Contribution of Green Propolis to the Antioxidant, Physical, and Sensory Properties of Fruity Jelly Candies Made with Sugars or Fructans
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Propolis Ethanolic Extract
2.3. Jelly Candy Manufacturing
2.4. Sample Extraction for Polyphenol Quantification and Antioxidant Assays
2.5. HPLC Analysis
2.6. Total Phenolic Content (TPC)
2.7. 2.2’-. Azinobis-3-ethylbenzothiazoline-6-sulfonic (ABTS•) Radical Cation Decolouration Assay
2.8. 2,2-. Diphenyl-1 Picrylhydrazyl (DPPH•) Radical-Scavenging Activity
2.9. Physical Assessment
2.10. Sensory Triangle Test with Consumers
3. Results
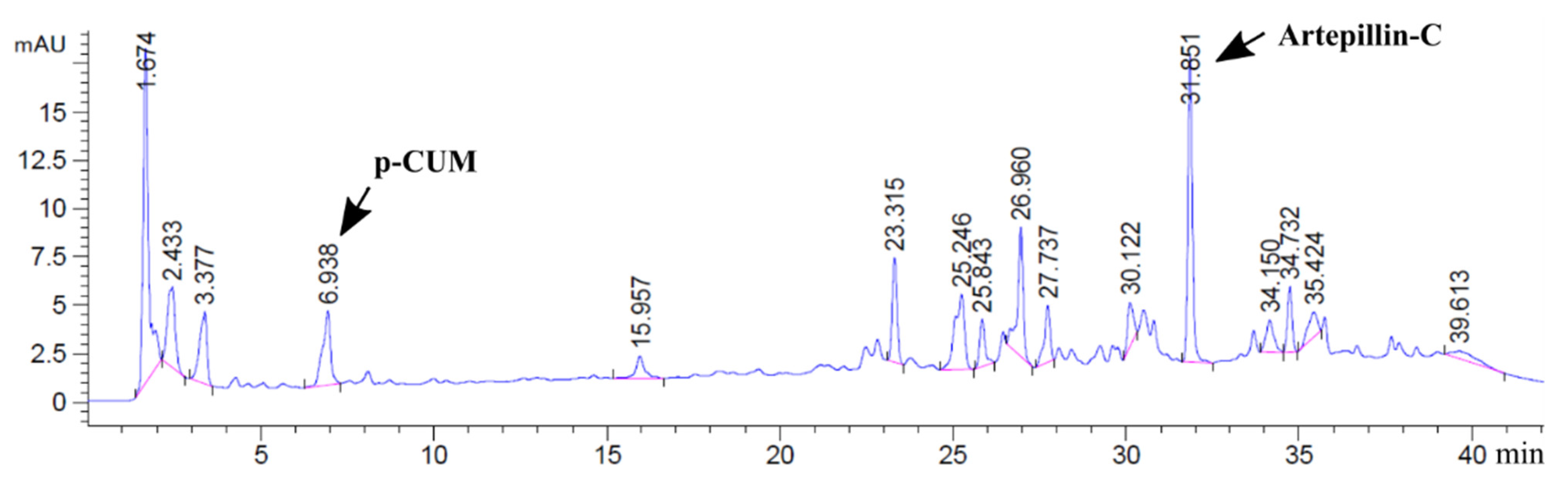
3.1. Quantitative Polyphenol Profile of PEE and Jelly Candies
3.2. Effects of Propolis on the Antioxidant Capacity of Jelly Candies
3.3. Effects of Propolis on the Physical Properties of Jelly Candies
3.4. Identification of Propolis Off-Flavour in Jelly Candies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Delgado, P.; Bañón, S. Effects of replacing starch by inulin on the physicochemical, texture and sensory characteristics of gummy jellies. CyTA—J. Food 2018, 16, 1–10. [Google Scholar] [CrossRef]
- Cedeño-Pinos, C.; Martínez-Tomé, M.; Murcia, M.A.; Jordán, M.J.; Bañón, S. Assessment of Rosemary (Rosmarinus officinalis L.) Extract as Antioxidant in Jelly Candies Made with Fructan Fibres and Stevia. Antioxidants 2020, 9, 1289. [Google Scholar] [CrossRef]
- Yan, B.; Davachi, S.M.; Ravanfar, R.; Dadmohammadi, Y.; Deisenroth, T.W.; Van Pho, T.; Odorisio, P.A.; Darji, R.H.; Abbaspourrad, A. Improvement of vitamin C stability in vitamin gummies by encapsulation in casein gel. Food Hydrocoll. 2020, 113, 106414. [Google Scholar] [CrossRef]
- Rivero, R.; Archaina, D.; Sosa, N.; Schebor, C. Development and characterization of two gelatin candies with alternative sweeteners and fruit bioactive compounds. LWT 2021, 141, 110894. [Google Scholar] [CrossRef]
- Cano-Lamadrid, M.; Calín-Sánchez, Á.; Clemente-Villalba, J.; Hernández, F.; Carbonell-Barrachina, Á.A.; Sendra, E.; Wojdyło, A. Quality parameters and consumer acceptance of jelly candies based on pomegranate juice “mollar de elche”. Foods 2020, 9, 516. [Google Scholar] [CrossRef]
- da Silva, L.B.; Annetta, F.E.; Alves, A.B.; Queiroz, M.B.; Fadini, A.L.; da Silva, M.G.; Efraim, P. Effect of differently processed açai (Euterpe oleracea Mart.) on the retention of phenolics and anthocyanins in chewy candies. Int. J. Food Sci. Technol. 2016, 51, 2603–2612. [Google Scholar] [CrossRef]
- Yenrina, R.; Sayuti, K.; Putri, R.A. Antioxidant activity and bioactivity (LC50) of soursop leaves jelly candy with addition of soursop fruit extract (Annona muricata L.). Pak. J. Nutr. 2015, 14, 259–262. [Google Scholar] [CrossRef][Green Version]
- Vergara, L.P.; Reissig, G.N.; Franzon, R.C.; Carvalho, I.R.; Zambiazi, R.C.; Rodrigues, R.S.; Chim, J.F. Stability of bioactive compounds in conventional and low-calorie sweet chewable candies prepared with red and yellow strawberry guava pulps. Int. Food Res. J. 2020, 27, 625–634. [Google Scholar]
- Mazur, L.; Gubsky, S.; Dorohovych, A.; Labazov, M. Antioxidant properties of candy caramel with plant extracts. Ukr. Food J. 2018, 7, 7–21. [Google Scholar] [CrossRef]
- Mandura, A.; Šeremet, D.; Ščetar, M.; Vojvodić Cebin, A.; Belščak-Cvitanović, A.; Komes, D. Physico-chemical, bioactive, and sensory assessment of white tea-based candies during 4-months storage. J. Food Process. Preserv. 2020, 44, e14628. [Google Scholar] [CrossRef]
- Kim, I.; Yang, M.; Cho, K.K.; Goo, Y.M.; Kim, T.W.; Park, J.H.; Cho, J.H.; Jo, C.; Lee, M.; Lee, O.H.; et al. Effect of medicinal plant extracts on the physicochemical properties and sensory characteristics of gelatin jelly. J. Food Process. Preserv. 2013, 38, 1527–1533. [Google Scholar] [CrossRef]
- Rodríguez-Zevallos, A.; Hayayumi-Valdivia, M.; Siche, R. Optimización de polifenoles y aceptabilidad de caramelos de goma con extracto de jengibre (Zingiber officinale R.) y miel con diseño de mezclas. Braz. J. Food Technol. 2018, 21. [Google Scholar] [CrossRef][Green Version]
- Sukandar, D.; Radiastuti, N.; Muawanah, A.; Hudaya, A. Antioxidant Activity From Water Extract Of Kecombrang Flower (Etlingera elatior) Leading To Jelly Candy Formulation. J. Kim. Val. 2011, 2, 393–398. [Google Scholar] [CrossRef]
- Charoen, R.; Savedboworn, W.; Phuditcharnchnakun, S.; Khuntaweetap, T. Development of Antioxidant Gummy Jelly Candy Supplemented with Psidium guajava Leaf Extract. KMUTNB Int. J. Appl. Sci. Technol. 2015, 8, 145–151. [Google Scholar] [CrossRef]
- Moise, A.R.; Bobiş, O. Baccharis dracunculifolia and dalbergia ecastophyllum, main plant sources for bioactive properties in green and red brazilian propolis. Plants 2020, 9, 1619. [Google Scholar] [CrossRef]
- Pasupuleti, V.R.; Sammugam, L.; Ramesh, N.; Gan, S.H. Honey, Propolis, and Royal Jelly: A Comprehensive Review of Their Biological Actions and Health Benefits. Oxid. Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef]
- Fan, Y.; Yi, J.; Hua, X.; Zhang, Y.; Yang, R. Preparation and characterization of gellan gum microspheres containing a cold-adapted β-galactosidase from Rahnella sp. R3. Carbohydr. Polym. 2017, 162, 10–15. [Google Scholar] [CrossRef]
- Ccana-Ccapatinta, G.V.; Mejía, J.A.A.; Tanimoto, M.H.; Groppo, M.; de Carvalho, J.C.A.S.; Bastos, J.K. Dalbergia ecastaphyllum (L.) Taub. and Symphonia globulifera L.f.: The Botanical Sources of Isoflavonoids and Benzophenones in Brazilian Red Propolis. Molecules 2020, 25, 2060. [Google Scholar] [CrossRef]
- Berretta, A.A.; Silveira, M.A.D.; Cóndor Capcha, J.M.; De Jong, D. Propolis and its potential against SARS-CoV-2 infection mechanisms and COVID-19 disease: Running title: Propolis against SARS-CoV-2 infection and COVID-19. Biomed. Pharmacother. 2020, 131, 110622. [Google Scholar] [CrossRef]
- Wang, K.; Hu, L.; Jin, X.L.; Ma, Q.X.; Marcucci, M.C.; Netto, A.A.L.; Sawaya, A.C.H.F.; Huang, S.; Ren, W.K.; Conlon, M.A.; et al. Polyphenol-rich propolis extracts from China and Brazil exert anti-inflammatory effects by modulating ubiquitination of TRAF6 during the activation of NF-κB. J. Funct. Foods 2015, 19, 464–478. [Google Scholar] [CrossRef]
- Directive. (EC), No 46/2002. Of the European Parliament and of the Council, of 10 June 2002, on the Approximation of the Laws of the Member States Relating to Food Supplements. Available online: https://eur-lex.erupa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32002L0046 (accessed on 3 January 2019).
- Brasilia, I.N.N. Ministério da Agricultura e do Abastecimento, Secretaria de Defesa agropecuária. 2001. Available online: https://www.defesa.agricultura.sp.gov.br/legislacoes/instrucao-normativa-n-30-de-26-de-junho-de-2001,1039.html (accessed on 21 March 2021).
- Burdock, G.A. Review of the biological properties and toxicity of bee propolis (propolis). Food Chem. Toxicol. 1998, 36, 347–363. [Google Scholar] [CrossRef]
- Berretta, A.A.; Arruda, C.; Galeti, F.; Baptista, N.; Piacezzi, A.; Marquele-Oliveira, F.; Issa, J.; da Silva, H.; Damasco, B.; Ramos, C.; et al. Functional properties of Brazilian propolis: From chemical composition until the market. In Superfood and Functional Food—Na Overview of Their Processing and Utilization; Shiomi, V.W.N., Ed.; IntechOpen: London, UK, 2017; pp. 55–93. ISBN 978-953-51-29-20-2. [Google Scholar]
- Galeotti, F.; Maccari, F.; Fachini, A.; Volpi, N. Chemical Composition and Antioxidant Activity of Propolis Prepared in Different Forms and in Different Solvents Useful for Finished Products. Foods 2018, 7, 41. [Google Scholar] [CrossRef]
- Regulation. (CE), N°1924/2006. Scientific Opinion on the Substantiation of Health Claims Related to Propolis (ID 1242, 1245, 1246, 1247, 1248, 3184) and Flavonoids in Propolis (ID 1244, 1644, 1645, 3526, 3527, 3798, 3799). Available online: https://www.efsa.europa.eu/es/efsajuornal/pub/181c (accessed on 10 February 2019).
- Seibert, J.B.; Bautista-Silva, J.P.; Amparo, T.R.; Petit, A.; Pervier, P.; dos Santos Almeida, J.C.; Azevedo, M.C.; Silveira, B.M.; Brandão, G.C.; de Souza, G.H.B.; et al. Development of propolis nanoemulsion with antioxidant and antimicrobial activity for use as a potential natural preservative. Food Chem. 2019, 287, 61–67. [Google Scholar] [CrossRef]
- Spinelli, S.; Conte, A.; Lecce, L.; Incoronato, A.L.; Del Nobile, M.A. Microencapsulated Propolis to Enhance the Antioxidant Properties of Fresh Fish Burgers. J. Food Process Eng. 2015, 38, 527–535. [Google Scholar] [CrossRef]
- Ezazi, A.; Javadi, A.; Jafarizadeh-Malmiri, H.; Mirzaei, H. Development of a chitosan-propolis extract edible coating formulation based on physico-chemical attributes of hens’ eggs: Optimization and characteristics edible coating of egg using chitosan and propolis. Food Biosci. 2021, 40. [Google Scholar] [CrossRef]
- Rivero, R.; Archaina, D.; Sosa, N.; Leiva, G.; Baldi Coronel, B.; Schebor, C. Development of healthy gummy jellies containing honey and propolis. J. Sci. Food Agric. 2020, 100, 1030–1037. [Google Scholar] [CrossRef]
- Osés, S.M.; Pascual-Maté, A.; Fernández-Muiño, M.A.; López-Díaz, T.M.; Sancho, M.T. Bioactive properties of honey with propolis. Food Chem. 2016, 196, 1215–1223. [Google Scholar] [CrossRef]
- Delgado, P.; Bañón, S. Determining the minimum drying time of gummy confections based on their mechanical properties. CyTA—J. Food 2015, 13, 329–335. [Google Scholar] [CrossRef]
- Veiga, R.S.; Mendonça, S.; Mendes, P.B.; Paulino, N.; Mimica, M.J.; Lagareiro Netto, A.A. Artepillin C and phenolic compounds responsible for antimicrobial and antioxidant activity of green propolis and Baccharis dracunculifolia DC. J. Appl. Microbiol. 2017, 122, 911–920. [Google Scholar] [CrossRef]
- Dias, L.G.; Pereira, A.P.; Estevinho, L.M. Comparative study of different Portuguese samples of propolis: Pollinic, sensorial, physicochemical, microbiological characterization and antibacterial activity. Food Chem. Toxicol. 2012, 50, 4246–4253. [Google Scholar] [CrossRef]
- Marcucci, M.C.; Sawaya, A.C.H.F.; Custodio, A.R.; Paulino, N.; Eberlin, M.N. HPLC and ESI-MS typification: New approaches for natural therapy with Brazilian propolis. In Scientific Evidence of the Use of Propolis in Ethnomedicine; Basic, N.O.I., Ed.; Transworld Research Network: Trivandrum, India, 2008; pp. 33–54. ISBN 978-81-7895-357-1. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Association of Official Agricultural Chemists. Official methods of analysis of AOAC International, 17th ed.; AOAC: Gainthersburg, MD, USA, 2000. [Google Scholar]
- International Standards Organization-ISO Sensory Analysis-Describes a Procedure for Determining Whether a Perceptible Sensory Difference or Similarity Exists between Samples of Two Products.ISO 4120. Geneva, Switzerland: The International Organization for Standardization. 2004. Available online: https://www.iso.org./standard/33495.html (accessed on 15 March 2019).
- Pereira Beserra, F.; Gushiken, L.F.S.; Hussni, M.F.; Ribeiro, V.P.; Bonamin, F.; Jackson, C.J.; Pellizzon, C.H.; Bastos, J.K. Artepillin C as an outstanding phenolic compound of Brazilian green propolis for disease treatment: A review on pharmacological aspects. Phyther. Res. 2020, 35, 2274–2286. [Google Scholar] [CrossRef]
- Marcucci, M.C.; Cunha, I.B.S.; Sanchez, E.M.S.; Passarelli-Gonçalves, C.; Cedeño-Pinos, C.; Bañón, S. Thermal Analysis of Brazilian propolis. Characteristics of Crude Resin, Ethanolic Extracts and Wax Isolated Compounds. Z Naturforsch C Biosci. 2021. submitted. [Google Scholar]
- Dikshit, R.; Tallapragada, P. Comparative study of natural and artificial flavoring agents and dyes; Elsevier Inc.: Amsterdam, The Netherlands, 2018; Volume 7, ISBN 9780128115183. [Google Scholar]
- Bassam, M.E.A.; Bassam, E.A.; Ali, M.F. Handbook of Industrial Chemistry: Organic Chemicals; McGraw-Hill: New York, NY, USA, 2005; ISBN 978-0-07-141037-3. [Google Scholar]
- Peshev, D.; Vergauwen, R.; Moglia, A.; Hideg, É.; Van Den Ende, W. Towards understanding vacuolar antioxidant mechanisms: A role for fructans? J. Exp. Bot. 2013, 64, 1025–1038. [Google Scholar] [CrossRef]
- Wölwer-Rieck, U. The leaves of Stevia rebaudiana (Bertoni), their constituents and the analyses thereof: A review. J. Agric. Food Chem. 2012, 60, 886–895. [Google Scholar] [CrossRef]
- da Silva, L.B.; Queiroz, M.B.; Fadini, A.L.; Fonseca, R.C.C.; Germer, S.P.M.; Efraim, P. Chewy candy as a model system to study the influence of polyols and fruit pulp (açai) on texture and sensorial properties. LWT-Food Sci. Technol. 2016, 65, 268–274. [Google Scholar] [CrossRef]
- Kim, H.; Cadwallader, K.R.; Kido, H.; Watanabe, Y. Effect of addition of commercial rosemary extracts on potent odorants in cooked beef. Meat Sci. 2013, 94, 170–176. [Google Scholar] [CrossRef]
- Wu, J.; Chiu, S.C.; Pearce, E.M.; Kwei, T.K. Effects of phenolic compounds on gelation behavior of gelatin gels. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 224–231. [Google Scholar] [CrossRef]
| Ingredients | S-Candies | F-Candies | All Candies |
|---|---|---|---|
| Sucrose | 21 | ||
| Glucose syrup 79 °B | 38.33 | ||
| Acidified thinned corn starch | 10 | ||
| Fructooligosaccharides syrup 72 °B | 70.58 | ||
| Inulin | 11.44 | ||
| Water * | 23.72 | 10.89 | |
| Stevia rebaudiana | 14 | ||
| Pork gelatine type “A” | 4.1 | ||
| Citric acid | 0.9 | ||
| Lactic acid | 0.6 | ||
| Sodium citrate | 0.6 | ||
| Flavour (menthe, orange, or strawberry) | 0.3 | ||
| Blue dye (Idacol) ** | 0.0175 | ||
| Orange dye (curcumin + carminic acid at 13:3 w/w) ** | 0.0259 | ||
| Red dye (carminic acid) ** | 0.0375 | ||
| Propolis ethanolic dry extract (PEE) | 0, 0.01 or 0.02 |
| Compounds | Formula | Theoretical m/z | Experimental m/z | Error | (% w/w) |
|---|---|---|---|---|---|
| Monocaffeoylquinic acid (1) | C16 H18 O9 | 353.0878 | 353.0878 | 0.02 | 0.08 |
| Caffeic acid | C9 H8 O4 | 179.0350 | 179.0355 | 2.89 | 0.11 |
| p-Coumaric acid (P-CUM) | C9 H8 O3 | 163.0401 | 163.0406 | 3.27 | 1.54 |
| 3,5-di-O – Caffeoylquinic acid (1) | C25 H24 O12 | 515.1195 | 515.1202 | 1.36 | 0.17 |
| 3,4-di-O – Caffeoylquinic acid (1) | C25 H24 O12 | 515.1195 | 515.1206 | 2.14 | 0.02 |
| Methyl-3,4-di-O – Caffeoylquinic acid (1), | C26 H26 O12 | 529.1351 | 529.1350 | 0.28 | 0.30 |
| Methyl-4,5-di-O – Caffeoylquinic acid (1) | C26 H26 O12 | 529.1351 | 529.1346 | 1.04 | 0.09 |
| 3-Prenyl-4-hydroxy cinnamic acid (2) | C14 H16 O3 | 231.1027 | 231.1035 | 3.60 | 1.52 |
| 2,2-dimethyl-2H-1-benzopyran-6-propenoic acid | C14 H14 O3 | 229.0870 | 229.0871 | 0.36 | |
| 4-Hydroxy-3(E)-(4-hydroxy-3-methyl-2-butenyl)-5-prenyl cinnamic acid (2) | C19 H24 O4 | 315.1602 | 315.1610 | 2.59 | 0.19 |
| Kaempferide | C16 H12 O6 | 299.0561 | 299.0569 | 2.64 | |
| 3-Prenyl-4-(2methylproprionyloxy)- cinnamic acid (2) | C18 H22 O4 | 301.1445 | 301.1449 | 1.22 | 0.15 |
| 3-Hydroxy-2,2-dimethyl-8-prenyl-2H-1-benzopyran-6-propenoic acid (2) | C19 H24 O4 | 315.1602 | 315.1610 | 2.59 | 0.01 |
| 3-Prenyl-4dihydrocinnamoyloxycinnamic acid (2) | C23 H24 O4 | 363.1602 | 363.1607 | 1.42 | 0.02 |
| (E)-3-{-4-hydroxy-3-[(E)-4(2,3-dihydrocinnamoyl oxy)-3methyl-2-butenyl]-5-prenylphenyl}-2-propenoic acid (2) | C28 H32 O5 | 447.2177 | 447.2181 | 0.90 | 0.08 |
| 3,5-Diprenyl-4-hydroxy cinnamic acid (2) (DHCA) | C19 H24 O3 | 299.1653 | 299.1658 | 1.78 | 3.41 |
| 2,2-Dimethyl-8-prenyl-2H-1-benzopiran-6-propenoic acid (2) | C19 H24 O3 | 299.1653 | 299.1662 | 3.11 | 0.81 |
| 3-Prenyl-4-dihydrocinnamoyloxy cinnamic acid (2) | C23 H24 O4 | 363.1602 | 363.1607 | 1.42 | 0.21 |
| Total polyphenols | 8.71 |
| S-Candies | F-Candies | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Menthe | Orange | Strawberry | Average | Menthe | Orange | Strawberry | Average | ||||
| PEE | Added | M | M | M | M | M | M | M | M | ||
| P-CUM | Untreated | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | ||
| μg/g | P1 | 1.54 | 1.61 b | 1.54 b | 2.04 b | 1.73 b | 1.79 b | 1.63 b | 1.66 b | 1.69 b | |
| P2 | 3.08 | 3.26 a | 3.31 a | 3.56 a | 3.38 a | 3.32 a | 3.21 a | 2.95 a | 3.16 a | ||
| SEM | 0.422 | 0.428 | 0.449 | 0.238 | 0.429 | 0.414 | 0.383 | 0.222 | |||
| DHCA | Untreated | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | ||
| μg/g | P1 | 3.41 | 3.20 b | 2.95 b | 3.84 b | 3.33 b | 3.21 b | 3.02 b | 3.49 b | 3.24 b | |
| P2 | 6.82 | 6.45 a | 6.93 a | 6.54 a | 6.64 a | 6.80 a | 6.49 a | 6.44 a | 6.58 a | ||
| SEM | 1.237 | 0.898 | 0.849 | 0.467 | 0.882 | 0.823 | 0.835 | 0.462 | |||
| S-Candies | F-Candies | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Menthe | Orange | Average | Menthe | Orange | Strawberry | Average | |||
| PEE | M | M | M | M | M | M | M | ||
| TPC | Untreated | 12.44 b | 11.92 c | 15.36 c | 13.18 c | 14.60 c | 21.67 b | 16.48 c | |
| mg GAE/100 g | P1 | 13.48 b | 17.51 b | 17.81 b | 21.27 b | 17.89 b | 20.83 b | 20.00 b | |
| P2 | 23.44 a | 22.53 a | 25.11 a | 27.93 a | 21.11 a | 32.60 a | 27.12 a | ||
| SEM | 1.658 | 1.374 | 0.964 | 1.938 | 0.858 | 1.711 | 0.991 | ||
| ABTS | Untreated | 1.24 c | 2.20 c | 3.29 c | 2.05 c | 1.14 c | 4.98 c | 2.73 c | |
| mg TE/100 g | P1 | 5.43 b | 7.25 b | 8.29 b | 4.68 b | 7.10 b | 12.35 b | 8.04 b | |
| P2 | 12.15 a | 16.69 a | 15.30 a | 6.90 a | 8.36 a | 15.33 a | 10.20 a | ||
| SEM | 1.421 | 1.899 | 0.945 | 0.627 | 0.996 | 1.377 | 0.742 | ||
| DPPH | Untreated | 4.25 b | 3.88 b | 5.57 c | 4.67 c | 2.58 c | 9.49 c | 5.58 c | |
| mg TE/100 g | P1 | 4.67 b | 4.22 b | 7.05 b | 5.41 b | 5.46 b | 11.02 b | 7.29 b | |
| P2 | 7.73 a | 10.79 a | 11.32 a | 6.30 a | 7.50 a | 14.42 a | 9.40 a | ||
| SEM | 0.491 | 1.006 | 0.299 | 0.212 | 0.640 | 0.652 | 0.560 | ||
| S-Candies | F-Candies | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Menthe | Orange | Strawberry | Average | Menthe | Orange | Strawberry | Average | |||
| PEE | M | M | M | M | M | M | M | M | ||
| Moisture | Untreated | 18.86 b | 17.18 b | 17.64 b | 18.28 b | 22.90 | 25.24 | 22.64 b | 23.60 | |
| % w/w | P1 | 19.97 a | 20.31 a | 19.67 a | 19.98 a | 22.83 | 25.04 | 25.37 a | 24.41 | |
| P2 | 19.45 a | 20.16 a | 19.31 a | 19.64 a | 23.58 | 24.71 | 26.84 a | 25.04 | ||
| SEM | 0.142 | 0.527 | 0.316 | 0.137 | 0.686 | 0.187 | 0.686 | 0.289 | ||
| pH | Untreated | 3.38 | 3.48 | 3.36 | 3.41 | 3.19 | 3.17 a | 3.23 b | 3.20 | |
| P1 | 3.36 | 3.49 | 3.33 | 3.39 | 3.20 | 3.08 b | 3.27 a,b | 3.19 | ||
| P2 | 3.34 | 3.50 | 3.33 | 3.39 | 3.23 | 3.06 b | 3.31 a | 3.20 | ||
| SEM | 0.009 | 0.005 | 0.009 | 0.014 | 0.008 | 0.021 | 0.014 | 0.016 | ||
| Lightness | Untreated | 17.38 | 50.63 | 25.33 | 31.11 | 26.21 b | 46.40 b | 23.86 b | 32.16 b | |
| CIE units | P1 | 17.80 | 50.09 | 24.12 | 30.67 | 28.25 a | 48.51 a | 28.10 a | 34.96 a | |
| P2 | 18.24 | 50.25 | 25.03 | 31.17 | 27.46 a | 47.89 a | 29.48 a | 34.95 a | ||
| SEM | 0.203 | 0.107 | 0.355 | 2.838 | 0.350 | 0.335 | 0.861 | 1.909 | ||
| a* | Untreated | 4.71 | 7.95 b | 30.64 b | 14.43 | −4.20 | 11.60 | 32.64 | 13.35 | |
| CIE units | P1 | 4.28 | 7.52 a | 29.06 a | 13.62 | −6.79 | 11.39 | 33.02 | 12.54 | |
| P2 | 4.40 | 9.01 a | 34.88 a | 16.10 | −6.60 | 11.69 | 33.31 | 12.80 | ||
| SEM | 0.562 | 0.227 | 0.388 | 2.368 | 0.425 | 0.254 | 0.261 | 3.121 | ||
| b* | Untreated | −17.61 | 31.08 | 4.37 a | 5.95 | −21.63 a | 27.38 a,b | 4.59 | 3.45 | |
| CIE units | P1 | −18.20 | 31.77 | 2.96 b | 5.51 | −25.28 b | 25.67 b | 3.97 | 1.45 | |
| P2 | −17.78 | 32.64 | 5.69 a | 6.85 | −22.83 a | 28.20 a | 3.19 | 2.85 | ||
| SEM | 0.192 | 0.876 | 0.402 | 3.992 | 0.568 | 0.451 | 0.226 | 4.041 | ||
| Hardness | Untreated | 5.89 a | 3.54 | 4.73 a | 4.72 | 4.71 | 2.71 a | 3.13 a,b | 3.38 a,b | |
| N | P1 | 4.56 b | 3.23 | 3.76 b | 4.58 | 4.13 | 2.33 b | 2.41 b | 2.90 b | |
| P2 | 4.80 a,b | 3.80 | 4.24 a,b | 4.68 | 4.43 | 2.51 a,b | 4.19 a | 3.86 a | ||
| SEM | 0.236 | 0.100 | 0.143 | 0.132 | 0.160 | 0.129 | 0.248 | 0.119 | ||
| Springiness | Untreated | 5.52 | 5.54 | 5.52 | 5.36 | 5.36 | 5.73 | 5.58 | 5.62 | |
| mm | P1 | 5.56 | 5.54 | 5.42 | 5.51 | 5.51 | 5.57 | 5.53 | 5.60 | |
| P2 | 5.51 | 5.52 | 5.28 | 5.42 | 5.42 | 5.63 | 5.53 | 5.84 | ||
| SEM | 0.020 | 0.020 | 0.062 | 0.029 | 0.030 | 0.029 | 0.022 | 0.015 | ||
| Cohesiveness | Untreated | 0.88 | 0.93 | 0.79 | 0.83 | 0.88 | 0.97 | 0.94 | 0.96 | |
| No units | P1 | 0.90 | 0.92 | 0.90 | 0.89 | 0.90 | 0.94 | 0.92 | 0.94 | |
| P2 | 0.86 | 0.90 | 0.83 | 0.84 | 0.87 | 0.99 | 0.88 | 0.93 | ||
| SEM | 0.009 | 0.009 | 0.014 | 0.007 | 0.012 | 0.010 | 0.016 | 0.008 | ||
| Chewiness | Untreated | 28.68 | 18.05 | 18.57 | 20.32 | 22.35 | 15.00 a,b | 16.30 a,b | 18.07 a | |
| N x mm | P1 | 22.76 | 16.43 | 18.43 | 22.30 | 20.50 | 12.17 b | 12.27 b | 15.14 b | |
| P2 | 22.53 | 18.80 | 18.40 | 20.7 | 22.35 | 14.00 a,b | 19.10 a,b | 19.49 a | ||
| SEM | 1.177 | 0.503 | 0.785 | 0.625 | 0.730 | 0.714 | 1.028 | 0.528 | ||
| S-Candies | F-Candies | ||||
|---|---|---|---|---|---|
| Consumer Trials | Correct Identifications | p-Value | Correct Identifications | p-Value | |
| Menthe | |||||
| Untreated vs. P1 | 40 | 14 | NS | 12 | NS |
| Untreated vs. P2 | 40 | 19 | * | 12 | NS |
| P1 vs. P2 | 40 | 9 | NS | 7 | NS |
| Orange | |||||
| Untreated vs. P1 | 40 | 10 | NS | 17 | NS |
| Untreated vs. P2 | 40 | 17 | NS | 20 | * |
| P1 vs. P2 | 40 | 13 | NS | 10 | NS |
| Strawberry | |||||
| Untreated vs. P1 | 40 | 14 | NS | 14 | NS |
| Untreated vs. P2 | 40 | 14 | NS | 12 | NS |
| P1 vs. P2 | 40 | 13 | NS | 9 | NS |
| Average | |||||
| Untreated vs. P1 | 120 | 38 | NS | 43 | NS |
| Untreated vs. P2 | 120 | 50 | * | 44 | NS |
| P1 vs. P2 | 120 | 35 | NS | 26 | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cedeño-Pinos, C.; Marcucci, M.C.; Bañón, S. Contribution of Green Propolis to the Antioxidant, Physical, and Sensory Properties of Fruity Jelly Candies Made with Sugars or Fructans. Foods 2021, 10, 2586. https://doi.org/10.3390/foods10112586
Cedeño-Pinos C, Marcucci MC, Bañón S. Contribution of Green Propolis to the Antioxidant, Physical, and Sensory Properties of Fruity Jelly Candies Made with Sugars or Fructans. Foods. 2021; 10(11):2586. https://doi.org/10.3390/foods10112586
Chicago/Turabian StyleCedeño-Pinos, Cristina, María Cristina Marcucci, and Sancho Bañón. 2021. "Contribution of Green Propolis to the Antioxidant, Physical, and Sensory Properties of Fruity Jelly Candies Made with Sugars or Fructans" Foods 10, no. 11: 2586. https://doi.org/10.3390/foods10112586
APA StyleCedeño-Pinos, C., Marcucci, M. C., & Bañón, S. (2021). Contribution of Green Propolis to the Antioxidant, Physical, and Sensory Properties of Fruity Jelly Candies Made with Sugars or Fructans. Foods, 10(11), 2586. https://doi.org/10.3390/foods10112586