 0.05 | 57.4 | 0.06 | ||
| SEM | 36.8 | 27.5 | ||||||
| p-Value | 0.001 | 0.001 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konkol, D.; Korzeniowska, M.; Różański, H.; Górniak, W.; Andrys, M.; Opaliński, S.; Popiela, E.; Korczyński, M. The Use of Selenium Yeast and Phytobiotic in Improving the Quality of Broiler Chicken Meat. Foods 2021, 10, 2558. https://doi.org/10.3390/foods10112558
Konkol D, Korzeniowska M, Różański H, Górniak W, Andrys M, Opaliński S, Popiela E, Korczyński M. The Use of Selenium Yeast and Phytobiotic in Improving the Quality of Broiler Chicken Meat. Foods. 2021; 10(11):2558. https://doi.org/10.3390/foods10112558
Chicago/Turabian StyleKonkol, Damian, Małgorzata Korzeniowska, Henryk Różański, Wanda Górniak, Marita Andrys, Sebastian Opaliński, Ewa Popiela, and Mariusz Korczyński. 2021. "The Use of Selenium Yeast and Phytobiotic in Improving the Quality of Broiler Chicken Meat" Foods 10, no. 11: 2558. https://doi.org/10.3390/foods10112558
APA StyleKonkol, D., Korzeniowska, M., Różański, H., Górniak, W., Andrys, M., Opaliński, S., Popiela, E., & Korczyński, M. (2021). The Use of Selenium Yeast and Phytobiotic in Improving the Quality of Broiler Chicken Meat. Foods, 10(11), 2558. https://doi.org/10.3390/foods10112558








