High Pressure Processing vs. Thermal Pasteurization of Whole Concord Grape Puree: Effect on Nutritional Value, Quality Parameters and Refrigerated Shelf Life
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Concord Grape Puree Preparation
2.3. Puree Preservation
2.3.1. High-Pressure Processing (HPP)
2.3.2. Heat Treatment (HT)
2.4. Refrigerated Storage
2.5. Microbial Analyses
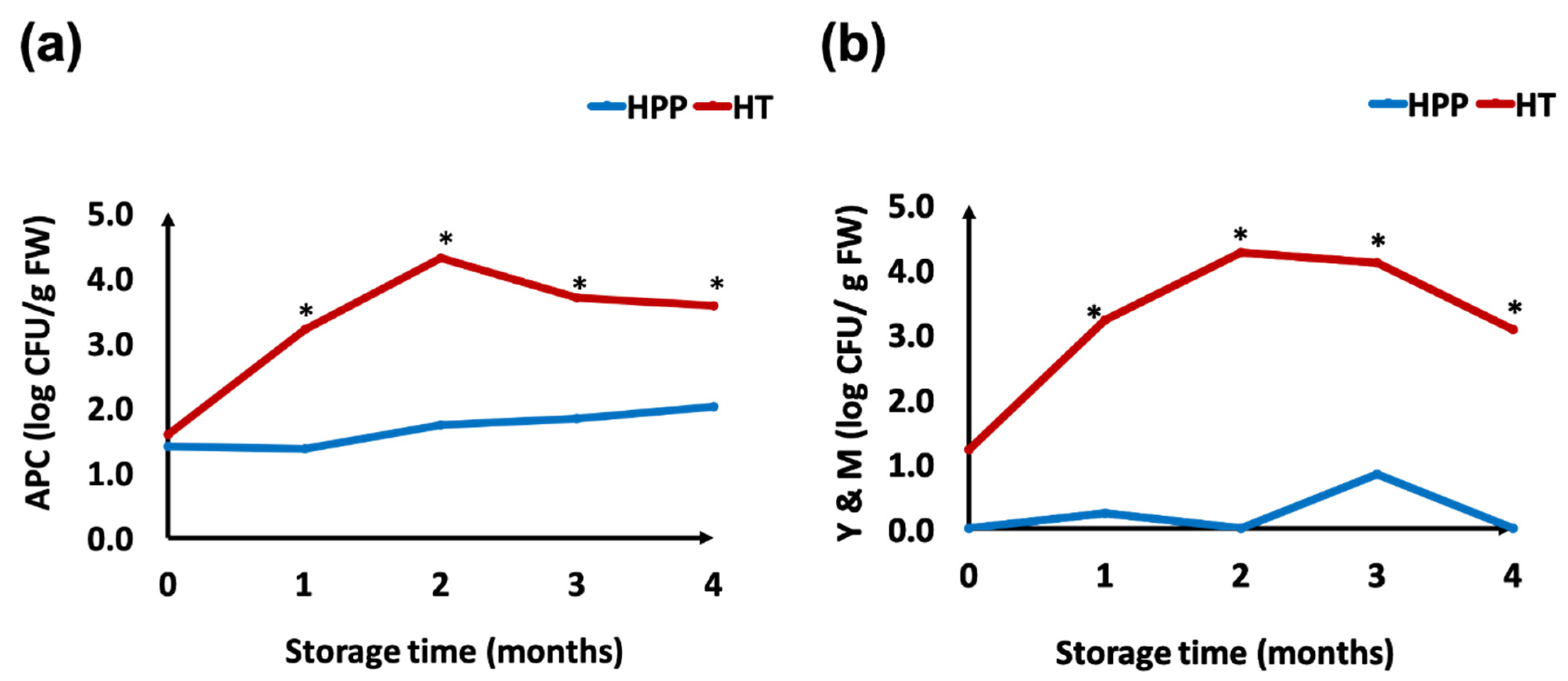
2.5.1. Total Aerobic Plate Count
2.5.2. Yeast and Mold Count
2.6. Physicochemical Properties Analyses
2.6.1. Total Soluble Solids Content (TSSC)
2.6.2. pH
2.6.3. Titratable Acidity (TA)
2.6.4. Color Measurement
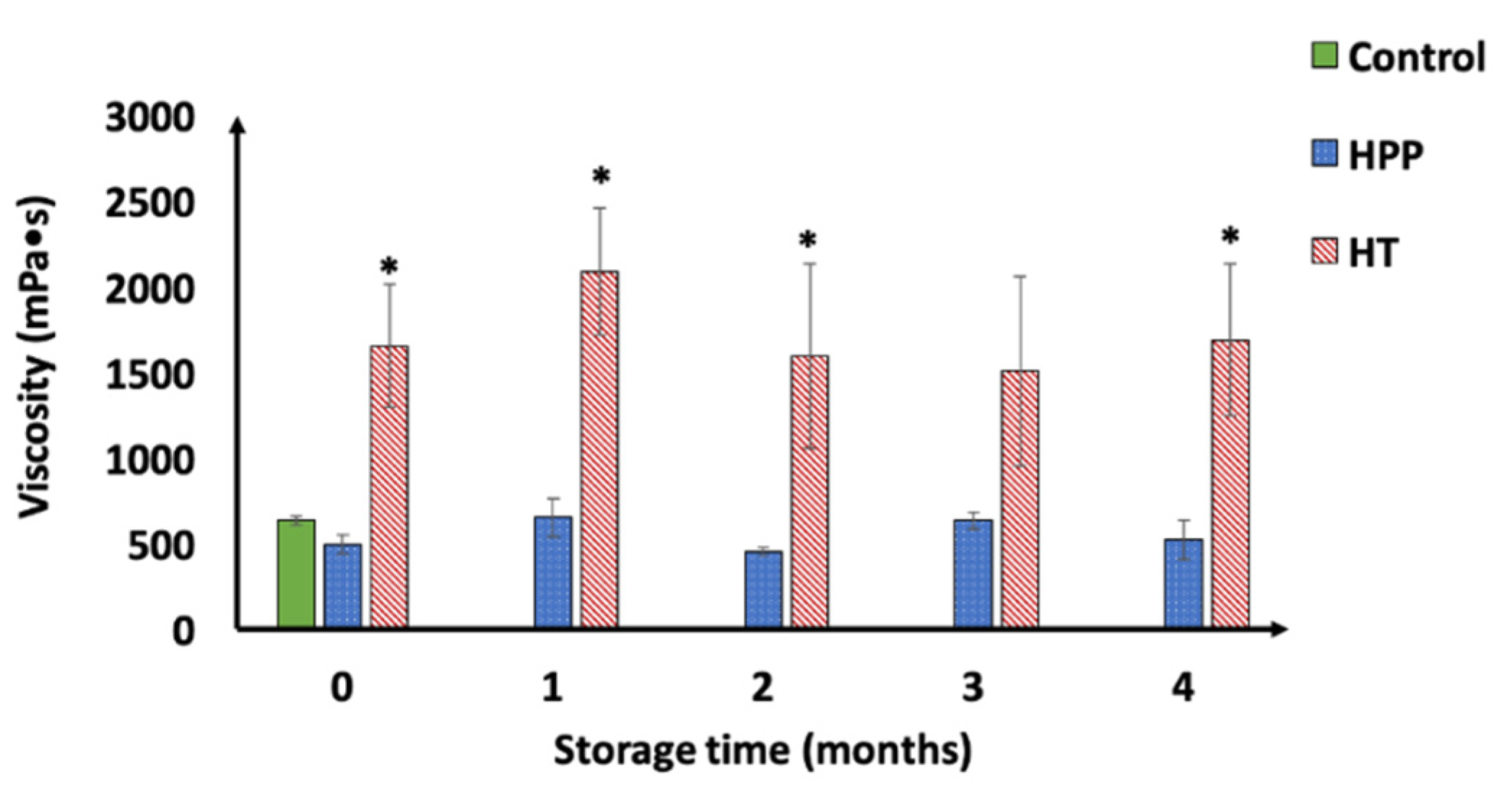
2.6.5. Particle Size Distribution (PSD), Serum Separation, and Viscosity
2.7. Phenolic Content and In Vitro Antioxidant Activity
2.7.1. Extraction of Total Phenols and Anthocyanins from Whole Concord Grape Puree
2.7.2. Total Phenolic Content (TP)
2.7.3. Total Monomeric Anthocyanin Content (TMA)
2.7.4. In Vitro Total Antioxidant Activity
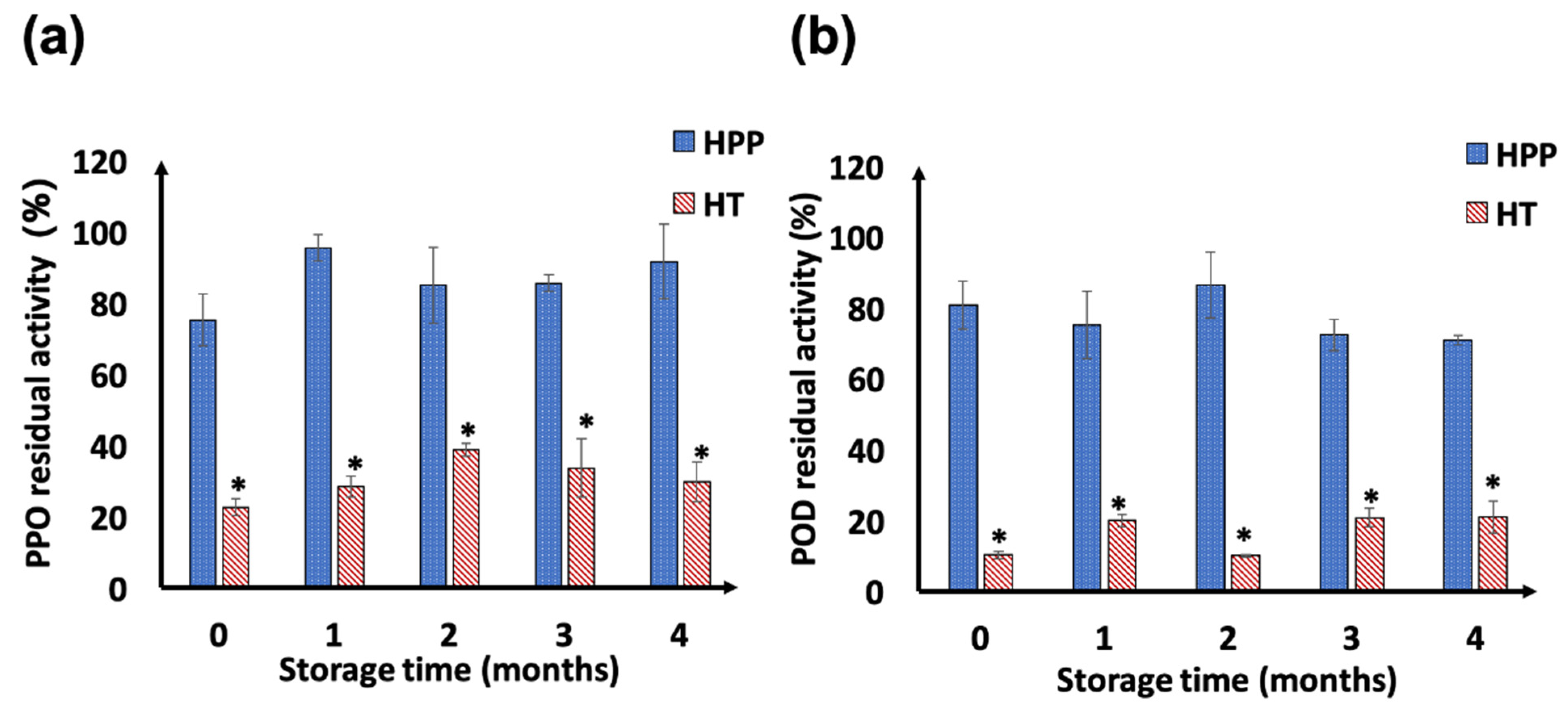
2.7.5. Enzymatic Activities: Polyphenoloxidases (PPO) and Peroxidases (POD)
2.8. Proximate Composition Analysis
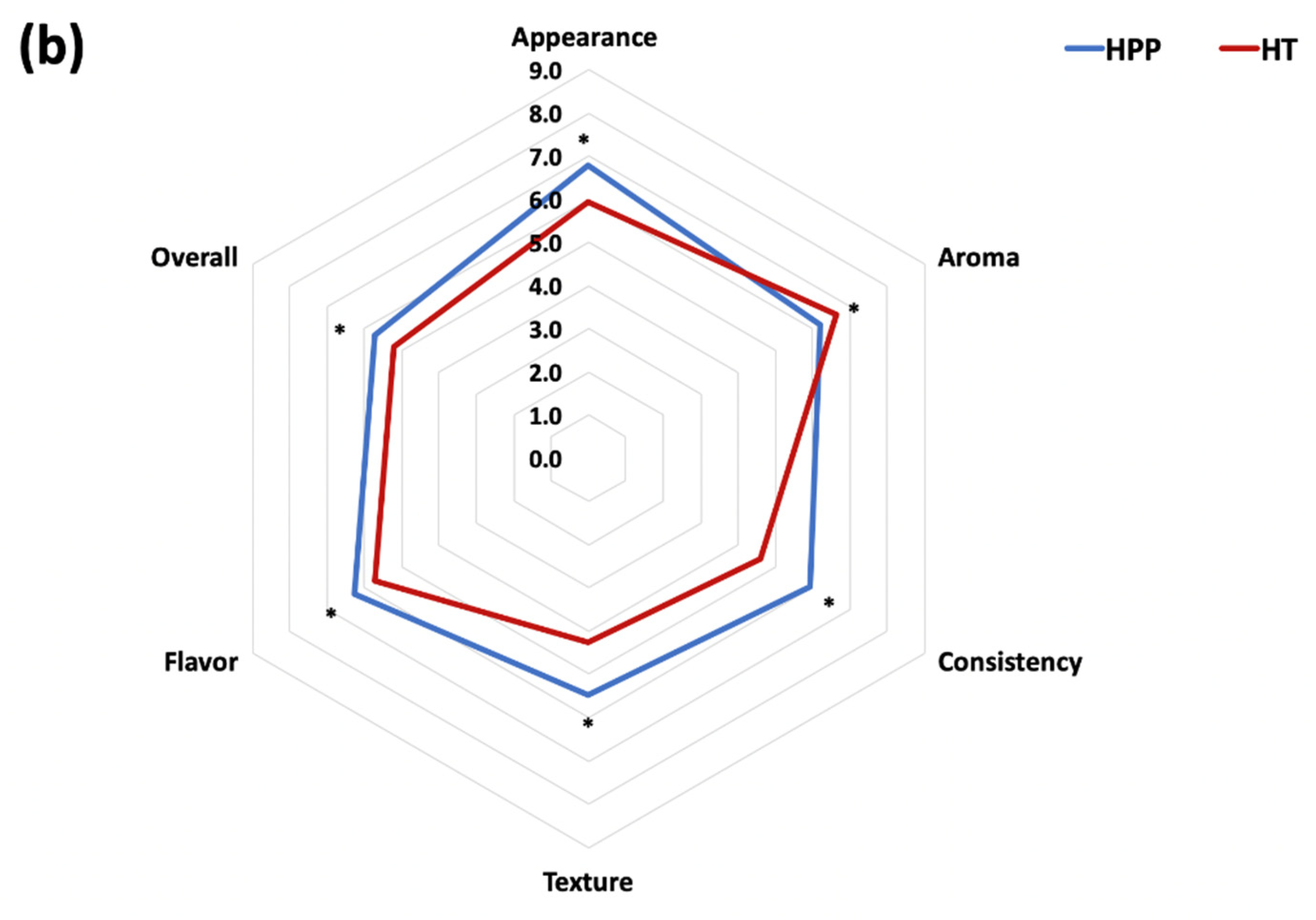
2.9. Sensory Study of HPP- and HT-Treated Purees
2.10. Statistical Analysis
3. Results and Discussion
3.1. Microbial Counts in Whole Concord Grape Puree during 5 Months Refrigerated Storage
3.2. Physicochemical Properties of Whole Concord Grape Puree
3.2.1. Total Soluble Solids Content (TSSC), pH, and Titratable Acidity (TA) of Whole Concord Grape Puree during 4 Months Refrigerated Storage
3.2.2. Effect of HPP and HT Treatments on Color Changes of Whole Concord Grape Puree
3.2.3. Effect of HPP and HT on Particle Size Distribution (PSD), Serum Separation Rate (SSR), and Viscosity of Concord Grape Puree
3.3. Total Phenolic Content (TP), Total Monomeric Anthocyanin Content (TMA), and Antioxidant Activity of Puree Samples
3.4. PPO and POD Enzyme Activity
3.5. Proximate Composition Analysis
3.6. Sensory Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/qc (accessed on 9 January 2021).
- Georgiev, V.; Ananga, A.; Tsolova, V. Recent Advances and Uses of Grape Flavonoids as Nutraceuticals. Nutrients 2014, 6, 391–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orak, H.H. Total antioxidant activities, phenolics, anthocyanins, polyphenoloxidase activities of selected red grape cultivars and their correlations. Sci. Hortic. 2007, 111, 235–241. [Google Scholar] [CrossRef]
- Anselm, E.; Chataigneau, M.; Ndiaye, M.; Chataigneau, T.; Schini-Kerth, V.B. Grape juice causes endothelium-dependent relaxation via a redox-sensitive Src- and Akt-dependent activation of eNOS. Cardiovasc. Res. 2007, 73, 404–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dohadwala, M.M.; Hamburg, N.M.; Holbrook, M.; Kim, B.H.; Duess, M.-A.; Levit, A.; Titas, M.; Chung, W.B.; Vincent, F.B.; Caiano, T.L.; et al. Effects of Concord grape juice on ambulatory blood pressure in prehypertension and stage 1 hypertension. Am. J. Clin. Nutr. 2010, 92, 1052–1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, Y.K.; Kim, J.-S.; Kang, M.-H. Concord grape juice supplementation reduces blood pressure in Korean hypertensive men: Double-blind, placebo controlled intervention trial. BioFactors 2004, 22, 145–147. [Google Scholar] [CrossRef]
- Shanmuganayagam, D.; Warner, T.F.; Krueger, C.G.; Reed, J.D.; Folts, J.D. Concord grape juice attenuates platelet aggregation, serum cholesterol and development of atheroma in hypercholesterolemic rabbits. Atherosclerosis 2007, 190, 135–142. [Google Scholar] [CrossRef]
- Siasos, G.; Tousoulis, D.; Kokkou, E.; Oikonomou, E.; Kollia, M.-E.; Verveniotis, A.; Gouliopoulos, N.; Zisimos, K.; Plastiras, A.; Maniatis, K.; et al. Favorable Effects of Concord Grape Juice on Endothelial Function and Arterial Stiffness in Healthy Smokers. Am. J. Hypertens. 2014, 27, 38–45. [Google Scholar] [CrossRef]
- Ho, L.; Ferruzzi, M.G.; Janle, E.M.; Lobo, J.; Chen, T.-Y.; Talcott, S.T.; Simon, J.; Wu, Q.L.; Wang, J.; Cheng, A.; et al. Bioavailability of grape-derived polyphenolics and implications in Alzheimer’s disease prevention and therapy. FASEB J. 2010, 24, 209.3. [Google Scholar] [CrossRef]
- Krikorian, R.; Nash, T.A.; Shidler, M.D.; Shukitt-Hale, B.; Joseph, J.A. Concord grape juice supplementation improves memory function in older adults with mild cognitive impairment. Br. J. Nutr. 2010, 103, 730–734. [Google Scholar] [CrossRef] [Green Version]
- Joseph, J.A.; Shukitt-Hale, B.; Willis, L.M. Grape Juice, Berries, and Walnuts Affect Brain Aging and Behavior. J. Nutr. 2009, 139, 1813S–1817S. [Google Scholar] [CrossRef] [Green Version]
- Shukitt-Hale, B.; Carey, A.; Simon, L.; Mark, D.A.; Joseph, J.A. Effects of Concord grape juice on cognitive and motor deficits in aging. Nutrition 2006, 22, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Singletary, K.W.; Stansbury, M.J.; Giusti, M.; van Breemen, R.B.; Wallig, M.; Rimando, A. Inhibition of Rat Mammary Tumorigenesis by Concord Grape Juice Constituents. J. Agric. Food Chem. 2003, 51, 7280–7286. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L. Phenolic Substances in Grapes and Wine, and Their Significance. 1969. Available online: https://agris.fao.org/agris-search/search.do?recordID=US201300459466 (accessed on 9 January 2021).
- Baydar, N.G.; Özkan, G.; Sağdiç, O. Total phenolic contents and antibacterial activities of grape (Vitis vinifera L.) extracts. Food Control 2004, 15, 335–339. [Google Scholar] [CrossRef]
- Raina, K.; Singh, R.P.; Agarwal, R.; Agarwal, C. Oral Grape Seed Extract Inhibits Prostate Tumor Growth and Progression in TRAMP Mice. Cancer Res. 2007, 67, 5976–5982. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.-Y.; Li, W.-G.; Wu, Y.-J.; Zheng, T.-Z.; Li, W.; Qu, S.-Y.; Liu, N.-F. Proanthocyanidin from grape seeds potentiates anti-tumor activity of doxorubicin via immunomodulatory mechanism. Int. Immunopharmacol. 2005, 5, 1247–1257. [Google Scholar] [CrossRef]
- Agarwal, C.; Veluri, R.; Kaur, M.; Chou, S.-C.; Thompson, J.A.; Agarwal, R. Fractionation of high molecular weight tannins in grape seed extract and identification of procyanidin B2-3,3′-di-O-gallate as a major active constituent causing growth inhibition and apoptotic death of DU145 human prostate carcinoma cells. Carcinogenesis 2007, 28, 1478–1484. [Google Scholar] [CrossRef] [Green Version]
- Kaur, M.; Singh, R.P.; Gu, M.; Agarwal, R.; Agarwal, C. Grape Seed Extract Inhibits In vitro and In vivo Growth of Human Colorectal Carcinoma Cells. Clin. Cancer Res. 2006, 12, 6194–6202. [Google Scholar] [CrossRef] [Green Version]
- Thomas, P.; Wang, Y.-J.; Zhong, J.-H.; Kosaraju, S.; O’Callaghan, N.J.; Zhou, X.-F.; Fenech, M. Grape seed polyphenols and curcumin reduce genomic instability events in a transgenic mouse model for Alzheimer’s disease. Mutat. Res. Mol. Mech. Mutagen. 2009, 661, 25–34. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Thomas, P.; Zhong, J.-H.; Bi, F.-F.; Kosaraju, S.; Pollard, A.; Fenech, M.; Zhou, X.-F. Consumption of Grape Seed Extract Prevents Amyloid-β Deposition and Attenuates Inflammation in Brain of an Alzheimer’s Disease Mouse. Neurotox. Res. 2009, 15, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Stockley, C.S.; Høj, P.B. Better wine for better health: Fact or fiction? Aust. J. Grape Wine Res. 2005, 11, 127–138. [Google Scholar] [CrossRef]
- Rattanathanalerk, M.; Chiewchan, N.; Srichumpoung, W. Effect of thermal processing on the quality loss of pineapple juice. J. Food Eng. 2005, 66, 259–265. [Google Scholar] [CrossRef]
- Rawson, A.; Patras, A.; Tiwari, B.K.; Noci, F.; Koutchma, T.; Brunton, N. Effect of thermal and non thermal processing technologies on the bioactive content of exotic fruits and their products: Review of recent advances. Food Res. Int. 2011, 44, 1875–1887. [Google Scholar] [CrossRef]
- Hogan, E.; Kelly, A.L.; Sun, D.-W. 1—High Pressure Processing of Foods: An Overview. In Emerging Technologies for Food Processing; Sun, D.-W., Ed.; Academic Press: London, UK, 2005; pp. 3–32. ISBN 978-0-12-676757-5. [Google Scholar]
- Martín, M.F.S.; Barbosa-Cánovas, G.V.; Swanson, B.G. Food Processing by High Hydrostatic Pressure. Crit. Rev. Food Sci. Nutr. 2002, 42, 627–645. [Google Scholar] [CrossRef]
- Butz, P.; Fernández García, A.; Lindauer, R.; Dieterich, S.; Bognár, A.; Tauscher, B. Influence of ultra high pressure processing on fruit and vegetable products. J. Food Eng. 2003, 56, 233–236. [Google Scholar] [CrossRef]
- Tauscher, B. Pasteurization of food by hydrostatic high pressure: Chemical aspects. Z. Für Lebensm.-Unters. Forsch. 1995, 200, 3–13. [Google Scholar] [CrossRef]
- Huang, H.-W.; Wu, S.-J.; Lu, J.-K.; Shyu, Y.-T.; Wang, C.-Y. Current status and future trends of high-pressure processing in food industry. Food Control 2017, 72, 1–8. [Google Scholar] [CrossRef]
- Suthanthangjai, W.; Kajda, P.; Zabetakis, I. The effect of high hydrostatic pressure on the anthocyanins of raspberry (Rubus idaeus). Food Chem. 2005, 90, 193–197. [Google Scholar] [CrossRef]
- Garcia-Palazon, A.; Suthanthangjai, W.; Kajda, P.; Zabetakis, I. The effects of high hydrostatic pressure on β-glucosidase, peroxidase and polyphenoloxidase in red raspberry (Rubus idaeus) and strawberry (Fragaria×ananassa). Food Chem. 2004, 88, 7–10. [Google Scholar] [CrossRef]
- Yuan, B.; Danao, M.-G.C.; Lu, M.; Weier, S.A.; Stratton, J.E.; Weller, C.L. High pressure processing (HPP) of aronia berry puree: Pilot scale processing and a shelf-life study. Innov. Food Sci. Emerg. Technol. 2018, 47, 241–248. [Google Scholar] [CrossRef]
- Yuan, B.; Danao, M.-G.C.; Stratton, J.E.; Weier, S.A.; Weller, C.L.; Lu, M. High pressure processing (HPP) of aronia berry purée: Effects on physicochemical properties, microbial counts, bioactive compounds, and antioxidant capacities. Innov. Food Sci. Emerg. Technol. 2018, 47, 249–255. [Google Scholar] [CrossRef]
- Landl, A.; Abadias, M.; Sárraga, C.; Viñas, I.; Picouet, P.A. Effect of high pressure processing on the quality of acidified Granny Smith apple purée product. Innov. Food Sci. Emerg. Technol. 2010, 11, 557–564. [Google Scholar] [CrossRef]
- Aaby, K.; Grimsbo, I.H.; Hovda, M.B.; Rode, T.M. Effect of high pressure and thermal processing on shelf life and quality of strawberry purée and juice. Food Chem. 2018, 260, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Bodelón, O.G.; Avizcuri, J.-M.; Fernández-Zurbano, P.; Dizy, M.; Préstamo, G. Pressurization and cold storage of strawberry purée: Colour, anthocyanins, ascorbic acid and pectin methylesterase. LWT-Food Sci. Technol. 2013, 52, 123–130. [Google Scholar] [CrossRef]
- Marszałek, K.; Mitek, M.; Skąpska, S. The effect of thermal pasteurization and high pressure processing at cold and mild temperatures on the chemical composition, microbial and enzyme activity in strawberry purée. Innov. Food Sci. Emerg. Technol. 2015, 27, 48–56. [Google Scholar] [CrossRef]
- Patras, A.; Brunton, N.P.; Da Pieve, S.; Butler, F. Impact of high pressure processing on total antioxidant activity, phenolic, ascorbic acid, anthocyanin content and colour of strawberry and blackberry purées. Innov. Food Sci. Emerg. Technol. 2009, 10, 308–313. [Google Scholar] [CrossRef]
- González-Cebrino, F.; Durán, R.; Delgado-Adámez, J.; Contador, R.; Ramírez, R. Changes after high-pressure processing on physicochemical parameters, bioactive compounds, and polyphenol oxidase activity of red flesh and peel plum purée. Innov. Food Sci. Emerg. Technol. 2013, 20, 34–41. [Google Scholar] [CrossRef]
- Chakraborty, S.; Rao, P.S.; Mishra, H.N. Effect of combined high pressure–temperature treatments on color and nutritional quality attributes of pineapple (Ananas comosus L.) puree. Innov. Food Sci. Emerg. Technol. 2015, 28, 10–21. [Google Scholar] [CrossRef]
- Palou, E.; López-Malo, A.; Barbosa-Cánovas, G.V.; Welti-Chanes, J.; Swanson, B.G. Polyphenoloxidase Activity and Color of Blanched and High Hydrostatic Pressure Treated Banana Puree. J. Food Sci. 1999, 64, 42–45. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, Y.; Ren, P.; Ni, Y.; Liao, X. Quality of Banana Puree During Storage: A Comparison of High Pressure Processing and Thermal Pasteurization Methods. Food Bioprocess Technol. 2016, 9, 407–420. [Google Scholar] [CrossRef]
- Castellari, M.; Matricardi, L.; Arfelli, G.; Rovere, P.; Amati, A. Effects of high pressure processing on polyphenoloxidase enzyme activity of grape musts. Food Chem. 1997, 60, 647–649. [Google Scholar] [CrossRef]
- Chang, Y.-H.; Wu, S.-J.; Chen, B.-Y.; Huang, H.-W.; Wang, C.-Y. Effect of high-pressure processing and thermal pasteurization on overall quality parameters of white grape juice. J. Sci. Food Agric. 2017, 97, 3166–3172. [Google Scholar] [CrossRef]
- van Wyk, S.; Farid, M.M.; Silva, F.V.M. SO2, high pressure processing and pulsed electric field treatments of red wine: Effect on sensory, Brettanomyces inactivation and other quality parameters during one year storage. Innov. Food Sci. Emerg. Technol. 2018, 48, 204–211. [Google Scholar] [CrossRef]
- van Wyk, S.; Silva, F.V.M. High pressure inactivation of Brettanomyces bruxellensis in red wine. Food Microbiol. 2017, 63, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Usaga, J.; Acosta, Ó.; Churey, J.J.; Padilla-Zakour, O.I.; Worobo, R.W. Evaluation of high pressure processing (HPP) inactivation of Escherichia coli O157:H7, Salmonella enterica, and Listeria monocytogenes in acid and acidified juices and beverages. Int. J. Food Microbiol. 2021, 339, 109034. [Google Scholar] [CrossRef]
- Tandon, K.; Worobo, R.W.; Churey, J.J.; Padilla-Zakour, O.I. Storage Quality of Pasteurized and Uv Treated Apple Cider. J. Food Process. Preserv. 2003, 27, 21–35. [Google Scholar] [CrossRef]
- Iland, P.; Brauer, N.; Edwards, G.; Weeks, S.; Wilks, E. Chemical Analysis of Grapes and Wines; Wine Promotions Pty Ltd.: Campbelltown, Australia, 2004; pp. 39–43, 45–48. [Google Scholar]
- Eliasson, A.-C.; Kim, H.R. Changes in Rheological Properties of Hydroxypropyl Potato Starch Pastes During Freeze-Thaw Treatments I. a Rheological Approach for Evaluation of Freeze-Thaw Stability. J. Texture Stud. 1992, 23, 279–295. [Google Scholar] [CrossRef]
- Jensen, J.S.; Blachez, B.; Egebo, M.; Meyer, A.S. Rapid Extraction of Polyphenols from Red Grapes. Am. J. Enol. Vitic. 2007, 58, 451–461. [Google Scholar]
- Waterhouse, A.L. Determination of Total Phenolics. Curr. Protoc. Food Anal. Chem. 2002, 6, I1.1.1–I1.1.8. [Google Scholar]
- Lee, J.; Durst, R.W.; Wrolstad, R.E.; Collaborators. Determination of Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines by the pH Differential Method: Collaborative Study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [Green Version]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Hall, M.B.; Hoover, W.H.; Jennings, J.P.; Webster, T.K.M. A method for partitioning neutral detergent-soluble carbohydrates. J. Sci. Food Agric. 1999, 79, 2079–2086. [Google Scholar] [CrossRef]
- Horwitz, W. Official Methods of Analysis of AOAC International. Volume I, Agricultural Chemicals, Contaminants, Drugs/Edited by William Horwitz; AOAC International 1997: Gaithersburg, MD, USA, 2010; ISBN 978-0-935584-67-7. [Google Scholar]
- O’Fallon, J.V.; Busboom, J.R.; Nelson, M.L.; Gaskins, C.T. A direct method for fatty acid methyl ester synthesis: Application to wet meat tissues, oils, and feedstuffs. J. Anim. Sci. 2007, 85, 1511–1521. [Google Scholar] [CrossRef] [Green Version]
- Petrus, R.; Churey, J.; Worobo, R. Searching for high pressure processing parameters for Escherichia coli O157:H7, Salmonella enterica and Listeria monocytogenes reduction in Concord grape juice. Br. Food J. 2019, 122, 170–180. [Google Scholar] [CrossRef]
- Barrett, D.M.; Beaulieu, J.C.; Shewfelt, R. Color, Flavor, Texture, and Nutritional Quality of Fresh-Cut Fruits and Vegetables: Desirable Levels, Instrumental and Sensory Measurement, and the Effects of Processing. Crit. Rev. Food Sci. Nutr. 2010, 50, 369–389. [Google Scholar] [CrossRef] [PubMed]
- Cash, J.N.; Sistrunk, W.A.; Stutte, C.A. Characteristics of Concord Grape Polyphenoloxidase Involved in Juice Color Loss. J. Food Sci. 1976, 41, 1398–1402. [Google Scholar] [CrossRef]
- Sistrunk, W.A.; Gascoigime, H.L. Stability of Color in‘Concord’Grape Juice and Expression of Color. J. Food Sci. 1983, 48, 430–433. [Google Scholar] [CrossRef]
- Bozkurt, H.; Göğüş, F.; Eren, S. Nonenzymic browning reactions in boiled grape juice and its models during storage. Food Chem. 1999, 64, 89–93. [Google Scholar] [CrossRef]
- Göğüş, F.; Bozkurt, H.; Eren, S. Kinetics of Maillard Reactions Between the Major Sugars and Amino Acids of Boiled Grape Juice. LWT-Food Sci. Technol. 1998, 31, 196–200. [Google Scholar] [CrossRef]
- Barba, F.J.; Esteve, M.J.; Frigola, A. Physicochemical and nutritional characteristics of blueberry juice after high pressure processing. Food Res. Int. 2013, 50, 545–549. [Google Scholar] [CrossRef]
- Cserhalmi, Z.; Sass-Kiss, Á.; Tóth-Markus, M.; Lechner, N. Study of pulsed electric field treated citrus juices. Innov. Food Sci. Emerg. Technol. 2006, 7, 49–54. [Google Scholar] [CrossRef]
- Ahmed, J.; Ramaswamy, H.S.; Hiremath, N. The effect of high pressure treatment on rheological characteristics and colour of mango pulp. Int. J. Food Sci. Technol. 2005, 40, 885–895. [Google Scholar] [CrossRef]
- Ditchfield, C.; Tadini, C.C.; Singh, R.; Toledo, R.T. Rheological Properties of Banana Puree at High Temperatures. Int. J. Food Prop. 2004, 7, 571–584. [Google Scholar] [CrossRef]
- Ilicali, C. Correlations for the consistency coefficients of apricot and pear purees. J. Food Process Eng. 1985, 8, 47–51. [Google Scholar] [CrossRef]
- Pelegrine, D.H.; Silva, F.C.; Gasparetto, C.A. Rheological Behavior of Pineapple and Mango Pulps. LWT-Food Sci. Technol. 2002, 35, 645–648. [Google Scholar] [CrossRef]
- Srisuvor, N.; Chinprahast, N.; Prakitchaiwattana, C.; Subhimaros, S. Effects of inulin and polydextrose on physicochemical and sensory properties of low-fat set yoghurt with probiotic-cultured banana purée. LWT-Food Sci. Technol. 2013, 51, 30–36. [Google Scholar] [CrossRef]
- Thakur, B.R.; Singh, R.K.; Nelson, P.E. Quality attributes of processed tomato products: A review. Food Rev. Int. 1996, 12, 375–401. [Google Scholar] [CrossRef]
- Terefe, N.S.; Buckow, R.; Versteeg, C. Quality-Related Enzymes in Fruit and Vegetable Products: Effects of Novel Food Processing Technologies, Part 1: High-Pressure Processing. Crit. Rev. Food Sci. Nutr. 2014, 54, 24–63. [Google Scholar] [CrossRef]
- Gonzalez, M.E.; Barrett, D.M. Thermal, High Pressure, and Electric Field Processing Effects on Plant Cell Membrane Integrity and Relevance to Fruit and Vegetable Quality. J. Food Sci. 2010, 75, R121–R130. [Google Scholar] [CrossRef] [Green Version]
- Lukhmana, N.; Kong, F.; Kerr, W.L.; Singh, R.K. Rheological and structural properties of tart cherry puree as affected by particle size reduction. LWT 2018, 90, 650–657. [Google Scholar] [CrossRef]
- Espinosa-Muñoz, L.; Renard, C.M.G.C.; Symoneaux, R.; Biau, N.; Cuvelier, G. Structural parameters that determine the rheological properties of apple puree. J. Food Eng. 2013, 119, 619–626. [Google Scholar] [CrossRef]
- Krikorian, R.; Boespflug, E.L.; Fleck, D.E.; Stein, A.L.; Wightman, J.D.; Shidler, M.D.; Sadat-Hossieny, S. Concord Grape Juice Supplementation and Neurocognitive Function in Human Aging. J. Agric. Food Chem. 2012, 60, 5736–5742. [Google Scholar] [CrossRef]
- Dohadwala, M.M.; Vita, J.A. Grapes and Cardiovascular Disease. J. Nutr. 2009, 139, 1788S–1793S. [Google Scholar] [CrossRef] [Green Version]
- O’Byrne, D.J.; Devaraj, S.; Grundy, S.M.; Jialal, I. Comparison of the antioxidant effects of Concord grape juice flavonoids α-tocopherol on markers of oxidative stress in healthy adults. Am. J. Clin. Nutr. 2002, 76, 1367–1374. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L. The Total Phenolic Content of Grape Berries during the Maturation of Several Varieties. Am. J. Enol. Vitic. 1966, 17, 126–134. [Google Scholar]
- Corrales, M.; Toepfl, S.; Butz, P.; Knorr, D.; Tauscher, B. Extraction of anthocyanins from grape by-products assisted by ultrasonics, high hydrostatic pressure or pulsed electric fields: A comparison. Innov. Food Sci. Emerg. Technol. 2008, 9, 85–91. [Google Scholar] [CrossRef]
- Vámos-Vigyázó, L.; Haard, N.F. Polyphenol oxidases and peroxidases in fruits and vegetables. CRC Crit. Rev. Food Sci. Nutr. 1981, 15, 49–127. [Google Scholar] [CrossRef] [PubMed]
- Ozgen, M.; Reese, R.N.; Tulio, A.Z.; Scheerens, J.C.; Miller, A.R. Modified 2,2-Azino-bis-3-ethylbenzothiazoline-6-sulfonic Acid (ABTS) Method to Measure Antioxidant Capacity of Selected Small Fruits and Comparison to Ferric Reducing Antioxidant Power (FRAP) and 2,2‘-Diphenyl-1-picrylhydrazyl (DPPH) Methods. J. Agric. Food Chem. 2006, 54, 1151–1157. [Google Scholar] [CrossRef]
- Arnous, A.; Makris, D.P.; Kefalas, P. Correlation of Pigment and Flavanol Content with Antioxidant Properties in Selected Aged Regional Wines from Greece. J. Food Compos. Anal. 2002, 15, 655–665. [Google Scholar] [CrossRef]
- Burns, J.; Gardner, P.T.; O’Neil, J.; Crawford, S.; Morecroft, I.; McPhail, D.B.; Lister, C.; Matthews, D.; MacLean, M.R.; Lean, M.E.J.; et al. Relationship among Antioxidant Activity, Vasodilation Capacity, and Phenolic Content of Red Wines. J. Agric. Food Chem. 2000, 48, 220–230. [Google Scholar] [CrossRef]
- Wang, S.Y.; Lin, H.-S. Antioxidant Activity in Fruits and Leaves of Blackberry, Raspberry, and Strawberry Varies with Cultivar and Developmental Stage. J. Agric. Food Chem. 2000, 48, 140–146. [Google Scholar] [CrossRef]
- Yıldırım, H.K.; Akçay, Y.D.; Güvenç, U.; Altındişli, A.; Sözmen, E.Y. Antioxidant activities of organic grape, pomace, juice, must, wine and their correlation with phenolic content. Int. J. Food Sci. Technol. 2005, 40, 133–142. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.-O.; Chung, S.-J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Kallithraka, S.; Mohdaly, A.A.-A.; Makris, D.P.; Kefalas, P. Determination of major anthocyanin pigments in Hellenic native grape varieties (Vitis vinifera sp.): Association with antiradical activity. J. Food Compos. Anal. 2005, 18, 375–386. [Google Scholar] [CrossRef]
- Yen, G.-C.; Lin, H.-T. Comparison of high pressure treatment and thermal pasteurization effects on the quality and shelf life of guava puree. Int. J. Food Sci. Technol. 1996, 31, 205–213. [Google Scholar] [CrossRef]
- Chakraborty, S.; Rao, P.S.; Mishra, H.N. Effect of pH on Enzyme Inactivation Kinetics in High-Pressure Processed Pineapple (Ananas comosus L.) Puree Using Response Surface Methodology. Food Bioprocess Technol. 2014, 7, 3629–3645. [Google Scholar] [CrossRef]
- Bravo, L.; Saura-Calixto, F. Characterization of Dietary Fiber and the In Vitro Indigestible Fraction of Grape Pomace. Am. J. Enol. Vitic. 1998, 49, 135–141. [Google Scholar]
- Saura-Calixto, F. Antioxidant Dietary Fiber Product: A New Concept and a Potential Food Ingredient. J. Agric. Food Chem. 1998, 46, 4303–4306. [Google Scholar] [CrossRef] [Green Version]
- Maier, T.; Schieber, A.; Kammerer, D.R.; Carle, R. Residues of grape (Vitis vinifera L.) seed oil production as a valuable source of phenolic antioxidants. Food Chem. 2009, 112, 551–559. [Google Scholar] [CrossRef]
- Garcia-Jares, C.; Vazquez, A.; Lamas, J.P.; Pajaro, M.; Alvarez-Casas, M.; Lores, M. Antioxidant White Grape Seed Phenolics: Pressurized Liquid Extracts from Different Varieties. Antioxidants 2015, 4, 737–749. [Google Scholar] [CrossRef] [Green Version]
- Lutterodt, H.; Slavin, M.; Whent, M.; Turner, E.; Yu, L.L. Fatty acid composition, oxidative stability, antioxidant and antiproliferative properties of selected cold-pressed grape seed oils and flours. Food Chem. 2011, 128, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Wijendran, V.; Hayes, K.C. Dietary n-6 and n-3 fatty acid balance and cardiovascular health. Annu. Rev. Nutr. 2004, 24, 597–615. [Google Scholar] [CrossRef] [PubMed]
- Šimunek, M.; Režek Jambrak, A.; Petrović, M.; Juretić, H.; Major, N.; Herceg, Z.; Hruškar, M.; Vukušić, T. Aroma Profile and Sensory Properties of Ultrasound-Treated Apple Juice and Nectar. Food Technol. Biotechnol. 2013, 51, 101–111. [Google Scholar]



| Storage Time (Months) | ||||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||
| TP (mg/g as GAE) | Control | 3.0 ± 0.1 a | - | - | - | - |
| HPP | 3.8 ± 0.5 bX | 3.0 ± 0.6 aY | 2.9 ± 0.2 aY | 3.2 ± 0.3 aXY | 2.6 ± 0.0 aY | |
| HT | 3.6 ± 0.0 abX | 3.6 ± 1.0 aX | 2.9 ± 0.3 aX | 3.2 ± 0.7 aX | 2.9 ± 0.3 aX | |
| TMA (mg/kg as CGE) | Control | 628 ± 35 a | - | - | - | - |
| HPP | 620 ± 110 aX | 590 ± 40 aX | 510 ± 50 aX | 520 ± 60 aX | 560 ± 30 aX | |
| HT | 790 ± 120 aX | 840 ± 40 bX | 650 ± 170 aX | 610 ± 180 aX | 610 ± 170 aX | |
| DPPH (TEAC µmol/g) | Control | 12.6 ± 0.3 a | - | - | - | - |
| HPP | 12.7 ± 0.1 aY | 13.2 ± 0.1 aX | 12.2 ± 0.2 aZ | 12.7 ± 0.2 aY | 12.6 ± 0.1 aY | |
| HT | 13.4 ± 0.1 aX | 13.2 ± 0.1 aX | 12.8 ± 0.2 aY | 12.8 ± 0.1 aY | 12.8 ± 0.1 aY | |
| ABTS (TEAC µmol/g) | Control | 34.7 ± 0.6 a | - | - | - | - |
| HPP | 38.1 ± 0.2 bW | 36.6 ± 0.5 aX | 32.3 ± 0.5 aZ | 36.6 ± 0.7 aX | 33.9 ± 0.5 aY | |
| HT | 37.8 ± 0.5 bY | 42.1 ± 0.6 bX | 36.1 ± 1.0 bY | 36 ± 0.4 aY | 33.6 ± 1.4 aZ | |
| Fresh Basis | ||||
|---|---|---|---|---|
| Control/W | Control/O | HPP/W | HT/W | |
| Moisture (%) | 75.90 ± 0.33 a | 78.40 ± 0.16 b | 75.33 ± 0.12 a | 75.07 ± 0.38 a |
| Dry matter (%) | 24.10 ± 0.33 a | 21.60 ± 0.16 b | 24.67 ± 0.12 a | 24.93 ± 0.38 a |
| Crude protein (%) | 0.93 ± 0.05 a | 0.70 ± 0.00 b | 0.90 ± 0.00 a | 0.97 ± 0.05 a |
| Crude Fiber (%) | 2.53 ± 0.05 a | 0.53 ± 0.05 b | 2.50 ± 0.00 a | 2.53 ± 0.09 a |
| WSC (%) | 14.43 ± 0.52 A | 13.97 ± 0.73 A | 14.67 ± 1.05 A | 15.80 ± 1.44 A |
| Total Fatty acids (%) | 0.29 ± 0.02 a | 0.06 ± 0.01 b | 0.28 ± 0.02 a | 0.32 ± 0.01 a |
| RUFAL (%) | 0.24 ± 0.02 a | 0.05 ± 0.01 b | 0.23 ± 0.02 a | 0.27 ± 0.01 a |
| Ash (%) | 0.93 ± 0.09 A | 1.14 ± 0.22 A | 0.90 ± 0.12 A | 1.13 ± 0.09 A |
| Calcium (%) | 0.02 ± 0.00 A | 0.01 ± 0.00 B | 0.02 ± 0.00 A | 0.02 ± 0.00 A |
| Phosphorus (%) | 0.02 ± 0.00 AB | 0.02 ± 0.00 A | 0.03 ± 0.00 B | 0.03 ± 0.00 B |
| Magnesium (%) | 0.01 ± 0.00 A | 0.01 ± 0.00 A | 0.01 ± 0.00 A | 0.01 ± 0.00 A |
| Potassium (%) | 0.34 ± 0.01 A | 0.35 ± 0.00 A | 0.32 ± 0.02 A | 0.36 ± 0.00 A |
| Sodium (%) | 0.00 ± 0.00 A | 0.00 ± 0.00 A | 0.00 ± 0.00 A | 0.00 ± 0.00 A |
| Iron (ppm) | 6.33 ± 3.30 A | 2.67 ± 0.94 A | 5.00 ± 0.82 A | 3.67 ± 0.47 A |
| Zinc (ppm) | <1.00 A | <1.00 A | <1.00 A | <1.00 A |
| Copper (ppm) | 1.00 ± 0.00 A | 1.00 ± 0.00 A | 2.00 ± 0.00 B | 1.67 ± 0.47 AB |
| Manganese (ppm) | 3.00 ± 0.00 A | 2.00 ± 0.00 B | 3.00 ± 0.00 A | 3.00 ± 0.00 A |
| Molybdenum (ppm) | <1.00 A | <1.00 A | <1.00 A | <1.00 A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Padilla-Zakour, O.I. High Pressure Processing vs. Thermal Pasteurization of Whole Concord Grape Puree: Effect on Nutritional Value, Quality Parameters and Refrigerated Shelf Life. Foods 2021, 10, 2608. https://doi.org/10.3390/foods10112608
Li Y, Padilla-Zakour OI. High Pressure Processing vs. Thermal Pasteurization of Whole Concord Grape Puree: Effect on Nutritional Value, Quality Parameters and Refrigerated Shelf Life. Foods. 2021; 10(11):2608. https://doi.org/10.3390/foods10112608
Chicago/Turabian StyleLi, Yuanyuan, and Olga I. Padilla-Zakour. 2021. "High Pressure Processing vs. Thermal Pasteurization of Whole Concord Grape Puree: Effect on Nutritional Value, Quality Parameters and Refrigerated Shelf Life" Foods 10, no. 11: 2608. https://doi.org/10.3390/foods10112608
APA StyleLi, Y., & Padilla-Zakour, O. I. (2021). High Pressure Processing vs. Thermal Pasteurization of Whole Concord Grape Puree: Effect on Nutritional Value, Quality Parameters and Refrigerated Shelf Life. Foods, 10(11), 2608. https://doi.org/10.3390/foods10112608






